ABSTRACT
The purpose of this study was to provide an up‐to‐date review for the accurate estimation of the efficacy of extracorporeal shock wave therapy (ESWT) on the healing of chronic wounds on the lower extremity (CWLE). A systematic review of 10 databases for clinical trials about ESWT in the management of CWLE published between 2000 and 2016 was performed. A total of 11 studies with 925 patients were found. Expert therapists assessed the methodological qualities of the selected studies using the Physiotherapy Evidence Database (PEDro) scale and categorised each study according to Sackett's levels of evidence. Eight studies were categorised as level II; two studies were categorised as level III and one study was categorised as level V. In conclusion, this review demonstrated mild to moderate evidence to support the use of ESWT as an adjuvant therapy with a standardised wound care programme. However, it is difficult to draw firm conclusions about the efficacy of ESWT. So, future researches with high methodological quality are required to assess the efficacy and cost‐effectiveness of this relatively new physical therapy application.
Keywords: Chronic wounds of the lower extremity, Extracorporeal shock wave therapy
Introduction
Chronic wounds are defined as wounds that are unresponsive to initial therapy or the failure of proper care to produce anatomic and functional integrity over a period of 3 months 1, 2. Chronic wounds of the lower extremities (CWLEs) have a significant effect on the amount of resources spent every year to treat, prevent or slow down the progress of the disease 1, 3, 4. CWLEs cause limitations in daily living activities, impaired mobility and decreased work capacity, restricted social activities and negative body image, loss of productivity and reduced quality of life 5, 6.
The most common types of CWLEs are described by their aetiology. These include venous, arterial, neurotrophic, lymphatic, malignant, infectious and inflammatory 7, 8. Currently, 15% of diabetic patients will develop CWLEs, and about 25% of those will have to undergo amputation 9, 10. However,
the most common cause of chronic ulceration of the lower extremities is venous insufficiency. Although the risk of amputation associated with venous ulceration is lower than diabetic ulceration, the prognosis for healing is only 40%, and the rate of recurrence averages 75% 11.
Treatment of chronic wounds involves various approaches to protect and promote healing. These include hyperbaric oxygen therapy (HBOT) 12, vacuum‐assisted wound closure 13, low‐level laser therapy 14 and electrical stimulation 15. However, results from these studies are inconsistent and reported limited success with no conclusive remark on its effect 12, 13, 14, 15, 16, 17.
A relatively new physical therapy application for chronic wounds is represented by extracorporeal shock wave therapy (ESWT). ESWs are biphasic acoustic waves, characterised by a high‐peak pressure (up to 100 MPa), short‐time duration (<1000 ns) and rapid rise in pressure (< 10 ns) 18, 19, 20, 21. For the past 20 years, ESWT has been used for the treatment of musculoskeletal disorders 22, 23, 24, 25.
In traditional ESW devices, an electromagnetic, electrohydraulic or piezoelectric source generates shock waves that concentrate (‘focus’) the acoustic energy beam on the target by means of a parabolic lens, with a penetration depth of approximately 12 cm 19, 20, 21. To treat larger surfaces of tissue loss (as in ulcers, chronic or complicated wounds), some ESW generators are designed and marketed to generate unfocused ESWs: the same biphasic wave assumes the form of a planar or defocused wave during application. Obviously, for these devices, the depth of penetration is lower so that their therapeutic indication is limited to the more superficial lesions, like cutaneous ulcers and related disorders 21. Pneumatic sources generate ball‐shaped waves, which propagate in a spherical way, thus driving the descriptive term of ‘radial waves,’ spreading across a wider target area with a penetration depth of approximately 3 cm. Compared to the conventional shock waves, radial waves differ for the centring of the focus (placed on the tip of the applicator instead of on the target site) and the shape of the waves themselves (showing a lower peak pressure and a very long rise).
ESWT is considered feasible for healing of the chronic wound in humans 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37. However, the findings of studies conducted so far are still inconclusive, and the clinical use for wound healing is under investigation. Despite the increasing use of ESWT, substantial uncertainty continues to exist because of insufficient and inconsistent supporting evidence. The limited scientific information available has been obtained from animal studies 38, 39, 40, case series studies 26, 31, 36, 37, single case reports 41, 42 and a few clinical trials 27, 28, 29, 30, 32, 33.
In the last 5 years, five systematic reviews 22, 25, 43, 44, 45 provided limited evidence to support the use of ESWT for the treatment of lower limb ulceration. However, ESWT may play an important role in wound care once evidence‐based practice guidelines are developed. For these reasons, the authors recognised the need for an up‐to‐date systematic review to detect a more accurate estimation of the efficacy of ESWT and their impact on the healing of chronic lower limb ulcers.
Materials and methods
Search strategy
As a first step in this review process, we framed our review questions using the PICOT (population, intervention/exposure, comparative, outcome, time frame) framework as in Table 1. The authors conducted a comprehensive search using the following electronic databases: OVID, MEDLINE, CINAHL, EMBASE, PubMed, Physiotherapy Evidence Database (PEDro), Scopus, Web of knowledge, the Cochrane Central Register of Controlled Trials (CENTRAL) and Clinical trial register. Only articles published in English since the 2000s were included.
Table 1.
The PICOT format
| Population | Male and/or females adult >18 years or older with chronic wounds of the lower extremities (CWLEs) including diabetic foot ulcer (DFU), traumatic wound (TW), venous and/or arterial leg ulcer (VLU, ALU), surgical wound (SW), burn wound (BW) and pressure ulcers (PU). |
|---|---|
| Intervention/exposure | Extracorporeal shock wave therapy (ESWT, focused/unfocused). |
| Comparative | Any standard wound care, sham and other physical modalities and different ESWT protocols. |
| Outcome |
Wound healing, measured objectively:
|
| Time frame | If more than one follow‐up set of data presented within the same category of timing of an outcome measure, only one set was considered (e.g. short‐term follow‐up refers to outcomes measured closest to 4 weeks after randomisation; intermediate follow‐up refers to measures taken at least 6 months after treatment, and long‐term follow‐up refers to measurement taken close to 2 years after treatment) 38. |
Keywords and Medical Subject Headings (MeSH) used included ‘foot ulcer’, ‘diabetic or neuropathic foot ulcer’, ‘diabetic wound’, ‘chronic wound’, ‘ulcer’,‘ traumatic wound’, ‘venous or arterial leg ulcers’, ‘Shock wave therapy (SWT)’ and ‘ESWT’. Upon completing the electronic search, one reviewer (MO) examined reference lists of all relevant articles to capture additional articles that met inclusion criteria and may not be indexed in the database.
Criteria for considering studies for this review
Type of studies
All randomised controlled trials (RCTs), quasi‐experimental, control before‐and‐after design and crossover design were eligible for inclusion in this review because of a limited number of studies.
Data extraction
Two reviewers independently screened all identified studies based on the titles and abstracts to detect possible eligibility. We retrieved the full reports of all related trials for further assessment of eligibility based on the inclusion criteria. Two reviewers (MO and RG) independently retrieved data from the included studies using a standardised form of data extraction: (i) characteristics of the trial (setting, location of care, country, level of evidence); (ii) participants (number of subjects, age, gender, type and size of the wound, duration of the wound, length of follow‐up); (iii) intervention, including ESWT protocol (type of generator, energy flux density, frequency, number of pulses per square centimetre, number of treatment sessions, time interval between each session); (iv) comparison intervention and (v) results of all relevant outcomes. The reviewers were not blind of studies authorship. In case of any disagreement, the third reviewer (AS) was involved, and an agreement was achieved to reach consensus.
Level of evidence
The studies were categorised according to Sackett's rules of evidence. Sackett's rules of evidence ranges from high certainty to decreasing certainty, from level I to V, respectively. Level I is a large, RCT (included 100 or more participants) with low false‐positive or false‐negative errors. Level II is a small RCT with high false‐positive or low false‐negative errors. Level III is a non‐randomised, concurrent cohort comparison between subjects who did and did not receive the intervention. Level IV is a non‐randomised, historical cohort comparison between current subjects who received the intervention and former subjects who did not receive the intervention. Level V is a case series without controls 46.
Assessment of methodological quality
Assessment of methodological quality was performed using the PEDro scale. This tool is reliable 47 and valid 48 for rating the quality of RCTs. Each of the 11 items of the PEDro scale was scored as ‘yes’ where quality met the specified criteria and ‘no’ where criteria were unclear. Two reviewers (MO and AS) independently assessed and scored each of the articles and presented their findings to the entire group. In case of any disagreement, the third reviewer (RG) was involved, and an agreement was achieved to reach consensus. Studies scoring 9–10 on the PEDro scale were considered methodologically to be of ‘excellent’ quality; scores ranging from 6 to 8 were considered to be of ‘good’ quality, studies scoring 4 or 5 of ‘fair’ quality, and studies scoring less than 4 were of ‘poor’ quality 47, 48, 49.
Results
Search results
Figure 1 illustrates the flow chart of the study selection for systematic review. A total of 594 studies were found in the databases. After reviewing the title and abstract using the inclusion and exclusion criteria, 583 studies were rejected, while the remaining 11 studies met the inclusion criteria for this review. Overall, five studies were conducted in Europe 30, 31, 35, 36, 37, five studies in Asia 26, 27, 28, 32, 34 and one study in Africa 29. These studies included seven RCTs 27, 28, 29, 30, 32, 34, 35, one clinical controlled trial (CCT) 36 and three case series studies (CSS) 26, 31, 37. The studies were critically evaluated. Eight studies were categorised as level II 27, 28, 29, 30, 32, 34, 35, 36, two studies as level III 31, 37 and one study as level V 26.
Figure 1.
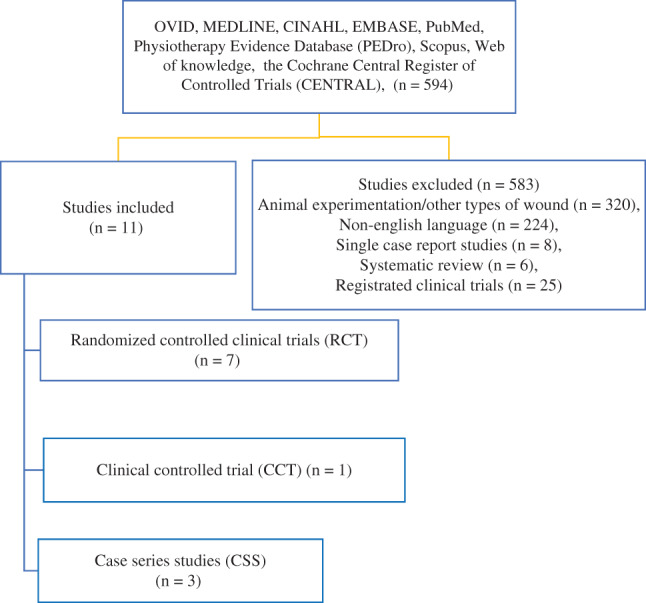
Study flow chart.
Participant characteristics
Table 2 summarises the participant characteristics and initial wound parameters. The number of participants included in the studies was 925 (range: 5–258), where 787 (85%) participants received ESWT, and 138 (15%) participants were placed in the comparison groups. The mean age of the participants was 59·5 years (range: 18–95 years). Gender distribution was not reported in two studies 32, 34. However, the majority of the participants in the remaining nine studies were male (58%) 26, 27, 28, 29, 30, 31, 35, 36, 37.
Table 2.
Summary of the participant characteristics and initial wound parameters of selected studies
| Authors | Design Level of evidence | Groups (n) | Mean age, y (range) Male/female, n | Wound parameters | ||
|---|---|---|---|---|---|---|
| Types, n | Size (cm2) | Duration (months) | ||||
| Variji et al., 2015 26 | CSS IV | ESWT group (n = 5) | 58·2 (33–75) 4/1 | DFU, n = 5 | 7·54 ± 5·05 | 19 ± 29 |
| Omar et al., 2014 27 | RCT II | ESWT group (n = 19) | 56 (45–65) 14/5 | DFU, n = 24 | 7·89 ± 2·97 | 11·97 ± 6·50 |
| Control group (n = 19) | 57 (45–65) 13/6 | DFU, n = 21 | 8·62 ± 3·47 | 10·81 ± 4·63 | ||
| Wang et al., 2014 28 | RCT II | DM group (n = 29) | 59·03 (33–81) 28/10 | DFU, n = 30 | 9·54 ± 15·44 | 20·9 ± 20·35 |
| NDM group (n = 28) | 60·75 (18–77) 15·14 | Non‐DFU, n = 30 | 8·49 ± 17·38 | 13·19 ± 15·36 | ||
| Nossair et al., 2013 29 | RCT II | ESWT group (n = 20) | 56·6y (44–64) 15/5 | DFU, n = 20 | 8·86 ± 3·41 | N/A |
| Control group (n = 20) | 55·15 (42–64) 14/6 | DFU, n = 20 | 8·32 ± 3·88 | N/A | ||
| Saggini et al., 2013 30 | RCT II | ESWT group (n = 62) | 62 (28–80) 39/23 |
DFU, n = 23; PU, n = 10; TW, n = 10; VLU, n = 19 |
3·85 | 10 (3–24) |
| ESWT group (n = 40) | 61 (33–77) 16/24 | DFU, n = 9; PU, n = 1; TW, n = 11; VLU, n = 14; cryoglobulinemia, n = 1 | 3·4 | |||
| Wolff et al., 2011 31 | CSS III | ESWT group (n = 258) | 63·5 (46–79) 140/118 | SW, n = 93; decubital ulcer, n = 13; TW, n = 86; cast ulcer, n = 9; VLU, n =38; AIU, n = 11; BW, n = 8 | 5 (0·5–300) | 76·36% < 3 12% >4 < 12 10·85% > 12 |
| Wang et al., 2011 32 | RCT II | ESWT group (n = 39) | 60·51 (20–81) N/A | DFU, n = 44 | 4 (1·5–9) | 6 (3–16) |
| HBOT group (n = 38) | 62·45 (23–88) N/A | DFU, n = 40 | 7 (2–12) | 6 (6–10) | ||
| Wang et al., 2009 34 | RCT II | ESWT group (n = 34) | 58·6 (33–79) N/A | DFU, n = 36 | 11·2 ± 20 | 22·7 ± 20·9 |
| HBOT group (n = 36) | 63·4 (39–81) N/A | DFU, n = 36 | 10·5 ± 20 | 19 ± 19·5 | ||
| Moretti et al., 2009 35 | RCT II | ESWT group (n = 15) | 56·2 (47–66) 9/6 | DFU, n = 15 | 2·97 ± 1·29 | 6 (3–24) |
| Control group (n = 15) | 56·8 (43–68y) 8/7 | DFU, n = 15 | 2·45 ± 1·09 | |||
| Saggini et al. 2008 36 | CCT II | ESWT group (n = 30) | 58·5 (24–79) 18/12 | VLU, n = 12; TW, n = 16; DFU, n = 4 VLU, n = 12; TW, n = 16; DFU, n = 4 | 5·15 (1–15·1) | 5·34 (3–8) |
| Control group (n = 10) | 66·6 (55–78) 6/4 | VLU, n = 5; TW, n = 2; DFU, n = 3 | 6·12 (1·9–12·2) | 5·2 (3–8) | ||
| Schaden et al., 2007 37 | CSS III | ESWT group (n = 208) | 61 (18–95) 109/99 | DWH, n = 82; AIU, n = 6; TW, n = 67BW, n = 7; PU, n = 21; VLU, n = 14 | 8·76 (3·7–16·1) | 79% ≤ 1 4% >1 < 12 14% > 12 |
ALU, arterial leg ulcers; BW, burn wound; CCT, control clinical trial; CSS, control series studies; DFU, diabetic foot ulcer; DM, diabetes mellitus; DWH, disturbed wound healing; ESWT, extracorporeal shock wave therapy; HBOT, hyperbaric oxygen therapy; N/A, not available; NDM, non‐diabetes mellitus; PU, pressure ulcers; RCT, randomised clinical trial; SW, surgical wound; TW, traumatic wound; VLU, venous leg ulcers.
A total of 947 lower limb ulcerations were available. These included diabetic foot ulcers (DFUs, n = 376; 39·6%), traumatic wounds (TWs, n = 192; 20·3%), venous leg ulcers (VLUs, n = 115; 12%), pressure ulcers (PUs, n = 54; 5·7%), arterial leg ulcers (ALUs, n = 17; 1·8%), acute burn wound (BWs, n = 15; 1·6%), disturbed wound healing (DWH, n = 85; 9%) and surgical wound (SWs, n = 93; 10%). The average size of wound surface area (WSA) was 6·83 ± 2·65 cm2 (range: 2·45–11·20 cm2). Non‐significant differences in WSA (6·79 ± 2·71 and 6·86 ± 2·75 cm2, P > 0·05) were observed between ESWT and the comparison groups, respectively. The duration of lower limb ulceration was reported in all studies except one 29 and ranged between 1 and 22 months. There was variability in the wound types, duration and characteristics across the studies. The majority of the treated wounds were chronic (>3 months) except in one study 37, while five studies included wounds of mixed aetiology 28, 30, 31, 36, 37.
Intervention parameters
Table 3 provides details of therapeutic parameters of the selected studies and follow‐up time. The majority of the studies included control groups. These groups received standard wound care (SWC) in four studies 27, 29, 35, 36 and HBOT in two studies 32, 34, while one study 30 compared two protocols of ESWT. In all studies 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, the wound dressing regimen and SWC remained unchanged after each treatment session.
Table 3.
Therapeutic parameters of ESWT of the selected studies and follow‐up time
| Authors | ESWT source | ESWT generators | EFD (mJ/mm2) | ESWT frequency (Hz) | Number of ESWT pulse (pulses/cm2) | Number of sessions | Time interval between sessions | Duration/follow‐up |
|---|---|---|---|---|---|---|---|---|
| Variji et al., 2015 26 | Electromagnetic | Unfocused | 0·25 | N/A | 600 | 6–8 | N/A | 6–8 wks/N/A |
| Omar et al., 2014 27 | Pneumatic | Unfocused | 0·11 | N/A | 100 | 8 | 1 wk | 8/20 wks |
| Wang et al., 2014 28 | Electromagnetic | Unfocused | 0·11 | 4 | At least 500 | 6 | 12/5 yrs | |
| Nossair et al., 2013 29 | Pneumatic | Unfocused | 0·1 | N/A | At least 500 | 3 | 1 wk | 12 wks/N/A |
| Saggini et al., 2013 30 | Electrohydraulic | Unfocused | 0·1 | 4 | 300–600 | 7 | 1 wk | 7 wks/N/A |
| 0·04 | ||||||||
| Wolff et al., 2011 31 | Electrohydraulic | Unfocused | 0·1 | 5 | 167 (100–300) | 1–10 | 2 wks | 15–17 wks /1 year |
| Wang et al., 2011 32 | Electromagnetic | Unfocused | 0·23 | 4 | at least 500 | 6 | 12 wks/N/A | |
| Wang et al., 2009 34 | Electrohydraulic | Focused | 0·11 | N/A | 300 + 100 | 3 | 2 wks | 6 wks/N/A |
| Moretti et al., 2009 35 | Electromagnetic | Focused | 0·03 | N/A | 100 | 3 | 3 days | 20 wks/N/A |
| Saggini et al., 2008 36 | Electrohydraulic | Unfocused | 0·037 | 4 | 100 | 4–10 | 2 wks | 8–20 wks/NA |
| Schaden et al., 2007 37 | Electrohydraulic | Unfocused | 0·1 | 5 | 100 | 3 (1–10) | 2 wks | 8 wks/N/A |
EFD, energy flux density; ESWT, extracorporeal shock wave therapy; Hz, hertz; mJ/mm2, mill joule per millimetre;N/A, not available; wk, week.
The source of the ESWT generator was electrohydraulic in five studies 30, 31, 34, 36, 37, electromagnetic in four studies 26, 28, 32, 35 and pneumatic in two studies 27, 29. Two types of ESWT generator heads were detected: unfocused ESWT in the majority of the studies 26, 27, 28, 29, 30, 31, 32, 36, 37 and focused ESWT in two studies 34, 35.
The dosage of ESW differs across the studies. It ranges between 0·03 and 0·25 mJ/mm2, with the most regular value set at 0·1 mJ/mm2 in four studies 29, 30, 31, 37 and 0·11 mJ/mm2 in three studies 27, 28, 34. The majority (82%) of reviewing studies 27, 28, 29, 30, 31, 34, 35, 36, 37 used low‐energy ESW (≤0·11 mJ/mm2), while only two studies 26, 32 used medium‐energy ESW (0·12–0·28 mmJ/mm2). Frequency was set at 4 Hz in four studies 28, 30, 32, 36 and 5 Hz in two studies 31, 37, while five studies did not describe that parameter 26, 27, 29, 34, 35. In most of the studies, the number of pulses in a single ESWT session ranged from 100 to 500 pulses/cm2 27, 28, 29, 31, 32, 34, 35, 36, 37, with the most frequent value being 100 pulses/cm2. The number of treatment sessions ranged between three and eight sessions in most of the studies 26, 27, 28, 29, 30, 32, 34, 35. However, in some studies 31, 36, 37, ESW application was continued for 10 sessions. The time interval between each session ranged from 3 days to 3 weeks, with a time interval of 1 and 2 weeks reported in 73% of the studies (21, 27, 29, 30, 31, 32, 34, 36, 37), while two studies used daily sessions of ESWT 28, 35. In all studies, the ESWT was performed without anaesthesia, and the wound was covered with a sterile plastic barrier 27, 28, 30, 32, 34, 37, single layer of sterilised gauze 36 or surgical draper over the wound 31, 35, and ultrasound gel was applied to the area of skin in contact with ESWT.
Outcome measurements
Summary of outcome measurements is presented in Tables 4 and 5. Assessment of wound healing varied across the studies. A total of 10 studies 27, 28, 29, 30, 31, 32, 34, 35, 36, 37 used percentage of wounds healed, while reduction of WSA was documented by four studies 26, 27, 29, 30. Healing time was used by five studies 26, 27, 31, 35, 37, and blood flow was recorded in three studies 28, 32, 34. The safety and adverse effects of ESWT are described in most of the studies 26, 27, 28, 30, 31, 32, 34, 36, 37. None of the studies provided any information regarding the reliability and validity for the outcome measures used.
Table 4.
Summary of the outcome measurements of the controlled clinical studies
| Authors | Mean time of reepithelisation (days) | Reduction in WSA | Proportion of wound healing (%) | Blood flow | Safety and adverse reaction | ||||
|---|---|---|---|---|---|---|---|---|---|
| ESWT | C‐group | ESWT | C‐group | ESWT | C‐group | ESWT | C‐group | ||
| Omar et al., 2014 27 | 64·5 ± 8·06* | 81·17 ± 4·35 | 83%* | 63% | 54% complete healing* 33·5% healed ≥ 50% 12·5% unchanged | 28·5% completed healing 19% healed ≥50% 52·5% unchanged | N/A | N/A | Not observed |
| Wang et al., 2014 28 | N/A | N/A | N/A | N/A | 43% (DM group), 71% (NDM group) completed | DM group 0·11 (0·04–0·3) | Not observed | ||
| healing 3% (DM group), 6% (NDM group) | NDM group 0·28* (0·07–0·8) | ||||||||
| healed ≥ 50% 27% (DM group), 10 (NDM group) | |||||||||
| unchanged 27% (DM group), 13 (NDM group) failure | |||||||||
| Nossair et al., 2013 29 | N/A | N/A | 78%* | 44% | 83% complete healing* | 49% complete healing | N/A | N/A | N/A |
| Saggini et al., 2013 30 | N/A | N/A | 80%* | 67% | 71% complete healing* 29% partially healed | 40% completed healing 37·5% partially healed 22·5% unchanged | N/A | N/A | Not observed |
| Wang et al., 2011 32 | N/A | N/A | N/A | N/A | 57% complete healing* 32% healed ≥ 50% 11% unchanged | 25% completed healing 15% healed ≥50% 60% unchanged | 0·61* (0·40–0·79) | 0·50 (0·11–0·53) | Not observed |
| Wang et al., 2009 34 | N/A | N/A | N/A | N/A | 31% complete healing* 58% improved 11% unchanged | 22% completed healing 50% improved 28% unchanged | 0·75* (0·46–1·28) | 0·58 (0·51–0·66) | Not observed |
| Moretti et al., 2009 35 | 60·8 ± 4·7* | 82·2 ± 4·7 | N/A | N/A | 53·3% complete healing* | 33·3% complete healing | N/A | N/A | N/A |
| Saggini et al., 2008 36 | N/A | N/A | N/A | N/A | 53·3% complete healing* | 10% complete healing | N/A | N/A | No adverse reaction |
C‐group, comparison group; DM, diabetes mellitus; ESWT, extracorporeal shock wave therapy; N/A, not available; NDM, non‐diabetes mellitus; WSA, wound surface area.
*P value ≤ 0·05.
Table 5.
Summary of the outcome measurements of case serious studies
| Mean time of reepithelisation (days) | Reduction in WSA | Proportion of compellation of wound healing (%) | Blood flow | Safety and adverse reaction | |
|---|---|---|---|---|---|
| Variji et al., 2015 26 | 6–8 weeks | Progressive reduction of WSA in four patients after 6–8 weekly treatment, mean reduction (1·21 ± 0·82 cm2) | N/A | N/A | Not observed |
| Wolff et al., 2011 31 | 2 wks (1–288) | N/A | 74% complete wound closure | N/A | Not observed |
| Schaden et al., 2007 37 | 44 days (39·4 days for <10 cm2, and 1 month duration – 164·6 days for wound > 10 cm2, and duration > 1 month) | N/A | 75% complete wound closure 81·0% complete wound closure in wound ≤10 cm2 versus 61·8% complete wound closure in wound ≥10 cm2 (P < 0·005) 83·0% complete wound closure for wound ≤ 1 month versus 57·1% for wound ≥1 month old (P < 0·001) 81·0% complete wound closure for acute wound versus 56·3% in chronic wound (P < 0·001) | N/A | Not observed |
N/A, not available; WSA, wound surface area.
Proportion of wound healing
There was a great variability in the percentage of wound healing among the studies. In 2014, Omar et al. 27 showed significant (P < 0·05) differences in wounds completely healed (54% and 2·5%), improved (33·5% and 19%) and unchanged (12·5% and 52·5%) at 20 weeks follow‐up in the ESWT group (n = 19) when compared to the control group (n = 19). Additionally, in the same year, Wang et al. 28 revealed the percentage of wounds that had healed completely (43% and 71%), had improved (3% and 6%) and were unchanged (27% and 13%) in diabetic and non‐diabetic foot ulcers treated with unfocused ESWT at 5 years follow‐up.
In 2013, Nossairet et al. 29 reported 83% wound closure in the ESWT‐treated group (n = 15) compared to 49% in the control group (n = 15) after 12 weeks of treatment. In the same year, Saggini et al. 30 reported complete wound healing in 71% and partial healing in 29% of cases in the unfocused ESWT‐treated group (n = 63), with an energy flux density of 0·01 mJ/mm2, compared with 40% completely healed, 37·5% improved and 22·5% unchanged in the unfocused ESWT group (n = 40), with energy density 0·04 mJ/mm2.
In 2011, Wang et al. 32 revealed significant differences (P = 0·005) in the proportion of wound healed; the authors reported completed healing in 57% and 25%, improved >50% (32% and 15%, P =0·005) and remained unchanged (11% and 60%, P = 0·071) in ESWT when compared with HBOT groups. Wolf et al. 31 showed complete healing in 74% of total participants (n = 258) with mixed wounds who received ESWT. The rate of healing was affected by wound surface and duration as 81·0% of wounds healed completely with WSA ≤10 cm2 versus 61·8% with WSA ≥10 cm2. Significant (P < 0·001) closure of the wounds was reported in 83·0% of cases with wound duration ≤1 month versus 57·1% with wound duration ≥1 month.
In a 2009 study by Wang et al. 34, the percentage of completely healed wounds was 31% and 22%, improved 58% and 50% and unchanged 11% and 28% in the ESWT and HBOT groups, respectively. Moreover, Moretti et al. 35 reported complete healing of DFU of 53·33% in the ESWT group (n = 15) compared with 33·33% in the control group (n = 15). Early work of Saggini et al. 36 reveals wound closure in 53·3% of the ESWT group (n = 30) compared to 10% in the control group (n = 10), while Schaden et al. 37 linked the completely healed wound with the wound area. The authors reported complete healing in 81% of wounds less than 10 cm2 and 61·8% of wounds larger than 10 cm2.
Time to healing
Time to complete healing was measured in days or months and presented as means with standard deviations or medians. Two randomised clinical trials by Omar et al. 27 and Moretti et al. 35 report short healing times in ESWT groups (64·50 ± 8·06 days and 60·80 ± 4·70 days, respectively) compared with the control groups (81·17 ± 4·35 and 82·20 ± 4·70 days, respectively). Early work of Schaden et al. 37 showed that the mean time to complete tissue epithelialisation is 44 days; the authors concluded that the time to complete healing was significantly associated with the wound surface area and duration of wound, where the time to complete healing was 39·4 days (WSA <10 cm2 and 1 month wound duration) and 164·6 days (WSA > 10 cm2 and duration >1 month). Moreover, Wolff et al. 31 reported complete healing of 74% of patients after a median duration of 31·8 months.
Reduction of wound surface area
A significant reduction of WSA was observed in four studies. Omar et al. 27 and Nossair et al. 29 reported reduction in WSA in the ESWT group (83% and 78%, respectively) when compared with the control group (63% and 44%, respectively). Saggini et al. 30 reported significant reduction in WSA of 80% in the ESWT group treated with energy flux density of 0·1 mJ/cm2 compared with 67% in the ESWT group treated with energy flux density of 0·04 mJ/cm2. A series case study reported mean reduction of WSA of 1·21 ± 0·82 cm2 from initial wound area in four out of five patients treated with ESWT 26.
Blood flow
The blood flow perfusion rate as an indicator of tissue viability was measured using laser Doppler perfusion imaging in three studies 28, 32, 34. Wang et al. 28 observed significant improvement in blood flow perfusion (0·07 ± 0·8, P < 0·04) in non‐diabetic patients treated with ESW compared with diabetic patients (0·11 ± 0·10) treated with the same protocol at 5‐year follow‐up, while there was no difference between groups at 1‐year follow‐up (0·67 ± 0·30 vs 0·65 ± 0·28, P = 0·94). Furthermore, Wang et al. 32 reported significant improvement in local blood perfusion in the participants with DFU and treated with ESW (0·61, P < 0·002) compared with those treated with HBOT (0·50). The authors failed to show such an effect in their earlier study 34. However, the change in the blood perfusion rate from baseline was significant (P < 0·05) in the ESWT group.
Safety and adverse effects of ESWT
Potential complications and adverse effects (e.g. pain, itching, skin irritation and pigmentation, infection) related to ESWT treatment were identified in all studies 26, 27, 28, 30, 31, 32, 34, 35, 36, 37 except one 29. Moretti et al. 35 reported symptoms of local infection in both groups (ESWT and SWC), and oral antibiotics were prescribed. This complication was resolved within 5–7 days. In two studies by Wang et al. 32, 34 comparing ESWT and HBOT, there were no adverse events and complications reported in the ESWT group. However, the HBOT group reported some complications, including middle ear barotraumas and sinus pain. All of these adverse reactions were resolved after the removal of chamber pressure. These results may reflect the superiority of ESWT.
Methodological quality
The methodological quality of the included studies according to the PEDro scale is shown in Table 6. The mean RCT score of PEDro is 5·37 (SD: 0·91, range: 4–7) for 8 studies out of 11. Classification of the trials according to the total PEDro score revealed three studies classified as good 27, 32, 34 and five as fair 28, 29, 30, 35, 36, while the remaining three studies were poor 26, 31, 37. These scores represent multiple sources of bias.
Table 6.
Quality assessment of the included studies according to the PEDro scale
| Authors | Vriji et al., 2015 26 | Omar et al., 2014 27 | Wang et al., 2014 28 | Nossair et al., 2013 29 | Saggini et al., 2013 30 | Wolff et al., 2011 31 | Wang et al., 2011 32 | Wang et al., 2009 34 | Moretti et al., 2009 35 | Saggini et al., 2008 36 | Schdaden et al., 2007 37 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Eligibility criteria | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2 | Random allocation | ‐‐‐‐ | √ | ‐‐‐‐‐ | √ | √ | ‐‐‐‐ | √ | √ | √ | √ | ‐‐‐‐‐ |
| 3 | Concealed allocation | ‐‐‐‐ | √ | ‐‐‐‐‐ | ‐‐‐‐‐‐‐ | ‐‐‐‐‐‐‐ | ‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐ | ‐‐‐‐ | ‐‐‐‐‐ |
| 4 | Baseline comparability | ‐‐‐‐ | √ | √ | √ | √ | ‐‐‐‐‐‐‐ | √ | √ | √ | √ | ‐‐‐‐‐ |
| 5 | Blinded subjects | ‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐ | ‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ |
| 6 | Blinded therapists | ‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ |
| 7 | Blinded assessors | ‐‐‐‐ | √ | ‐‐‐‐‐‐‐ | ‐‐‐‐‐ | √ | ‐‐‐‐‐‐‐ | √ | √ | ‐‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐‐ |
| 8 | Adequate follow‐up | √ | √ | √ | √ | ‐‐‐‐‐‐‐ | √ | √ | √ | √ | √ | √ |
| 9 | Intention‐to‐treat analysis | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐‐‐ | ‐‐‐‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐‐ | ‐‐‐‐ | ‐‐‐‐‐‐ | ‐‐‐‐‐ |
| 10 | Between‐group analysis | ‐‐‐‐‐‐ | √ | √ | √ | √ | ‐‐‐‐‐‐‐‐ | √ | √ | √ | √ | ‐‐‐‐‐‐ |
| 11 | Point estimates of variability | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Total scores (10) | 2 | 7 | 4 | 5 | 5 | 2 | 6 | 6 | 5 | 5 | 2 |
In these studies, the worst scored criteria of quality were lack of blinding as all the studies 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37 failed to obtain a positive score for both therapists blinding and subject blinding, and four studies reported assessors blinding 27, 30, 32, 34. Moreover, intention‐to‐treat analysis achieved negative results in all studies 26, 27, 28, 29, 30, 31, 32, 34, 35, 36, 37, while concealed allocation was undertaken by one study 27. The best scored criteria were related to the statistical analysis of the results. All the studies reported between‐group statistical analysis using both point measures and measures of variability except in three studies 26, 31, 37. Eight studies 27, 29, 30, 32, 33, 34, 35, 36 reported random allocation. However, detailed explanations of randomisation were not reported adequately. Nine studies 27, 28, 29, 30, 32, 33, 34, 35, 36 had similar groups at baseline. Adequate follow‐up was described in all studies except one 30, and two of them 28, 29 had 100% follow‐up because of the short duration of the study.
Discussion
The purpose of this systematic review is to evaluate evidence of effectiveness of ESWT on chronic lower limb ulcers. For this purpose, 11 papers were evaluated. The evidence showed the clinical effectiveness regarding rate and time of wound healing and decreasing wound size. ESWT appears to be safe and associated with a low rate of complications during its application to treat chronic lower limb ulcers in both short‐ and mid‐term follow‐up periods.
The levels of evidence and the quality of the methodology of the identified studies must be considered before making conclusions about the effectiveness of ESWT for the management of chronic lower limb ulcers. In the current review, eight studies 27, 28, 29, 30, 32, 34, 35, 36 with level II evidence showed mild to moderate methodological quality (score ≥ 5). There were no significant differences in patient demographics between the treatment groups in any of the eight included studies. The number of patients enrolled in each study ranged from 15 to 62, which is acceptable. Patient selection criteria were reported in all the included studies. This allows us to have confidence in the recommendations of these studies. However, two studies were graded as level III 31, 37 because they were non‐randomised clinical controlled studies. The final study 26 was graded as level V as it was reported as a clinical case series and had no controls. These findings do not suggest which treatment approach used in level III and V studies is less effective but only that the research designs used were less rigorous. Therefore, there is diminished confidence that the treatments themselves created the change in the outcome measures.
None of the 11 studies addressed all criteria of methodological quality suggested in this review. All studies are reported poor in blinding (therapist and patient), intention‐to‐treat analysis and concealed allocation except one 27. This might influence the internal validity of the studies and prevents researchers from incorporating expectations of the outcome while evaluating participants, and random allocation attempts to control extraneous factors in subject pools and balance variables throughout the groups.
Wound characteristics revealed the lack of uniform classification of ulceration (e.g., aetiology, duration, grade and size) across the identified studies. ESWs have been used to treat varieties of chronic wounds, such as DFU, PU, VLU, ALU and acute wounds, including BW, TW and SW. The duration of an ulcer varied across identified studies (1–24 months), and the initial ulcer size also varied (1 cm up to 10 cm2). Although the included studies focus on the aetiology and duration of different wounds , the way in which they heal is similar (e.g. inflammation, proliferation, epithelialisation, etc.); therefore, it is appropriate to group them based on the measurement of the therapeutic effects of ESWT. However, the discrepancy across the studies represents the sources of bias.
In the current review, the relevant clinical outcomes were focused on wound healing and reepithelialisation time. However, the methods used to define them are varied. The common approach used to determine wound closure included observation and/or photographic documentation. The observation of wound closure and/or over 95% reepithelialisation was definecbd as the clinical endpoint by all studies 27, 28, 29, 30, 31, 32, 34, 35, 36, 37 except one 26 during the follow‐up and assessment period. In all studies, reepithelialisation and time to wound closure were significantly lower in the ESWT groups compared with standard therapy or HBOT. Additionally, the percentages of reduction in WSA were calculated in four studies 26, 27, 29, 30. The authors believe that using the rate of complete healing is clinically more useful than reporting the percentage of reduction in WSA.
Furthermore, blood flow perfusion was detected before and after the intervention in many studies 28, 32, 34. The reepithelialisation index that was quantified as mm2 per day was used in the study by Moretti et al. 35. However, a clear definition of the reepithelialisation index has not been identified; therefore, it may be problematic to compare results across the included studies. All reported studies did not show validity or reliability of the data for the outcome measures chosen. Objective measures of wound healing are necessary to reduce bias and to determine whether the treatment findings are because of the ESWT intervention or whether they result from measurement error.
All included studies provide sufficient details to allow the repetition of the intervention protocol. However, there are differences in the generator, dosage, frequency and duration of ESW protocols. The heterogeneity in such parameters made it difficult to compare studies and standardise the application of different protocols. Intensity assessment is important for the analysis of ESWT study outcomes, and it is considered a major aspect of negative outcomes and adverse effects. To date, to our knowledge, no study has successfully achieved an intensity within the recommended range of the previous reports. Several other important aspects of ESWT should be considered for further analysis (e.g. total energy, frequency of intervention, types of ESW generators). Unfortunately, many key parameters are incompletely reported in the studies we reviewed, such as frequency. However, most of the studies suggest that low‐intensity ESWT is a preferred treatment of chronic wounds. In addition, the follow‐up period of all included trials is relatively short, except one study that had a 5‐year follow‐up 28. Therefore, the long‐term efficacy remains unknown. Furthermore, the number of the trials comparing ESWT with other therapies is small, causing a lack of further understanding of the efficacy of ESWT.
None of the included studies reported adverse reactions secondary to ESWT application. However, one study 35 reported local signs of infection that has been resolved after 7 days of antibiotic administration. Even if none of the included studies have considered the cost‐effectiveness of ESWT, all show a significant reduction in time required for wound closure when using ESWT. This might be administered to potentially reduce the need for prolonged wound dressing and care.
This review has some limitations. Even if not in our opinion, as a systematic review of small trials, it could be potentially unreliable in some fields of medicine for several reasons. This could be partly because of the restriction on English‐language publications during the bibliographical research; thus, although we used a detailed search strategy, we still cannot be sure that all relevant trials were found. Selecting, publishing and reporting are other major causes of bias that should be considered. Probably not as a consequence of this, we included a limited number of studies (n = 11) with relatively small sample sizes. Although the quality of the studies included in this review was satisfactory, poor methodological quality of some studies could affect the reliability of our results and limit the overall conclusion about the efficacy of ESWT on managing chronic lower limb ulcers; this highlights the importance of future research that should follow the standardised guidelines of clinical trials.
Conclusions
There is mild to moderate evidence to support the use of ESWT as an adjuvant therapy with a standardised wound care programme. This will promote wound closure and reepithelialisation, reduce WSA and improve blood flow perfusion and the time required to complete healing. However, it is difficult to draw firm conclusions about the efficacy of ESWT. Thus, future research with high methodological quality is required to assess the efficacy and cost‐effectiveness of this relatively new physical therapy application.
References
- 1. Spentzouris G, Labropoulos N. The evaluation of lower extremity ulceration. Semin Intervent Radiol 2009;26:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. J Plast Reconstr Surg 2006;117:355–415. [DOI] [PubMed] [Google Scholar]
- 3. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Gent WB, Wilschut ED, Wittens C. Management of venous ulcer disease. BMJ 2010;341:1092–6. [DOI] [PubMed] [Google Scholar]
- 5. Agale SV. Chronic leg ulcers: epidemiology, aetiopathogessis, and management. Ulcers 2013;413604:9. [Google Scholar]
- 6. Consuegra RV, Verdu J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs 2011;67:926–44. [DOI] [PubMed] [Google Scholar]
- 7. Werdin F, Tenenhaus M, Rennekampff HO. Chronic wound care. Lancet 2008;372:1860–2. [DOI] [PubMed] [Google Scholar]
- 8. Moloney MC, Grace P. Understanding the underlying causes of chronic leg ulceration. J Wound Care 2004;13:215–8. [DOI] [PubMed] [Google Scholar]
- 9. Willrich A, Pinzur M, McNeil M, Juknelis D, Lavery L. Health related quality of life, cognitive function, and depression in diabetic patients with foot ulcer or amputation. A preliminary study. Foot Ankle Int 2005;26:128–34. [DOI] [PubMed] [Google Scholar]
- 10. Apelqvist J, Ragnarson‐Tennvall G, Persson U, Larsson J. Diabetic foot ulcers in a multidisciplinary setting. An economic analysis of primary healing and healing with amputation. J Intern Med 1994;235:463–71. [DOI] [PubMed] [Google Scholar]
- 11. Abbade LP, Lastoria S, Rolla HD, Stolf HD. A sociodemographic, clinical study of patients with venous ulcer. Int J Dermatol 2005;44:989–92. [DOI] [PubMed] [Google Scholar]
- 12. Kranke P, Bennett MH, Martyn‐St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 2015:– CD00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg 2008;95:685–92. [DOI] [PubMed] [Google Scholar]
- 14. Saltmarche AE. Low level laser therapy for healing acute and chronic wounds – the extendicare experience. Int Wound J 2008;5:351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Houghton PE, Kincaid CB, Lovell M, Campbell KE, Keast DH, Woodbury MG, Harris KA. Effect of electrical stimulation on chronic leg ulcer size and appearance. Phys Ther 2003;83:17–28. [PubMed] [Google Scholar]
- 16. Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol Assess 2001;5:1–221. [DOI] [PubMed] [Google Scholar]
- 17. Dyson M. Adjuvant therapies; ultrasound, laser therapy, electrical stimulation, hyperbaric oxygen and VAC‐therapy. In: Morrison MJ, Moffatt CJ, Franks PJ, editors. Leg Ulcers: A Problem Based Learning Approach. Philadelphia: Mosby, Elsevier, 2007:429–51. [Google Scholar]
- 18. Qureshi AA, Ross KM, Ogawa R, Orgill DP. Shock wave therapy in wound healing. Plast Reconstr Surg 2011;128:721e–7. [DOI] [PubMed] [Google Scholar]
- 19. Shrivastava SK, Kailash. Shock wave treatment in medicine. J Biosci 2005;30:175–269. [DOI] [PubMed] [Google Scholar]
- 20. Speed C. A systematic review of shockwave therapies in soft tissue conditions: focusing on the evidence. Br J Sports Med 2014;48:1538–42. [DOI] [PubMed] [Google Scholar]
- 21. Mittermayr R, Antonic V, Hartinger J, Kaufmann H, Redl H, Téot L, Stojadinovic A, Schaden W. Extracorporeal shock wave therapy (ESWT) for wound healing: technology, mechanisms, and clinical efficacy'. Wound Repair Regen 2012;20:456–65. [DOI] [PubMed] [Google Scholar]
- 22. Gerdesmeyer L, Frey C, Vester J, Maier M, Weil L, Russlies M, Stienstra J, Scurran B, Fedder K, Diehl P, Lohrer H, Henne M, Gollwitzer H. Radial extracorporeal shock wave therapy is safe and effective in the treatment of chronic recalcitrant plantar fasciitis: results of a confirmatory randomized placebo‐controlled multicenter study. Am J Sports Med 2008;36:2100–9. [DOI] [PubMed] [Google Scholar]
- 23. Gerdesmeyer L, Wagenpfeil S, Haake M, Maier M, Loew M, Lampe R, et al. Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA 2003;290:2573–80. [DOI] [PubMed] [Google Scholar]
- 24. Ko JY, Chen HS, Chen LM. Treatment of lateral epicondylitis of the elbow with shock waves. Clin Orthop Relat Res 2001;387:60–7. [DOI] [PubMed] [Google Scholar]
- 25. Wang CJ. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J 2003;26:220–32. [PubMed] [Google Scholar]
- 26. Variji Z, Aghazadeh N, Hasanzadeh H, Flrooz A. Extracorporeal shock wave therapy in the treatment of non‐healing diabetic ulcer: a pilot study. J Clin Exp Dermatol Res 2015;6:289. [Google Scholar]
- 27. Omar MT, Alghadir A, Al‐Wahhabi KK, Al‐Askar AB. Efficacy of shock wave therapy on Ccronic diabetic foot ulcer: a single‐blinded randomized controlled clinical trial. Diabetes Res Clin Pract 2014;106:548–54. [DOI] [PubMed] [Google Scholar]
- 28. Wang CJ, WuCT YYJ, Liu RT, Kuo YR. Long‐term outcomes of extracorporeal shockwave therapy for chronic foot ulcers. J Surg Res 2014;189:e366–72. [DOI] [PubMed] [Google Scholar]
- 29. Nossair AA, Eid MM, Salama AB. Advanced Protocol of shock wave therapy for diabetic foot ulcer. J Am Sci 2013;9:633–8. [Google Scholar]
- 30. Saggini R, Fioramonti P, Bellomo RG, DiStefano A, Scarcello L, DiPancrazio L, Iodice R, Sagginp A, Scuderi N. Chronic ulcers: treatment with unfocused extracorporeal shock waves. Eur J Inflamm 2013;11:99–509. [Google Scholar]
- 31. Wolff KS, Wibmer A, Pusch M, et al. The influence of comorbidities and etiologies on the success of extracorporeal shock wave therapy for chronic soft tissue wounds: midterm results. Ultrasound Med Biol 2011;37:1111–9. [DOI] [PubMed] [Google Scholar]
- 32. Wang CJ, Wu RW, Yang YJ. Treatment of diabetic foot ulcers: a comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy. Diabetes Res Clin Pract 2011;92:187–93. [DOI] [PubMed] [Google Scholar]
- 33. Larking AM, Duport S, Clinton M, Hardy M, Andrews K. Randomized control of extracorporeal shock wave therapy versus placebo for chronic decubitus ulceration. Clin Rehabil 2010;24:222–9. [DOI] [PubMed] [Google Scholar]
- 34. Wang CJ, Kuo YR, Wu RW, Liu RT, Hsu CS, Wang FS, Yang KD. Extracorporeal shockwave treatment for chronic diabetic foot ulcers. J Surg Res 2009;152:96. [DOI] [PubMed] [Google Scholar]
- 35. Moretti B, Notarnicola A, Maggio G, Moretti L, Pascone M, Tafui S, et al. The management of neuropathic ulcers of the foot in diabetes by shock wave therapy. BMC Musculoskelet Disord 2009;10:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saggini R, Figus A, Troccola A, Cocco V, Saggini A, Scuderi N. Extracorporeal shock wave therapy for management of chronic ulcers in the lower extremities. Ultrasound Med Biol 2008;34:1261–71. [DOI] [PubMed] [Google Scholar]
- 37. Schaden W, Thiele R, Kolpl C, Pusch M, Nissan A, Attinger CE, et al. Shock wave therapy for acute and chronic soft tissue wounds. A feasibility study. J Surg Res 2007;143:1–12. [DOI] [PubMed] [Google Scholar]
- 38. Yan X, Zeng B, Chai Y, Luo C, Li X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low‐energy shockwave therapy in random‐pattern skin flap model. Ann Plast Surg 2008;61:646–53. [DOI] [PubMed] [Google Scholar]
- 39. Cwykiel JM, Klimczak A, Krokowicz L, Siemionow M. Pre‐and postischemic pulsed acoustic cellular expression conditioning modulates expression of inflammation factors in cremaster ischemia/reperfusion injury model. Microsurgery 2013;33:134–40. [DOI] [PubMed] [Google Scholar]
- 40. Stojadinovic A, Elster EA, Anam K, Tadaki D, Amare M, Zins S, Davis TA. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis 2008;11:369–80. [DOI] [PubMed] [Google Scholar]
- 41. Jankovic D. Case study: shock waves treatment of diabetic foot gangrene. Int Wound J 2011;8:206–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stieger M, Schmid JP, Bajrami S, Hunziker T. Extracorporeal shock wave therapy in the treatment of non‐healing chronic venous leg ulcer. Hautarzt 2013;46:493–6. [DOI] [PubMed] [Google Scholar]
- 43. Butterworth PA, Walsh TP, Pennis YD, Chesne AD, Schmitz C, Nancarrow SA. The effectiveness of extracorporeal shock wave therapy for the treatment of lower limb ulceration: a systematic review. J Foot Ankle Res 2015;8:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dymarek R, halski T, Ptaszkowski K, Slupska L, Rosinczuk J, Taradaj J. Exracoporeal shock wave therapy as adjunct wound treatment: a systematic review of the literature. Ostomy Wound Manage 2014;60:26–39. [PubMed] [Google Scholar]
- 45. Antonic W, Mitermayr R, Schaden W, Stojadinovic A. Evidence supporting extracorporeal shockwave therapy for acute and chronic soft tissuewounds. Wounds 2011;23:204–15. [PubMed] [Google Scholar]
- 46. Sackett DL. Rules of evidence and clinical recommendations of the use of antithrombotic agents. Chest 1989;95:2S–4. [PubMed] [Google Scholar]
- 47. Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003;83:713–21. [PubMed] [Google Scholar]
- 48. De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 2009;55:129–33. [DOI] [PubMed] [Google Scholar]
- 49. Sherrington C, Herbert RD, Maher CG, Moseley AM. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man Ther 2000;5:223–6. [DOI] [PubMed] [Google Scholar]


