Abstract
Soft tissue sarcomas occur most commonly in the lower and upper extremities. The standard treatment is limb salvage surgery combined with radiotherapy. Postoperative radiotherapy is associated with wound complications. This systematic review aims to summarise the available evidence and review the literature of the last 10 years regarding postoperative wound complications in patients who had limb salvage surgical excision followed by direct closure vs flap coverage together with postoperative radiotherapy and to define the optimal timeframe for adjuvant radiotherapy after soft tissue sarcomas resection and flap reconstruction. A literature search was performed using PubMed. The following keywords were searched: limb salvage, limb‐sparing, flaps, radiation therapy, radiation, irradiation, adjuvant radiotherapy, postoperative radiotherapy, radiation effects, wound healing, surgical wound infection, surgical wound dehiscence, wound healing, soft tissue sarcoma and neoplasms. In total, 1045 papers were retrieved. Thirty‐seven articles were finally selected after screening of abstracts and applying dates and language filters and inclusion and exclusion criteria. Plastic surgery provides a vast number of reconstructive flap procedures that are directly linked to decreasing wound complications, especially with the expectant postoperative radiotherapy. This adjuvant radiotherapy is better administered in the first 3–6 weeks after reconstruction to allow timely wound healing and avoid local recurrence.
Keywords: postoperative radiotherapy, reconstruction, soft tissue sarcoma, wound complications
ABBREVIATIONS
- APR
abdominoperineal resection
- BRT
brachytherapy
- EBRT
external beam radiotherapy
- ILP
isolated limb perfusion
- PMMF
pectoralis major myocutaneous flap
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RT
radiotherapy
- STS
soft tissue sarcoma.
1. INTRODUCTION
Soft‐tissue sarcomas (STS) are rare mesenchymal malignant tumors.1 They represent less than 1% of all malignancies.2 The annual incidence in Europe is 1500–2000 cases. The incidence increases in elderly patients, peaking above 50 years of age.3 STS may occur at any part of the body, but the most common sites are the lower and upper extremities.4 The most recent classification provided in the World Health Organization fourth edition 2013 includes clinical, histological and genetic data. It classifies each histological category as either benign or malignant, with some categories having an additional intermediate malignancy group. The most common types of extremity STS in adults are undifferentiated pleomorphic sarcoma (previously known as malignant fibrous histiocytoma), liposarcoma and synovial sarcoma.5
Historically, the treatment of choice was aggressive surgical approaches, such as compartmental resection or even amputation.6 Nowadays, the aim of surgery is local tumour control with limb‐preserving surgery and negative safety margins.7 This frequently requires additional (neo‐) adjuvant modalities such as pre‐ or postoperative radiation. This leads to similar local control and overall survival rates comparable to major amputation and allows preservation of limb function so that amputations are rarely needed.8 Accordingly, the combination of adjuvant radiotherapy and wide surgical excision led to the concept of limb salvage therapy, which is the standard therapy for STS nowadays.4, 9
However, no one can deny that radiation therapy is associated with specific perioperative morbidity, especially wound‐related complications. Consequently, many research groups have focused on radiation‐related wound complications and evaluated their potential causes as well as options for prevention and treatment.10
The choice of combining either preoperative or postoperative radiotherapy with limb salvage surgery is still debatable. Postoperative radiotherapy can be applied as brachytherapy (BRT) or external beam radiotherapy (EBRT).11 BRT usually starts on the fifth day postoperatively, while EBRT starts after 4 weeks. The latter results in less wound complications because of the longer postoperative interval allowing wound healing.10 Schwartz et al12 demonstrated that local control rates decreased when adjuvant radiotherapy for extremity STS was postponed for more than 4 months. Conversely, preoperative radiotherapy uses a lower radiation dose to decrease the size of the tumour before surgical excision, leading to fewer long‐term complications and morbidity but unfortunately resulting in higher rates of acute wound complications than postoperative radiotherapy.13 High perioperative morbidity rates of 35% are described for preoperative radiation protocols when compared to 18% for postoperative radiation.14 On the contrary, similar acute wound complications were found by others after neoadjuvant or adjuvant radiotherapy, while long‐term morbidity was higher in patients after postoperative EBRT.15
Limb salvage therapy relies on wide surgical resection of the tumour potentially resulting in large‐sized defects that are probably associated with the exposure of important structures such as bone, vessels or nerves, and this introduces the essential role of plastic reconstructive techniques in the multidisciplinary management of STS. Plastic surgery provides a wide armamentarium of vascularised tissue transfers. This not only allows defect reconstruction, limb preservation and function but may also improve wound healing and subsequently allows adjuvant radiotherapy. In this context, either pedicled flaps or free microvascular flaps can be applied depending on the size and localisation of the defect. Microsurgical reconstruction of the oncological defects could be the sole option when local flaps cannot be used due to previous radiation or surgery at the donor site of the flap.7, 11, 16 Usui17 was the first who described the structured use of microsurgical free flaps for reconstruction after STS resection.
Microsurgical reconstruction may even be indicated when primary wound closure is possible but is associated with tension, and adjuvant treatment options such as radiation therapy are required.18, 19 Vascularised tissue transfer is additionally preferred for irradiated sarcoma defects as perioperative morbidity can be reduced.20
Treatment for patients suffering from STS of the extremities requires multimodal and multidisciplinary protocols. The aim of STS treatment is oncological safety as well as minimal morbidity and limb salvage. However, the literature on some aspects of consecutive protocols is still sparse. Here, the indications for vascularised tissue transfer to reduce wound complications are still debatable and frequently lead to interdisciplinary discussions. Additionally, the timing of postoperative radiation protocols is frequently discussed as early radiation therapy may lead to perioperative complications, and postponed protocols may decrease local control rates.
The aim of this review is to summarise the available evidence on these aspects and to review the literature of the last 10 years regarding postoperative wound complications in patients who had limb salvage surgical excision followed by direct closure vs flap coverage together with postoperative EBRT and to define the optimal timeframe for adjuvant radiotherapy after STS resection and flap reconstruction.
2. METHODS
2.1. Search strategy
A systematic search was performed using the PubMed database on August 5, 2016. The Boolean operators AND and OR were used in different combinations for the following keywords as both text words and Medical Search Headings (MeSH terms): limb salvage, limb‐sparing, flaps, radiation therapy, radiation, irradiation, adjuvant radiotherapy, postoperative radiotherapy, radiation effects, wound healing, surgical wound infection, surgical wound dehiscence, wound healing, soft tissue sarcoma and neoplasms.
The following search string was used: ([radiation therapy OR radiation OR adjuvant radiotherapy OR postoperative radiotherapy OR Radiotherapy, Adjuvant[mh] OR irradiation OR irradiated] AND [soft tissue sarcoma OR Soft Tissue Neoplasms] AND [surgical wound infection OR surgical wound dehiscence OR wound healing OR wound therapy OR radiation injuries OR Surgical Flaps OR limb‐sparing OR flaps OR limb salvage] AND [limb OR extremity OR extremities]).
2.2. Eligibility criteria
Filters were applied to the search results to restrict the studies to the period between 1996 and 2016. Similarly, only articles in English, German and French were included. The inclusion criteria were studies reporting on postoperative complications in 10 or more patients aged over 18 years diagnosed with extremity STS and treated with postoperative EBRT. On the other hand, systematic reviews or studies reporting on intraoperative radiotherapy were excluded. The retrieved studies were qualitatively analysed regarding timing of irradiation and the rate of postoperative wound complication. This systematic review was performed according to PRISMA statement.21 However, a detailed bias assessment according to the PRISMA criteria was not possible due to the inconsistency of the existing literature.
2.3. Ethical considerations
This article does not contain any studies with human participants or animals performed by any of the authors.
3. RESULTS
The initial literature search resulted in 1045 articles. After applying the abovementioned filters, 735 articles remained. After reading titles and abstracts, another 484 articles were excluded. Finally, inclusion and exclusion criteria were applied, yielding 37 articles as shown in Figure 1.
Figure 1.
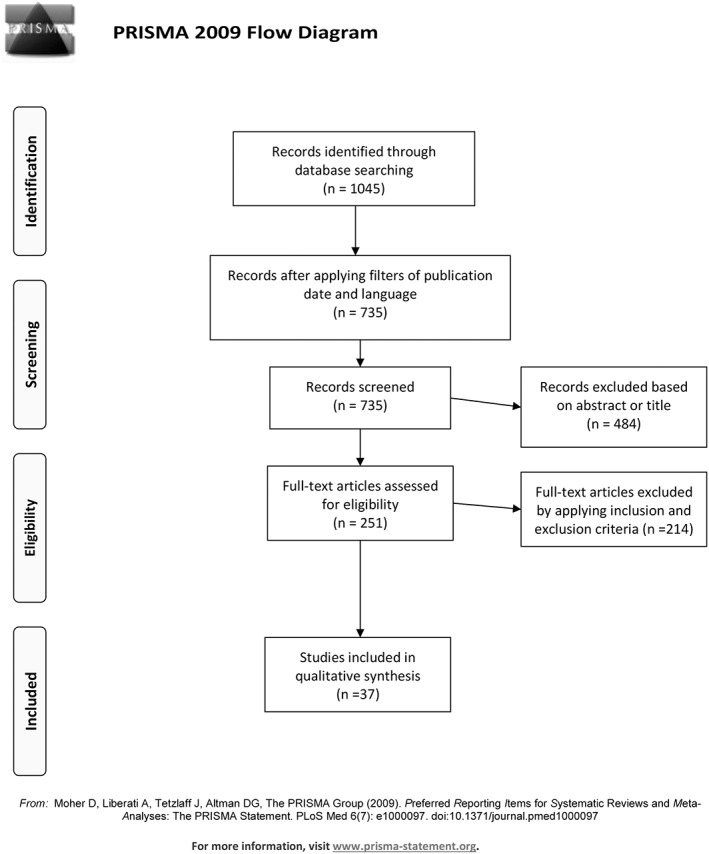
Showing the flow diagram of the results of the literature search according to PRISMA statement
Overall, the published data regarding oncological resection, flap reconstruction and postoperative radiation are characterised by a relatively low level of evidence. All the reviewed studies were retrospective except two studies; one was a prospective multicentre randomised controlled trial,14 and the other was a retrospective analysis of prospectively collected data.22
4. TIMING OF RADIOTHERAPY AFTER STS RESECTION
No consensus regarding the timing of postoperative radiation protocols is available in the literature. Different protocols are described, but almost no studies compare different strategies in homogenous patient cohorts.
Additionally, different types of complications are reported. These include persistent pain, oedema, fibrosis, pathological fracture, joint stiffness, osteoradionecrosis, osteomyelitis, chronic radiation dermatitis and wound‐related complications.22, 23, 24, 25 The timing of radiation therapy mostly influences wound‐related complications, such as wound healing disorders, infection, seroma or haematoma. The other complications are probably not related to radiation or the timing of radiation. Consequently, we focused on wound‐related morbidity rates. Overall, only 15 of the 37 studies report on exact timeframes, as seen in Table 1. Four of these studies started radiation therapy within the first four postoperative weeks. Wound‐healing problems occurred in 3–70% of cases in these series. These predominantly were infection, seroma, haematoma and wound dehiscence.26, 27, 28, 29
Table 1.
Studies showing details of the postoperative interval before radiotherapy and complications
| Postoperative interval for starting radiotherapy | Number of studies | Author and mean postoperative interval | Wound complications rates | Types of wound complications |
|---|---|---|---|---|
| First postoperative month | 4 | 3 wk: Karakousis et al29 | 3 | Infection, seroma, haematoma, wound dehiscence |
| 3 wk: Thacker et al28 | 6 | Wound dehiscence | ||
| 3 wk: Shapeero et al27 | 70 | Seromas | ||
| 4 wk: Leidinger et al26 | 21 | Wound healing disorder | ||
| Second postoperative month | 9 | 4.71 wk: Cannon et al31 | 16 | Wound dehiscence, infection, seroma |
| 3‐6 wk: O'Sullivan et al14 | 17 | Wound problems requiring secondary operation and wound breakdown requiring prolonged dressings | ||
| 4‐6 wk: Spierer et al10 | 4 | Wound infection requiring debridement | ||
| 5.28 wk: Penna et al33 | 21 | Major (complete flap loss or partial flap loss or dehiscence), minor (minor wound dehiscence) | ||
| 5.7 wk: McGee et al30 | 3 | Delayed wound healing | ||
| 6 wk: Lehane et al32 | 12 | Haematoma and wound infection | ||
| 6 wk: Vrouenraets et al34 | 60 | Non‐healing wounds, delayed wound healing, lymphocele and erysipelas | ||
| 6.5 wk: Miller et al35 | 15 | Wound healing complications requiring secondary operative intervention or hospital admission for intravenous antibiotics | ||
| 7.2 wk: Chao et al15 | 13 | Total flap loss, donor site seroma, recipient site (abscess, dehiscence, haematoma, partial skin graft loss, pedicle thrombosis) | ||
| Third postoperative month | 2 |
8.4 wk: Merimsky et al36 |
7 | Wound dehiscence |
| 10 wk: Grainger et al37 | 39 | Major wound complications requiring surgical debridement or moderate requiring dressings and antibiotics | ||
Nine studies started radiation therapy within the second postoperative month.10, 14, 15, 30, 31, 32, 33, 34, 35 Here, wound‐related complication rates ranged from 3% to 18.5%. Only one study reported higher morbidity rates of 60%, which may be addressed by the fact that all patients additionally received isolated limb perfusion (ILP).34 Two more groups applied radiation therapy in the third postoperative month after an average of 8.4 weeks36 and 10 weeks, respectively.37 This resulted in wound complication rates of 7% and 39%, respectively.36, 37 However, 39% were observed in a study including infectious complications that were reported from other hospitals.37
In summary, the available literature suggests that early radiation within the first postoperative month is associated with the highest morbidity, whereas complication rates decrease with time. On the other hand, postponed radiation may lead to oncological compromises12 and should therefore be applied within the second month. Some studies reported that the onset of radiation therapy had to be postponed for up to more than 12 weeks in a subset of patients due to postoperative wound healing problems.26, 35 Therefore, strategies to reduce the risk of wound break down should be included in the multimodal treatment regimen.
5. COMPLICATIONS OF RADIOTHERAPY WITH FREE FLAPS COMPARED TO PRIMARY CLOSURE
High‐quality literature regarding plastic reconstruction after extremity STS resection and adjuvant EBRT is sparse. To the authors' knowledge, there is no prospective randomised trial comparing STS with and without plastic reconstruction after radiation.
Moreover, even retrospective studies evaluating reconstruction after STS resection do not always report perioperative wound complication rates in relation to the method of wound closure (primary closure vs vascularised reconstruction).22, 24, 26, 27, 29, 30, 32, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46
The mean number of patients in the included studies was 61 (range 10–315), and the mean period of follow up was 64 months (range 8–214) as shown in Table 2.
Table 2.
Details of the studies reviewed
| Author | Number of patients | Period of study | Length of follow up in months: Mean (range) | Number of flaps performed (%) | Radiation dose (mean Gy) | Fractionation (Gy) | Chemotherapy % | Isolated limb perfusion number (%) | Perioperative wound complications number (%) |
|---|---|---|---|---|---|---|---|---|---|
| Muller et al23 | 315 | 1994‐2013 | 45 (12‐255) | 61 (20%) | 70 (45–81) | Not mentioned | 37 | None | 62 (20%) |
| Miller et al35 | 75 | 1998‐2013 | 52 (7‐150) | 6 (8%) | 60 (45–70.2) | 57%(1.8 Gy),43%(2 Gy) | 39 | None | 11 (15%) |
| Lehane et al32 | 34 | 1995‐2014 | 51 | None | 60–66 | 1.8–2 | 26 | None | 4 (12%) |
| Jakob et al54 | 29 | 1998‐2010 | 39.5 (3‐165) | 20 (22%) | 60–66 | Not mentioned | None | 29 (100%) | Could not be calculated in the RT group (because of the other group without RT). |
| Folkert et al24 | 280 | 1996‐2010 | 90 (range, 3‐187) | None | 63 (18–70.2) | 1.8 | Could not be calculated in the postoperative RT group | None | 207 (65%) for both the preoperative and postoperative RT groups. |
| Cassidy et al38 | 25 | 1977‐2010 | 138 | None | 61.8 | Twice daily | 4 | None | 11 (30%) for both the preoperative and postoperative groups |
| Beane et al22 | 30 | 1983‐1991 | 214 (12‐348) | None | 45 followed by a boost of 18 | 1.8 | 97 | None | 8 (27%) |
| Felderhof et al39 | 118 | 1995‐2010 | 93.4 (9‐192) | None | 60 while 6 patients received 66 | 2 | 10 | None | 8 (7%) |
| Agrawal et al48 | 62 | 2004‐2010 | Not mentioned | Used, but could not be calculated | Not mentioned | Not mentioned | Could not be calculated in the postoperative RT group | None | 94 (100%) in the pre‐2007 group and 112 (75%) in the post‐2007 group. |
| McGee et al30 | 173 | 1970‐2008 | 124.8 (3.6‐385.2) | None | 65 (49‐74) | 47%: Twice daily 53%: Once daily | 4 | None | 6 (3%) |
| Emory et al25 | 130 | 1990‐2009 | 40 (3‐155) | 18 (9%) | 60.9 | 1.8 | None | None | 50 (35%) for both the preoperative and postoperative groups. |
| Chao et al15 | 14 | 2001‐2010 | 38.0 ± 29.8 | 100% | 58.2 ± 9.8 | Not mentioned | 6.5 | None | 16 (13%) |
| Penna et al33 | 26 | 1999‐2009 | 59 (9‐137) | 100% | Not mentioned | Not mentioned | None | None | 7 (21%) with and without RT |
| Shirmali et al40 | 29 | 2000‐2005 | 45 (17‐90) | None | 50 | 2 (twice daily) | None | None | 16 (55%) |
| Rohde et al55 | 13 | 1995‐2006 | 84 | Used, but could not be calculated | 60.7 (45‐131.4) | Not mentioned | None | None | Could not be calculated (because of the other RT groups than postoperative EBRT) |
| Barner‐Rasmussen et al52 | 10 | 1990‐2006 | 60 | 100% | 50 | Not mentioned | Could not be calculated in the postoperative RT group | None | 4 (20%) in all groups with preoperative RT or postoperative RT or without RT |
| Shapeero et al27 | 13 | N/A | 45 (6‐132) | None | 56 | Not mentioned | None | None | 9 (70%) |
| Rimner et al41 | 79 | 1982‐2002 | 71 | None | 64.8 (39.6‐75) | 1.8–2 | Could not be calculated in the postoperative RT group | None | 3 (3.9%) |
| Barner‐Rasmussen et al51 | 33 | 1985‐2009 | 65.9 (0‐244) | 100% | 50 ± 10 boost | Not mentioned | Could not be calculated in the postoperative RT group | None | 19 (26%) in all groups with preoperative RT or postoperative RT or without RT |
| Thacker et al28 | 18 | 1981‐2003 | 99 (24‐216) | 27% | Not mentioned | Not mentioned | 33 | None | 1 (6%) |
| Müller et al23 | 10 | 1995‐2005 | 42 | 6 (30%) | Not mentioned | Not mentioned | 40 | None | 3 (30%) |
|
Alektiar et al43 |
34 |
2002‐2005 | 35 | None | 63 (59.4‐66.6) | 1.8 | 35 | None | 10 (24%) for both the preoperative and postoperative groups. |
| Pritsch et al42 | 17 | 1980‐2003 | 79 (4‐192) | None | Not mentioned | Not mentioned | 47 | None | 4 (24%) |
| Alektiar et al49 | 24 | 2002‐2005 | 23 (6‐56) | 7 (23%) | 63 (59.4‐66.6) | 1.8 | 38 | None | 4 (17%) |
| Livi et al44 | 58 | 1990‐2000 | 54 (3‐120) | None | 62.4 (45‐70) | Not mentioned | 12 | None | 2 (3%) |
| Leidinger et al26 | 33 | 1999‐2000 | 35.8 (4‐120) | None | 60 (58‐66) | 1.8–2 | None | None | 7 (21%) |
| Cannon et al31 | 143 | 1960‐2003 | 111 (14.4‐372) | 18 (13%) | 60 (50‐72) | Not mentioned | Could not be calculated in the postoperative RT group | None | 22 (16%) |
| Merimsky et al36 | 133 | 1994‐2002 | 48 | None | 63 or 70 in case of marginal excision | 1.8 | 17 | None | 9 (7%) |
| Fontanesi et al45 | 16 | 1980‐1999 | 60.5 (23‐192) | None | 45‐68 | Not mentioned | Could not be calculated in the postoperative RT group | None | 1 (6%) |
| Spierer et al10 | 27 | 1982‐2000 | 32 (1‐186) | 100% | 63 (41‐70) | Not mentioned | None | None | 1 (4%) |
| O'Sullivan et al14 | 89 | 1994‐1997 | 39.6 (3.24‐67.2) | 19 (20%) | 66‐70 | 2 | None | None | 16 (17%) |
| Lohman et al53 | 19 | 1992‐1997 | 33 | 6 (32%) | 50 (25‐70) | Not mentioned | Could not be calculated in the postoperative RT group | None | 11 (38%) in the flap group while in direct closure group 17 (24%) |
| Karakousis et al29 | 33 | 1977‐1995 | Not mentioned | None | 60 | 2 | None | None | 1 (3%) |
| Grainger et al37 | 46 | 1989‐1999 | Not mentioned | None | Not mentioned | Not mentioned | None | None | 18 (39%) |
| Vrouenaets et al34 | 10 | 1992‐1994 | 8 (4‐17) | None | 50 followed by a boost of 10‐20 | 2 | None | 10 (100%) | 6 (60%) |
| Colterjohn et al50 | 12 | 1987‐1994 | 52 (24‐109) | 3 (25%) | 66 | 2 | Could not be calculated in the postoperative RT group | None | 6 (17%) with or without RT |
| Cheng et al46 | 64 | 1979‐1993 | 63 (16‐192) | None | 62.8 | Not mentioned | None | None | 5 (8%) |
The risk of wound healing problems increases with radiation doses or with different fractionation regimens. The mean total dose in the reviewed literature was relatively homogenous and ranged from 50 to 70 Gray (Gy), with details shown in Table 2. It was not mentioned in six studies.28, 33, 37, 42, 47, 48 The fractionation of the radiotherapy dose was either 1.8 or 2 Gy as shown in Table 2. It was mentioned only in 16 articles.14, 22, 24, 25, 26, 29, 32, 34, 35, 36, 39, 40, 41, 43, 49, 50 No correlation could be detected between the rates of complications and the radiotherapy dose or fractionation. Other factors disturbing wound healing are tension on wound edges, large dead spaces or compromised wound vicinity. The risk even increases with additional radiotherapy and leads to wound healing disorders, including dehiscence, infection, seroma and haematoma.48 Most of these problems can potentially be overcome by tension‐free reconstruction using vascularised tissue transfer. However, these positive effects pose an extra risk of specific flap‐related complications, such as anastomotic problems as well as partial or complete flap loss.51 Consequently, risk reduction of wound morbidity must be weighed against flap complications. Reviewing the literature, it is of utmost importance to distinguish between flap‐related vs wound morbidity. The latter can be classified either according to Common Terminology Criteria for Adverse Events38, 41, 43, 49 or as major and minor where the major wound complications require operative intervention or hospital admission unlike their minor counterparts.48
In theory, flap reconstruction after STS resection and radiation should decrease perioperative morbidity rates. Matching results were described in some studies showing increased morbidity rates in patients treated with direct closure rather than vascularised flap reconstruction.14, 31, 48 O'Sullivan et al14 found that complication rates after direct closure were 15%, compared to 2% with additional vascularised tissue transfer. These results were similar to those described by Cannon and colleagues in 2006. This group reported morbidity rates of 13% following primary closure vs 3% after additional flap reconstruction.31 Agrawal et al48 described two patient cohorts; one was before a plastic surgery service was introduced in their institution and the other cohort came thereafter. The authors described a decreased incidence of wound infection and dehiscence, seroma or haematoma after plastic surgery was included in the treatment. However, flap‐related complications, such as fat or partial flap necrosis, were increased: 4.44% before plastic surgery and 7.58% thereafter.
Other studies did not compare their results after flap reconstruction to a primary closure group. In these studies, only patients requiring vascularised tissue transfer were included. These patients generally had a higher risk profile for wound problems with larger tumours; unfavourable STS location; and exposure of vital structures such as tendons, nerves, bone or vessels.7 However, reported wound‐related morbidity rates were still satisfying and ranged from 13% to 30%.15, 33, 47, 51, 52 Spierer et al10 reported uncomplicated wound healing in all but one patient (4%), who required debridement before radiotherapy. Barner‐Rasmussen et al51 reviewed the STS database of Helsinki University central hospital in 2008 and extracted the data for 72 lower extremity cases that underwent microsurgical reconstruction and found a complication rate of 26%. Similarly, the same authors reviewed 20 upper extremity cases that underwent microsurgical reconstruction in 2010 and found a complication rate of 20%.52 The complications mentioned in the two studies were immediate reoperations for anastomotic revision, reoperations for haematoma and minor debridements.51, 52
Only two of the studies in this review reported higher complication rates in the patients treated by flaps opposed to patients treated by direct closure.35, 53 Lohman et al53 found complication rates of 38% in the flap group in comparison to 24% in the direct closure group. Likewise, another study reported complication rates of 27% in the flap group, rather than 20% in the direct closure group.35 However, the patients requiring flap reconstruction were observed with a significantly higher risk profile for wound healing disorders as the average tumour size was higher, more patients received radiation therapy and general risk factors such as smoking were more frequent. Moreover, a closer look on this group's data showed that major wound complications requiring readmission or reoperation all occurred after direct closure, whereas conservative wound complication management was predominantly possible in cases after vascularised flap reconstruction. Major complications in the latter group were mainly flap related, such as two revisions of thrombosed microvascular anastomoses, one haematoma drainage and one flap loss.53
Other groups reported on complication rates in mixed patient cohorts, including cases with and without vascularised reconstruction, but did not separately describe morbidity for the different subsets of patients. Here, complications were observed in 6–35% of the patients.23, 25, 28, 49, 50, 54, 55 However, these studies suggested that flap reconstruction might help to reduce complication rates because the highest number of complications was found with the least number of flaps performed (9%).25 With the exception of two studies,54, 55 it was found that the higher the percentage of flaps used, the lower the incidence of complications as shown in Table 3.23, 25, 28, 49, 50
Table 3.
Comparison of wound complications rates after different closure methods
| Wound complications after different closure methods | Articles | Complication rates | |
|---|---|---|---|
| Articles not reporting complications after STS resection in relation to the method of wound closure. | 19 articles.22, 24, 26, 27, 29, 30, 32, 34, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 | Not mentioned | |
| Articles reporting higher morbidity rates in direct closure group than in flap group. | O'Sullivan et al14 | 15% after direct closure vs 2% after flaps. | |
| Cannon et al31 | 13% after direct closure vs 3% after flaps. | ||
| Agrawal et al48 | 100% after direct closure vs 75% after flaps. | ||
| Articles reporting higher morbidity rates in flap group than in direct closure group. | Lohman et al53 | 38% after flaps vs 24% after direct closure. | |
| Miller et al35 | 27% after flaps vs 20% after direct closure. | ||
| Articles reporting complications in patients who all had flap coverage. | Chao et al15 | 13% | |
| Barner‐Rasmussen et al52 | 20% | ||
| Penna et al33 | 21% | ||
| Barner‐Rasmussen et al51 | 26% | ||
| Muller et al47 | 30% | ||
| Articles reporting complication rates in mixed patient cohorts with and without flaps. | Complications | Flaps utilized | |
| Thacker et al28 | 6% | 27% | |
| Colterjohn et al50 | 17% | 25% | |
| Alektiar et al49 | 24% | 23% | |
| Muller et al23 | 20% | 20% | |
| Emory et al25 | 35% | 9% | |
| Jakob et al54 | Could not be calculated | 22% | |
| Rohde et al55 | Could not be calculated | Used, but could not be calculated | |
Radiation therapy is not the only factor influencing wound healing in STS patients. Other treatment options in multimodal regimens such as chemotherapy or ILP may likewise lead to increased morbidity rates. Chemotherapy was used in 25 of the reviewed articles. The percentage of patients who received chemotherapy ranged from 4% up to 47%, with details listed in Table 2.22, 27, 28, 32, 36, 41, 42, 43, 49, 50, 51, 53 ILP was used in 100% of the patients in only two studies.34, 54The indication was either primary intermediate and high‐grade lesions54 or irresectable lesions.34
6. DISCUSSION
The effect of radiation on the wound healing process depends on several factors, such as the interval between surgery and radiotherapy, the use of preoperative or postoperative radiotherapy, rate of dose accumulation, type and energy of radiation used, the volume of normal tissues subjected to radiation and the site being treated.56, 57, 58
Radiation causes cellular injury by damaging the DNA and by generating free radicals.58, 59 This adversely affects all steps of wound healing.59 Skin irradiation suppresses the inflammatory reactions, inhibits angiogenesis and decreases formation of collagen.60 Radiation damages the vascular endothelial cells, leading to their oedema, and activates the coagulation system, leading to thrombosis with consequent vessel occlusion.59 Likewise, radiation inhibits functions of fibroblasts such as division and movement. This results in decreased formation and maturation of collagen, eventually leading to decreased tensile wound strength. All these derangements lead to wound healing complications such as dehiscence, infection, ulcers and unstable scars or even malignant transformation.58, 59
The introduction of vascularised tissue can counteract some of the harmful effects of radiation. This is mediated by improving tissue oxygenation, providing neutrophils, fibroblasts and macrophages to the local tissues, which are essential for wound healing.20 Geller et al61 mentioned that the prophylactic use of flaps for defects after STS resection resulted in less wound complications.
The tension exerted on the wound edges during closure is another detrimental factor for wound healing. Defects resulting from sarcoma excision can be closed directly or otherwise require vascularised tissue transfer. Direct closure can be performed if there is minimal wound tension. On the other hand, flaps are indicated if there is a large defect or excessive tension on the wound edges or exposed important structures. As radiation as well as tension increases the risk of wound break down, flap reconstruction should be liberally indicated in patients requiring adjuvant radiotherapy.62
Likewise, creation of dead space after surgical resection leads to impaired wound healing by providing a space for haematoma or seroma formation with possible bacterial infection.63 Additionally, radiotherapy leads to loss of tissue pliability, thus accentuating the effect of dead space formation. Hence, introducing vascularised tissue into these wounds obliterates the dead space even if there is no wound tension, thus combating surgical‐ and radiation‐induced wound healing problems.53
The fact that complication rates can be reduced by vascularised flap reconstruction in oncological patients undergoing radiation therapy has been demonstrated for different tumour entities. Devulapalli et al64 published a meta‐analysis analysis comparing primary closure with myocutaneous flaps for pelvic reconstruction following abdominoperineal resection (APR) and pelvic exenteration. They found that the use of myocutaneous flaps like vertical rectus abdominis muscle and gracilis muscle led to significant statistical and clinical decrease of perineal wound complications compared to primary closure. Likewise, another systematic review showed that myocutaneous flap reconstruction following APR in irradiated patients reduces perineal wound complications.65
Similarly, another group compared the rates of pharyngocutaneous fistula in irradiated total laryngectomy with primary closure vs closure by pectoralis major myocutaneous flap (PMMF). They found a higher fistula rate in the primary closure group of 36% compared to 14% in the PMMF group.66
STS resection with postoperative radiation is a good example of the fact that the historic concept of the ladder of reconstruction cannot be applied in all patients. Primary closure may be possible in a subset of patients but may, however, not be the best therapeutic option when radiation therapy is planned. Furthermore, the attempt to close a wound primarily may lead to oncological compromises that would not be necessary when plastic reconstructive techniques are included in the treatment plan. Similarly, skin grafts can be used to achieve wound healing but may lead to significant postoperative morbidity when additional radiation therapy is planned.14
In our review, three studies showed lower complication rates in the reconstruction group.14, 31, 48 The two studies reporting higher complication rates in the flap group vs direct closure showed selection bias that was related to the general clinical setting. The different groups were not comparable as flaps had a certain indication, and patients were not randomised to the method of wound closure. Prospective randomised trials are not possible in the context of STS treatment because it would be ethically unacceptable to randomise a patient to the direct closure group if this is impossible. Consequently, flaps are only applied if tumours are large and direct closure is impossible or if the tumour is located unfavourably (e.g. at the lower leg). If additional risk factors such as the percentage of patients undergoing radiation therapy are additionally excluded, it becomes obvious that flaps still reduce morbidity rates in a patient cohort with a high risk profile due to the STS characteristics.35, 53 This is specially illustrated by Lohmann et al53 who demonstrated that postoperative radiation had to be postponed in a subset of patients after STS resection and primary closure, whereas timely EBRT was possible in all patients after additional flap reconstruction. As the main goal in STS resection is local tumour control, treatment regimens should be adapted to avoid wound healing problems.
Another daily question for the treatment team in STS patients is the optimal timing of radiation after reconstruction. This question has been fully investigated and addressed in head and neck cancer.67 Likewise, Schwartz et al12 studied the effect of delaying adjuvant RT on local control in extremity STS patients. They divided the patients into two cohorts, a short one with a median delay of 1 month and a long cohort with a median delay of 5 months. The local recurrence was 34% in the long delay cohort in contrast to 11.5% in the short delay cohort.12 RT is usually initiated 3–6 weeks postoperatively to allow for both adequate wound healing and effective local tumour control.68 Therefore, treatment regimens should ensure uneventful wound healing within this timeframe.
The conclusion of our review regarding flap reconstruction as well as optimal timing of radiation therapy after reconstruction is hampered mainly by the limited quality of existing evidence. Different definitions, different cut‐off values regarding the operative timing, varying inclusion criteria and predominantly retrospective nature render it difficult to perform an assessment according to the PRISMA criteria. The analysis of bias was especially not feasible due to the quality of the existing literature. Therefore, this review is just summarising the available literature, and necessary future studies should be based on this knowledge.
7. CONCLUSION
Postoperative radiotherapy together with limb salvage surgery is an already established protocol in the treatment of extremity STS. However, radiotherapy is associated with perioperative wound complications. Surgical excision of the STS lesion leaves a defect that may be closed directly or by flaps. Flaps help to decrease wound complications by introducing vascularised tissue that can withstand the effects of RT. Adjuvant radiotherapy should not be delayed for more than 6 weeks because this appears to compromise local control. As uneventful wound healing is mostly accomplished after 3 weeks, radiation therapy after STS resection should be allowed between 3 and 6 weeks. Plastic surgery is an integral part of the multidisciplinary team for extremity STS and may allow reduced perioperative morbidity rates. Consequently, plastic reconstructive options should be involved in all treatment stages for patients suffering from STS.
Conflicts of interest
The authors declare that they have no conflict of interest.
Abouarab MH, Salem IL, Degheidy MM, et al. Therapeutic options and postoperative wound complications after extremity soft tissue sarcoma resection and postoperative external beam radiotherapy. Int Wound J. 2018;15:148–158. 10.1111/iwj.12851
REFERENCES
- 1. Steinau HU, Daigeler A, Langer S, et al. Limb salvage in malignant tumors. Semin Plast Surg. 2010;24(01):018–033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lehnhardt M, Daigeler A, Hauser J, et al. The value of expert second opinion in diagnosis of soft tissue sarcomas. J Surg Oncol. 2008;97(1):40–43. [DOI] [PubMed] [Google Scholar]
- 3. Momeni A, Kalash Z, Stark GB, Bannasch H. The use of the anterolateral thigh flap for microsurgical reconstruction of distal extremities after oncosurgical resection of soft‐tissue sarcomas. J Plastic Reconst Aesthetic Surg. 2011;64(5):643–648. [DOI] [PubMed] [Google Scholar]
- 4. Davis LE, Ryan CW. Preoperative therapy for extremity soft tissue sarcomas. Curr Treat Options in Oncol. 2015;16(6):25. [DOI] [PubMed] [Google Scholar]
- 5. Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss's soft tissue tumors. Elsevier Health Sci. 2013;1:3–5. [Google Scholar]
- 6. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 7. Chao AH, Mayerson JL, Chandawarkar R, Scharschmidt TJ. Surgical management of soft tissue sarcomas: extremity sarcomas. J Surg Oncol. 2015;111(5):540–545. [DOI] [PubMed] [Google Scholar]
- 8. Haas RL, Delaney TF, O'Sullivan B, et al. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? Int J Radiat Oncol Biol Phys. 2012;84(3):572–580. [DOI] [PubMed] [Google Scholar]
- 9. Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: Improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21(14):2719–2725. [DOI] [PubMed] [Google Scholar]
- 10. Spierer MM, Alektiar KM, Zelefsky MJ, Brennan MF, Cordiero PG. Tolerance of tissue transfers to adjuvant radiation therapy in primary soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2003;56(4):1112–1116. [DOI] [PubMed] [Google Scholar]
- 11. Townley WA, Mah E, O'Neill AC, et al. Reconstruction of sarcoma defects following pre‐operative radiation: Free tissue transfer is safe and reliable. J Plastic Reconst Aesthetic Surg. 2013;66(11):1575–1579. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz DL, Einck J, Hunt K, et al. The effect of delayed postoperative irradiation on local control of soft tissue sarcomas of the extremity and torso. Int J Radiat Oncol Biol Phys. 2002;52(5):1352–1359. [DOI] [PubMed] [Google Scholar]
- 13. Davis A, Osullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75(1):48–53. [DOI] [PubMed] [Google Scholar]
- 14. O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft‐tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–2241. [DOI] [PubMed] [Google Scholar]
- 15. Chao AH, Chang DW, Shuaib SW, Hanasono MM. The effect of neoadjuvant versus adjuvant irradiation on microvascular free flap reconstruction in sarcoma patients. Plast Reconstr Surg. 2012;129(3):675–682. [DOI] [PubMed] [Google Scholar]
- 16. Papagelopoulos PJ, Mavrogenis AF, Mastorakos DP, Vlastou C, Vrouvas J, Soucacos PN. Free vascularised tissue transfer and brachytherapy for soft‐tissue sarcomas of the extremities. Injury. 2008;39(Suppl 3):S83–S89. [DOI] [PubMed] [Google Scholar]
- 17. Usui M, Ishii S, Yamamura M, Minami A, Sakuma T. Microsurgical reconstructive surgery following wide resection of bone and soft tissue sarcomas in the upper extremities. J Reconstr Microsurg. 1986;2(02):77–84. [DOI] [PubMed] [Google Scholar]
- 18. Popov P, Tukiainen E, Asko‐Seljavaara S, et al. Soft‐tissue sarcomas of the upper extremity: surgical treatment and outcome. Plast Reconstr Surg. 2004;113(1):222–230. discussion 31‐2. [DOI] [PubMed] [Google Scholar]
- 19. Hidalgo DA, Carrasquillo IM. The treatment of lower extremity sarcomas with wide excision, radiotherapy, and free‐flap reconstruction. Plast Reconstr Surg. 1992;89(1):96–101. discussion 2. [PubMed] [Google Scholar]
- 20. Barwick WJ, Goldberg JA, Scully SP, Harrelson JM. Vascularized tissue transfer for closure of irradiated wounds after soft tissue sarcoma resection. Ann Surg. 1992;216(5):591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. W64. [DOI] [PubMed] [Google Scholar]
- 22. Beane JD, Yang JC, White D, Steinberg SM, Rosenberg SA, Rudloff U. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20‐year follow‐up of a randomized prospective trial. Ann Surg Oncol. 2014;21(8):2484–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muller DA, Beltrami G, Scoccianti G, Frenos F, Capanna R. Combining limb‐sparing surgery with radiation therapy in high‐grade soft tissue sarcoma of extremities ‐ is it effective? Eur J Surg Oncol. 2016;42(7):1057–1063. [DOI] [PubMed] [Google Scholar]
- 24. Folkert MR, Singer S, Brennan MF, et al. Comparison of local recurrence with conventional and intensity‐modulated radiation therapy for primary soft‐tissue sarcomas of the extremity. J Clin Oncol. 2014;32(29):3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Emory CL, Montgomery CO, Potter BK, Keisch ME, Conway SA. Early complications of high‐dose‐rate brachytherapy in soft tissue sarcoma: a comparison with traditional external‐beam radiotherapy. Clin Orthop Relat Res. 2012;470(3):751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leidinger B, Heyse T, Schuck A, et al. High incidence of metastatic disease in primary high grade and large extremity soft tissue sarcomas treated without chemotherapy. BMC Cancer. 2006;6(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shapeero LG, De Visschere PJ, Verstraete KL, et al. Post‐treatment complications of soft tissue tumours. Eur J Radiol. 2009;69(2):209–221. [DOI] [PubMed] [Google Scholar]
- 28. Thacker MM, Potter BK, Pitcher JD, Temple HT. Soft tissue sarcomas of the foot and ankle: impact of unplanned excision, limb salvage, and multimodality therapy. Foot Ankle Int. 2008;29(7):690–698. [DOI] [PubMed] [Google Scholar]
- 29. Karakousis CP, Zografos GC. Radiation therapy for high grade soft tissue sarcomas of the extremities treated with limb‐preserving surgery. Eur J Surg Oncol. 2002;28(4):431–436. [DOI] [PubMed] [Google Scholar]
- 30. McGee L, Indelicato DJ, Dagan R, et al. Long‐term results following postoperative radiotherapy for soft tissue sarcomas of the extremity. Int J Radiat Oncol Biol Phys. 2012;84(4):1003–1009. [DOI] [PubMed] [Google Scholar]
- 31. Cannon CP, Ballo MT, Zagars GK, et al. Complications of combined modality treatment of primary lower extremity soft‐tissue sarcomas. Cancer. 2006;107(10):2455–2461. [DOI] [PubMed] [Google Scholar]
- 32. Lehane C, Ho F, Thompson SR, et al. Neoadjuvant chemoradiation (modified Eilber protocol) versus adjuvant radiotherapy in the treatment of extremity soft tissue sarcoma. J Med Imaging Radiat Oncol. 2016;60(4):539–544. [DOI] [PubMed] [Google Scholar]
- 33. Penna V, Iblher N, Momeni A, Stark GB, Bannasch H. Free tissue transfer in reconstruction following soft tissue sarcoma resection. Microsurgery. 2011;31(6):434–440. [DOI] [PubMed] [Google Scholar]
- 34. Vrouenraets BC, Keus RB, Nieweg OE, Kroon BB. Complications of combined radiotherapy and isolated limb perfusion with tumor necrosis factor alpha +/− interferon gamma and melphalan in patients with irresectable soft tissue tumors. J Surg Oncol. 1997;65(2):88–94. [DOI] [PubMed] [Google Scholar]
- 35. Miller ED, Mo X, Andonian NT, et al. Patterns of major wound complications following multidisciplinary therapy for lower extremity soft tissue sarcoma. J Surg Oncol. 2016;114(3):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Merimsky O, Soyfer V, Kovner F, et al. Limb sparing approach: adjuvant radiation therapy in adults with intermediate or high‐grade limb soft tissue sarcoma. Radiotherapy Oncol J Eur Soc Ther Radiol Oncol. 2005;77(3):295–300. [DOI] [PubMed] [Google Scholar]
- 37. Grainger MF, Grimer RJ, Carter SR, Tillman RM. Wound complications following resection of adductor compartment tumours. Sarcoma. 2001;5(4):203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cassidy RJ, Indelicato DJ, Gibbs CP, Scarborough MT, Morris CG, Zlotecki RA. Function preservation after conservative resection and radiotherapy for soft‐tissue sarcoma of the distal extremity: utility and application of the Toronto extremity salvage score (TESS). Am J Clin Oncol. 2016;39(6):600–603. [DOI] [PubMed] [Google Scholar]
- 39. Felderhof JM, Creutzberg CL, Putter H, et al. Long‐term clinical outcome of patients with soft tissue sarcomas treated with limb‐sparing surgery and postoperative radiotherapy. Acta Oncol. 2013;52(4):745–752. [DOI] [PubMed] [Google Scholar]
- 40. Shrimali RK, Correa PD, Lee KC, Lai CN, Kakumanu SA, Cowie F. Adjuvant radiotherapy with 50 Gy after limb‐sparing surgery for soft‐tissue sarcoma‐‐west of Scotland experience. Clin Oncol (R Coll Radiol). 2010;22(4):322–323. [DOI] [PubMed] [Google Scholar]
- 41. Rimner A, Brennan MF, Zhang Z, Singer S, Alektiar KM. Influence of compartmental involvement on the patterns of morbidity in soft tissue sarcoma of the thigh. Cancer. 2009;115(1):149–157. [DOI] [PubMed] [Google Scholar]
- 42. Pritsch T, Bickels J, Winberg T, Malawer MM. Popliteal sarcomas: presentation, prognosis, and limb salvage. Clin Orthop Relat Res. 2007;455:225–233. [DOI] [PubMed] [Google Scholar]
- 43. Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity‐modulated radiation therapy on local control in primary soft‐tissue sarcoma of the extremity. J Clin Oncol. 2008;26(20):3440–3444. [DOI] [PubMed] [Google Scholar]
- 44. Livi L, Santoni R, Paiar F, et al. Late treatment‐related complications in 214 patients with extremity soft‐tissue sarcoma treated by surgery and postoperative radiation therapy. Am J Surg. 2006;191(2):230–234. [DOI] [PubMed] [Google Scholar]
- 45. Fontanesi J, Mott MP, Lucas DR, Miller PR, Kraut MJ. The role of irradiation in the management of locally recurrent non‐metastatic soft tissue sarcoma of extremity/trunkal locations. Sarcoma. 2004;8(2‐3):57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheng EY, Dusenbery KE, Winters MR, Thompson RC. Soft tissue sarcomas: preoperative versus postoperative radiotherapy. J Surg Oncol. 1996;61(2):90–99. [DOI] [PubMed] [Google Scholar]
- 47. Muller M, Bickert B, Germann G, Sauerbier M. Soft‐tissue sarcoma of the forearm and hand. Plastic surgical management. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen. 2008;79(7):682–688. [DOI] [PubMed] [Google Scholar]
- 48. Agrawal N, Wan D, Bryan Z, Boehmler J, Miller M, Tiwari P. Outcomes analysis of the role of plastic surgery in extremity sarcoma treatment. J Reconstr Microsurg. 2013;29(2):107–111. [DOI] [PubMed] [Google Scholar]
- 49. Alektiar KM, Hong L, Brennan MF, Della‐Biancia C, Singer S. Intensity modulated radiation therapy for primary soft tissue sarcoma of the extremity: preliminary results. Int J Radiat Oncol Biol Phys. 2007;68(2):458–464. [DOI] [PubMed] [Google Scholar]
- 50. Colterjohn NR, Davis AM, O'Sullivan B, Catton CN, Wunder JS, Bell RS. Functional outcome in limb‐salvage surgery for soft tissue tumours of the foot and ankle. Sarcoma. 1997;1(2):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barner‐Rasmussen I, Popov P, Bohling T, Tarkkanen M, Sampo M, Tukiainen E. Microvascular reconstruction after resection of soft tissue sarcoma of the leg. Br J Surg. 2009;96(5):482–489. [DOI] [PubMed] [Google Scholar]
- 52. Barner‐Rasmussen I, Popov P, Bohling T, Blomqvist C, Tukiainen E. Microvascular reconstructions after extensive soft tissue sarcoma resections in the upper limb. Eur J Surg Oncol. 2010;36(1):78–83. [DOI] [PubMed] [Google Scholar]
- 53. Lohman RF, Nabawi AS, Reece GP, Pollock RE, Evans GR. Soft tissue sarcoma of the upper extremity: a 5‐year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer. 2002;94(8):2256–2264. [DOI] [PubMed] [Google Scholar]
- 54. Jakob J, Tunn PU, Hayes AJ, Pilz LR, Nowak K, Hohenberger P. Oncological outcome of primary non‐metastatic soft tissue sarcoma treated by neoadjuvant isolated limb perfusion and tumor resection. J Surg Oncol. 2014;109(8):786–790. [DOI] [PubMed] [Google Scholar]
- 55. Rohde RS, Puhaindran ME, Morris CD, et al. Complications of radiation therapy to the hand after soft tissue sarcoma surgery. J Hand Surg. 2010;35(11):1858–1863. [DOI] [PubMed] [Google Scholar]
- 56. Wang J, Boerma M, Fu Q, Hauer‐Jensen M. Radiation responses in skin and connective tissues: effect on wound healing and surgical outcome. Hernia. 2006;10(6):502–506. [DOI] [PubMed] [Google Scholar]
- 57. Denham JW, Hauer‐Jensen M. The radiotherapeutic injury‐‐a complex 'wound. Radiotherapy Oncol J Eur Soc Ther Radiol Oncol. 2002;63(2):129–145. [DOI] [PubMed] [Google Scholar]
- 58. Dormand EL, Banwell PE, Goodacre TE. Radiotherapy and wound healing. Int Wound J. 2005;2(2):112–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Janis JE, Harrison B. Wound healing: part I. Basic science. Plast Reconstr Surg. 2016;138(3 Suppl):9S–17S. [DOI] [PubMed] [Google Scholar]
- 60. Jagetia GC, Rajanikant GK. Acceleration of wound repair by curcumin in the excision wound of mice exposed to different doses of fractionated gamma radiation. Int Wound J. 2012;9(1):76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Geller DS, Hornicek FJ, Mankin HJ, Raskin KA. Soft tissue sarcoma resection volume associated with wound‐healing complications. Clin Orthop Relat Res. 2007;459:182–185. [DOI] [PubMed] [Google Scholar]
- 62. Kane JM 3rd, Gibbs JF, McGrath BE, Loree TR, Kraybill WG. Large, deep high‐grade extremity sarcomas: when is a myocutaneous flap reconstruction necessary? Surg Oncol. 1999;8(4):205–210. [DOI] [PubMed] [Google Scholar]
- 63. Peat BG, Bell RS, Davis A, et al. Wound‐healing complications after soft‐tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980–987. [DOI] [PubMed] [Google Scholar]
- 64. Devulapalli C, Jia Wei AT, DiBiagio JR, et al. Primary versus flap closure of Perineal defects following oncologic resection: a systematic review and meta‐analysis. Plast Reconstr Surg. 2016;137(5):1602–1613. [DOI] [PubMed] [Google Scholar]
- 65. Howell AM, Jarral OA, Faiz O, Ziprin P, Darzi A, Zacharakis E. How should perineal wounds be closed following abdominoperineal resection in patients post radiotherapy‐‐primary closure or flap repair? Best evidence topic (BET). Int J Surg. 2013;11(7):514–517. [DOI] [PubMed] [Google Scholar]
- 66. Gendreau‐Lefevre AK, Audet N, Maltais S, Thuot F. Prophylactic pectoralis major muscle flap in prevention of pharyngocutaneous fistula in total laryngectomy after radiotherapy. Head Neck. 2015;37(9):1233–1238. [DOI] [PubMed] [Google Scholar]
- 67. Trotti A, Klotch D, Endicott J, Ridley M, Cantor A. Postoperative accelerated radiotherapy in high‐risk squamous cell carcinoma of the head and neck: long‐term results of a prospective trial. Head Neck. 1998;20(2):119–123. [DOI] [PubMed] [Google Scholar]
- 68. Nystrom LM, Reimer NB, Reith JD, et al. Multidisciplinary management of soft tissue sarcoma. Sci World J. 2013;2013:852462. [DOI] [PMC free article] [PubMed] [Google Scholar]


