Abstract
Accumulating evidence shows that circRNAs play critical roles in the development of human tumors. We observed that circ_0000527 was overexpressed in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) compared in hFOB1.19 cells. We demonstrated that the circ_0000527 level was higher in osteosarcoma specimens than in non-tumor specimens. The ectopic expression of circ_0000527 was shown to induce cell growth, cell cycle progression and the secretion of inflammatory mediators, including IL-1β, IL-6, IL-8 and TNF-α. We demonstrated that circ_0000527 sponges miR-646 in osteosarcoma cells and that ARL2 is a target gene of miR-646. MiR-646 expression was decreased and ARL2 was overexpressed in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) compared to hFOB1.19 cells. Overexpression of circ_0000527 was demonstrated to induce ARL2 expression in MG-63 cells. We showed that miR-646 was downregulated in osteosarcoma specimens compared to that of non-tumor specimens and that the level of circ_0000527 was negatively correlated with miR-646 expression in osteosarcoma specimens. The elevated expression of circ_0000527 was shown to promote cell growth and cell cycle progression by modulating miR-646 expression. The ectopic expression of circ_0000527 was shown to promote cell growth, cell cycle progression and the secretion of inflammatory mediators by modulating ARL2. The present study suggested that the circ_0000527/miR-646/ARL2 axis may be a potential treatment target for osteosarcoma.
Keywords: osteosarcoma, circ_0000527, ARL2, miR-646
INTRODUCTION
Osteosarcoma is the most frequent type of primary malignant bone tumor in adolescents and children [1–5]. The predicted worldwide incidence is four million cases every year, with a high incidence at 15–19 years of age [6–9]. Osteosarcoma is locally destructive with a high rate of metastasis to other organs, especially to the lung [10–12]. Despite the rapid advances in treatment strategies, including adjuvant chemotherapy, radiotherapy and wide local tumor excision, the 5-year survival rate remains unsatisfactory [13–16]. Thus, it is urgent to study the fundamental mechanisms and find novel diagnostic and therapeutic targets for osteosarcoma.
Accumulating evidence has shown that circRNAs can modulate gene expression by acting as competing endogenous RNAs (ceRNAs) for miRNAs and their target genes [17–21]. CircRNAs are deregulated in several tumors, such as nasopharyngeal carcinoma, retinoblastoma, glioma, cervical cancer and osteosarcoma [22–26]. CircRNAs play critical roles in many cell functions, including metabolism, development, differentiation, invasion and apoptosis [27–29]. Recently, Zhang et al. [30] have illustrated that circ_0000527 is upregulated in retinoblastoma cells and tissues, inhibits cell apoptosis and induces cell invasion, growth and migration. However, its function in osteosarcoma remains elusive.
We demonstrated that the circ_0000527 level is higher in osteosarcoma specimens than in non-tumor specimens. We showed that the ectopic expression of circ_0000527 induces cell growth, cell cycle progression, invasion and secretion of inflammatory mediators, including IL-1β, IL-6, IL-8 and TNF-α.
RESULTS
circ_0000527, miR-646 and ARL2 expression in osteosarcoma cells
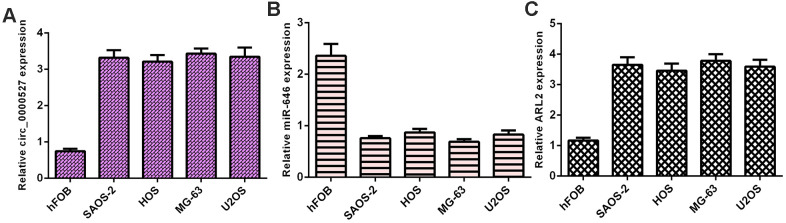
We observed that circ_0000527 was overexpressed in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) compared to hFOB1.19 cells (Figure 1A). MiR-646 expression was decreased in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) compared to hFOB1.19 cells (Figure 1B). ARL2 was overexpressed in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) compared to hFOB1.19 cells (Figure 1C).
Figure 1.
circ_0000527, miR-646 and ARL2 expression in osteosarcoma cells. (A) The expression of circ_0000527 in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) and hFOB1.19 was determined by qRT-PCR assay. (B) miR-646 was decreased in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) compared to hFOB1.19 cells. (C) The expression of ARL2 in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) and hFOB1.19 was measured using qRT-PCR assay.
Circ_0000527 expression is higher in osteosarcoma specimens
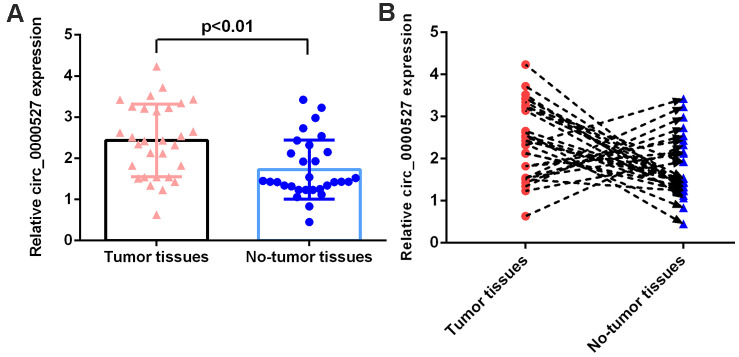
Then, we determined the expression of circ_0000527 in the osteosarcoma specimens. We observed that the circ_0000527 level was higher in the osteosarcoma specimens than in the non-tumor specimens (Figure 2A).
Figure 2.
circ_0000527 expression in osteosarcoma specimens. (A) The level of circ_0000527 in osteosarcoma specimens and non-tumor specimens was measured by a qRT-PCR assay. (B) The level of circ_0000527 was upregulated in 20 cases (20/30, 66.7%) compared to non-tumor specimens.
The level of circ_0000527 was upregulated in 20 cases (20/30, 66.7%) compared to the non-tumor specimens (Figure 2B).
Ectopic expression of circ_0000527 induces cell growth and cell cycle progression
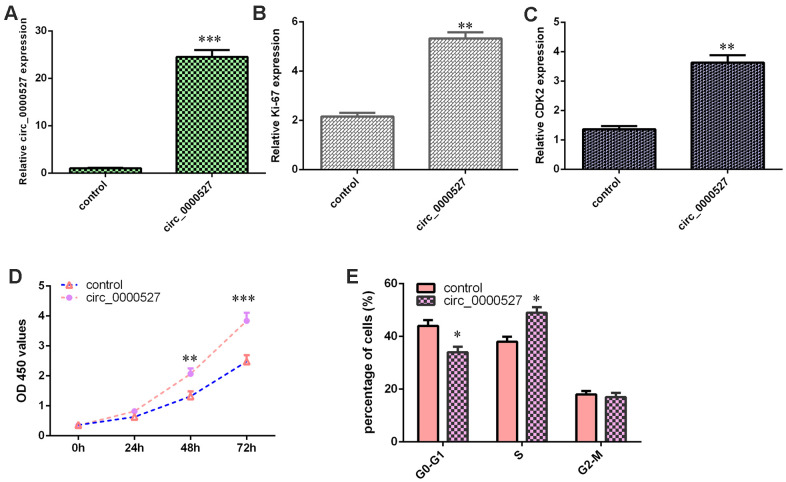
The level of circ_0000527 was upregulated in MG-63 cells after treatment with pcDNA-circ_0000527 compared to that of the control group (Figure 3A). The elevated expression of circ_0000527 enhanced the expression of ki-67 (Figure 3B) and CDK2 (Figure 3C) in MG-63 cells. The ectopic expression of circ_0000527 induced cell growth in MG-63 cells (Figure 3D). The overexpression of circ_0000527 induced cell cycle progression in MG-63 cells (Figure 3E).
Figure 3.
Ectopic expression of circ_0000527 induced cell growth, cell cycle progression and invasion. (A) The level of circ_0000527 was determined by a qRT-PCR assay. (B) The elevated expression of circ_0000527 enhanced ki-67 expression in MG-63 cells. (C) The level of CDK2 was determined using qRT-PCR analysis. (D) The ectopic expression of circ_0000527 induced cell growth in MG-63 cells. (E) The overexpression of circ_0000527 induced cell cycle progression in MG-63 cells. *p<0.05, ** p<0.01, ***p<0.001.
Overexpression of circ_0000527 promotes the secretion of inflammatory mediators
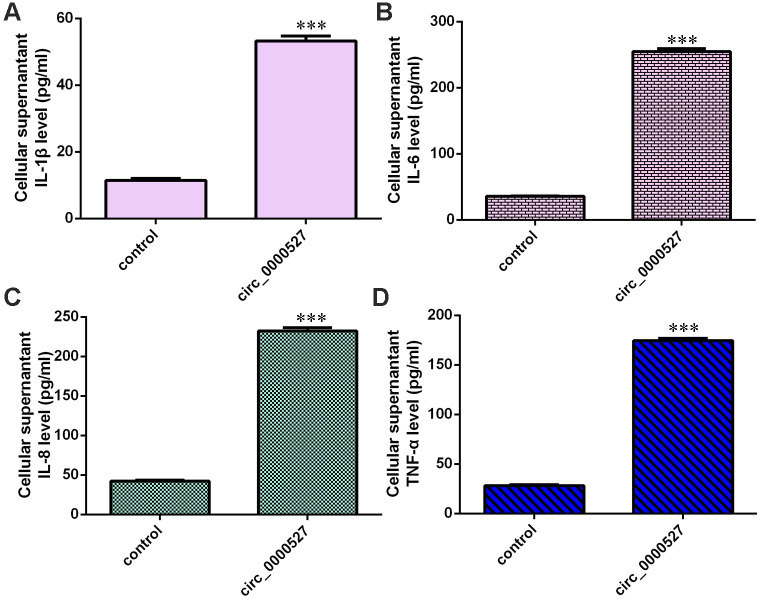
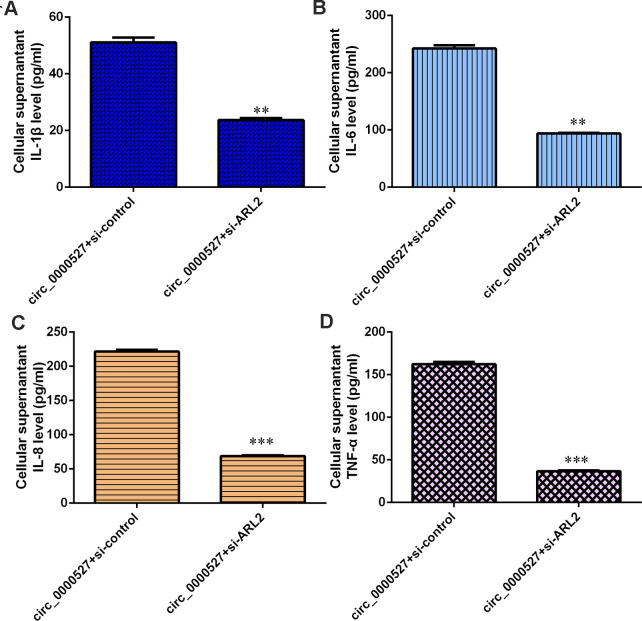
The elevated expression of circ_0000527 induced IL-1β expression in MG-63 cells (Figure 4A). The overexpression of circ_0000527 promoted IL-6 expression in MG-63 cells (Figure 4B). The ectopic expression of circ_0000527 enhanced the expression of IL-8 (Figure 4C) and TNF-α (Figure 4D) in MG-63 cells.
Figure 4.
Overexpression of circ_0000527 promoted secretion of inflammatory mediators. (A) The elevated expression of circ_0000527 induced IL-1β expression in MG-63 cells. (B) The expression of IL-6 was determined using an ELISA assay. (C) The ectopic expression of circ_0000527 enhanced IL-8 expression. (D) The expression of TNF-α was determined by ELISA assay. ***p<0.001.
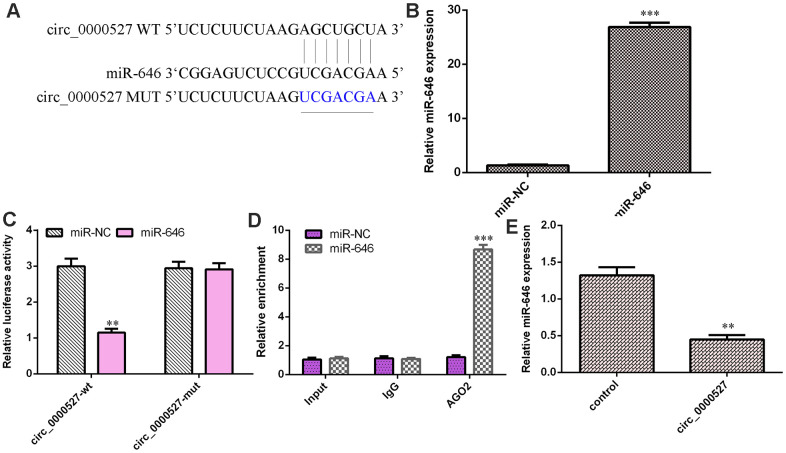
Circ_0000527 sponges miR-646 in osteosarcoma cells
As predicted by Starbase, circ_0000527 might be regulated by miR-646 (Figure 5A). The level of miR-646 was upregulated in MG-63 cells after treatment with the miR-646 mimic compared to the control group (Figure 5B). We observed that overexpression of miR-646 significantly decreased the luciferase value of WT circ_0000527 but did not have an effect on mut circ_0000527 (Figure 5C). The RIP assay illustrated that expression of miR-646 could be enriched with circ_0000527 (Figure 5D). The elevated expression of circ_0000527 suppressed miR-646 expression in MG-63 cells (Figure 5E).
Figure 5.
circ_0000527 sponged miR-646 expression in osteosarcoma cells. (A) As predicted by Starbase, circ_0000527 might be regulated by miR-646. (B) The level of miR-646 was measured by a qRT-PCR assay. (C) The overexpression of miR-646 significantly decreased the luciferase value of WT circ_0000527 but did not have an effect on mut circ_0000527. (D) A RIP assay illustrated that overexpression of miR-646 could be enriched with circ_0000527. (E) The elevated expression of circ_0000527 suppressed miR-646 expression in MG-63 cells. ** p<0.01.
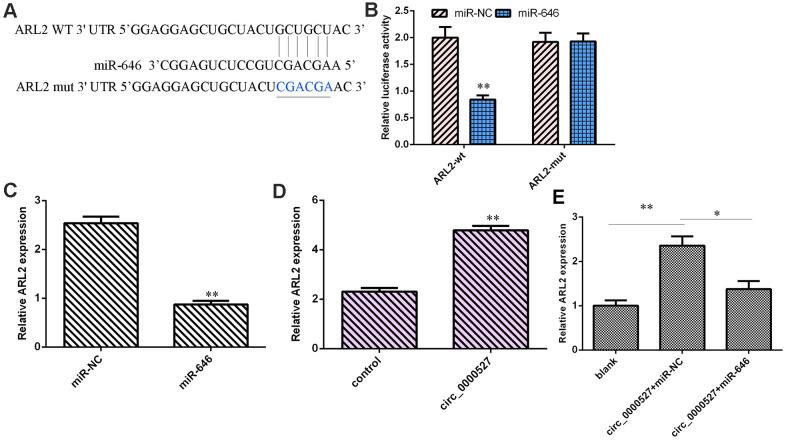
ARL2 is a target gene of miR-646
As predicted by Targetscan, ARL2 expression might be regulated by miR-646 (Figure 6A). We observed that overexpression of miR-646 significantly decreased the luciferase value of WT ARL2 but did not have an effect on mut ARL2 (Figure 6B). The elevated expression of miR-646 suppressed ARL2 expression in MG-63 cells (Figure 6C). The overexpression of circ_0000527 induced ARL2 expression in MG-63 cells (Figure 6D). The ectopic expression of circ_0000527 promoted ARL2 expression, but the overexpression of miR-646 could decrease its expression (Figure 6E).
Figure 6.
ARL2 was a target gene of miR-646. (A) As predicted by Targetscan, ARL2 might be regulated by miR-646. (B) The overexpression of miR-646 significantly decreased luciferase value of WT ARL2 but did not have an effect on mut ARL2. (C) The elevated expression of miR-646 suppressed ARL2 expression in MG-63 cells. (D) The expression of ARL2 was measured using a qRT-PCR assay. (E) The expression of ARL2 was assessed by qRT-PCR analysis. ** p<0.01.
MiR-646 expression is downregulated in osteosarcoma
Furthermore, we demonstrated that miR-646 was downregulated in osteosarcoma specimens compared to non-tumor specimens (Figure 7A). The level of miR-646 was upregulated in 17 cases (17/30, 56.7%) compared to non-tumor specimens (Figure 7B). The level of circ_0000527 was negatively correlated with miR-646 expression in osteosarcoma specimens (Figure 7C).
Figure 7.
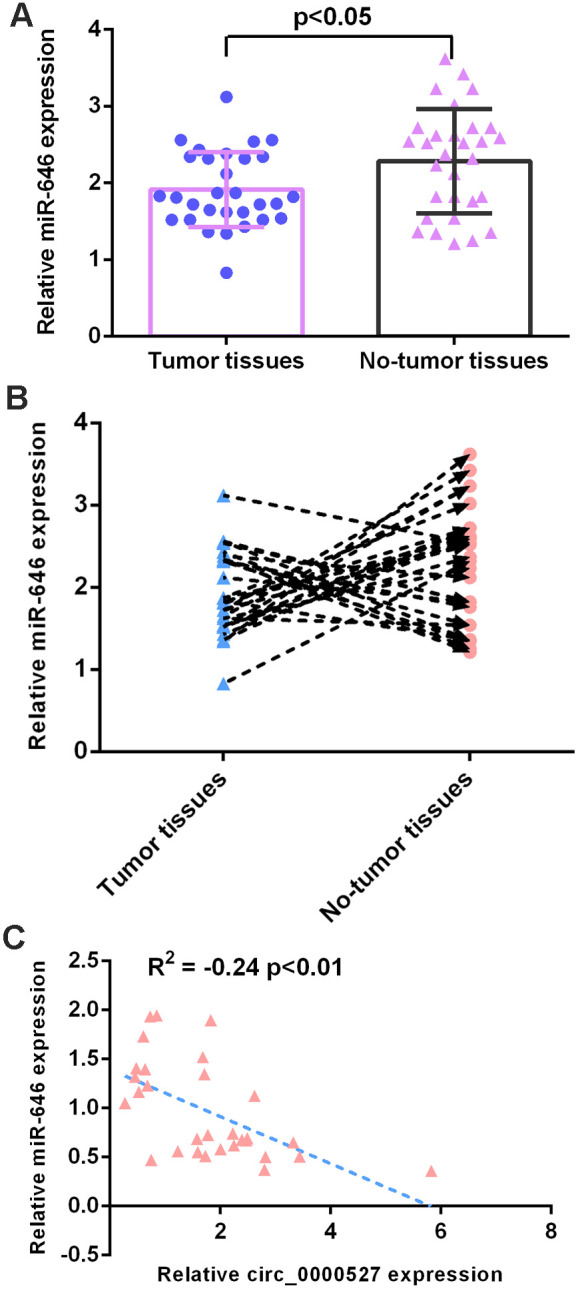
miR-646 expression was downregulated in osteosarcoma. (A) miR-646 was downregulated in osteosarcoma specimens compared to non-tumor specimens. The expression of miR-646 was detected by a qRT-PCR assay. (B) The level of miR-646 was upregulated in 17 cases (17/30, 56.7%) compared to non-tumor specimens. (C) The level of circ_0000527 was negatively correlated with miR-646 expression in osteosarcoma specimens.
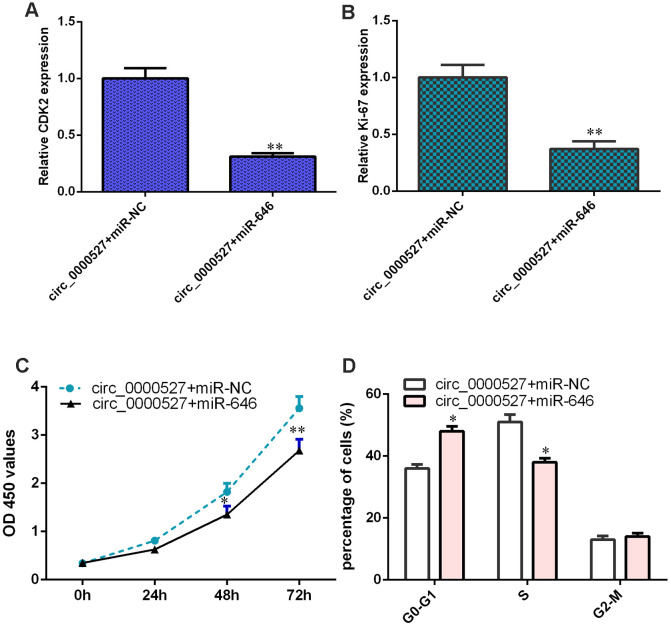
Ectopic expression of circ_0000527 promotes cell growth and cell cycle progression by modulating miR-646
The elevated expression of miR-646 decreased expression of CDK2 (Figure 8A) and ki-67 (Figure 8B) in the circ_0000527-overexpressing MG-63 cells. Overexpression of miR-646 suppressed cell proliferation (Figure 8C) and cell cycle progression (Figure 8D) in the circ_0000527-overexpressing MG-63 cells.
Figure 8.
Ectopic expression of circ_0000527 promoted cell growth and cycle via modulating miR-646. (A) The level of CDK2 was measured by a qRT-PCR assay. (B) The expression of ki-67 was determined using a qRT-PCR assay. (C) Cell growth was assessed by CCK-8 analysis. (D) Cell cycle progression was determined by flow cytometry. *p<0.05, ** p<0.01, ***p<0.001.
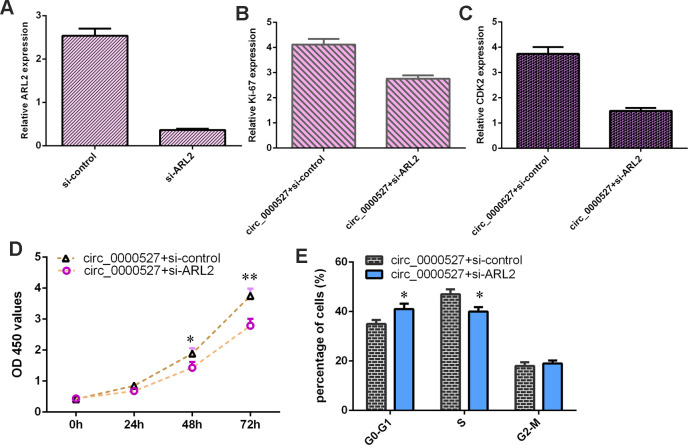
Ectopic expression of circ_0000527 promotes cell growth and cell cycle progression by modulating ARL2
The level of ARL2 was downregulated in MG-63 cells after treatment with ARL2 siRNA compared to that of the control group (Figure 9A). Knockdown of ARL2 decreased the expression of ki-67 (Figure 9B) and CDK2 (Figure 9C) in the circ_0000527-overexpressing MG-63 cells. Inhibition of ARL2 suppressed cell proliferation (Figure 9D) and cell cycle progression (Figure 9E) in the circ_0000527-overexpressing MG-63 cells.
Figure 9.
Ectopic expression of circ_0000527 promoted cell growth, cycle and invasion via modulating ARL2. (A) The expression of ARL2 was determined using qRT-PCR assay. (B) The expression of ki-67 was detected using qRT-PCR analysis. (C) The level of CDK2 was measured by qRT-PCR assay. (D) Inhibition expression of ARL2 suppressed cell proliferation in circ_0000527-overexpressing MG-63 cell. (E) Cell cycle was determined by flow cytometry. *p<0.05,** p<0.01, ***p<0.001.
Elevated expression of circ_0000527 promotes secretion of inflammatory mediators by regulating ARL2
Knockdown of ARL2 suppressed IL-1β expression (Figure 10A) and IL-6 expression (Figure 10B) in the circ_0000527-overexpressing MG-63 cells. Inhibition of ARL2 inhibited expression of IL-8 expression (Figure 10C) and TNF-α (Figure 10D) in the circ_0000527-overexpressing MG-63 cells.
Figure 10.
Elevated expression of circ_0000527 promoted secretion of inflammatory mediators through regulating ARL2. (A) The knockdown of ARL2 suppressed IL-1β expression in circ_0000527-overexpressing MG-63 cells. (B) The expression of IL-6 was assessed by ELISA. (C) The expression of IL-8 was assessed by ELISA. (D) The expression of TNF-α was assessed by ELISA. ** p<0.01, ***p<0.001.
DISCUSSION
Increasing evidence has shown that circRNAs play critical roles in the development of human tumors [31–33]. Existing studies illustrate that circRNAs can modulate multidrug resistance, metastasis and growth in human malignancies [34–37]. For instance, Ding et al. [38] illustrated that circ_0005909 was overexpressed in osteosarcoma cells and specimens and was correlated with a poor survival rate. The inhibition of circ_0005909 has been shown to suppress cell viability, invasion, EMT and migration in vitro and to decrease cancer growth in vivo partially by regulating miR-936/HMGB1. Lin et al. [39] demonstrated that circEIF4G2 was overexpressed in osteosarcoma cells and specimens and that the knockdown of circEIF4G2 inhibited osteosarcoma cell invasion, growth and migration through modulating miR-218. Li and colleague illustrated that circ_0003732 was overexpressed in specimens and that overexpression of circ_0003732 induced cell proliferation by regulating the CCNA2/miR-545 axis [40]. Wu et al. [41] indicated that circUBAP2 was overexpressed in osteosarcoma cells and specimens and that knockdown of circUBAP2 inhibited cellular EMT, invasion and growth through modulating the miR-641/ YAP1 axis. Xu et al. [42] illustrated that circTUBGCP3 was overexpressed in osteosarcoma specimens and that overexpression of circTUBGCP3 induced cell migration, survivability and proliferation through sponging miR-30b. Recently, Zhang et al. [30] illustrated that circ_0000527 was upregulated in retinoblastoma cells and tissues and that circ_0000527 overexpression inhibited cell apoptosis and induced cell invasion, growth and migration. Furthermore, Chen et al. [43] also observed that ectopic expression of circ_0000527 enhanced retinoblastoma cell migration, viability and invasion. Exposure to inflammatory cytokines is the principal reason for tumorigenesis, and the regulation of inflammation is an important method for preventing cancer progression [44]. We observed that circ_0000527 is overexpressed in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) compared to hFOB1.19 cells. We demonstrated that the circ_0000527 level is higher in the osteosarcoma specimens than in the non-tumor specimens. We showed that the ectopic expression of circ_0000527 induces cell growth, cell cycle progression, invasion and secretion of inflammatory mediators, including IL-1β, IL-6, IL-8 and TNF-α.
Previously published studies have shown that circRNAs can act as ceRNAs of miRNAs to decrease the activity of miRNAs and modulate gene expression, transcription and binding with these proteins [45, 46]. For example, Zhang et al. [47] noted that circ_0136666 overexpression promoted osteosarcoma tumorigenesis by regulating the ZEB2/miR-593-3p pathway. Fang et al. [48] showed that circ_0000337 was upregulated in osteosarcoma specimens and that the knockdown of circ_0000337 inhibited osteosarcoma cell migration and growth by modulating miR-4458/BACH1. Zhang et al. [49] noted that circ_0002052 induced osteosarcoma progression through regulating the miR-382/STX6 axis. A previous study showed that overexpression of circ_0000527 enhanced retinoblastoma cell migration, viability and invasion by modulating miR-646 [30]. We demonstrated that circ_0000527 sponges miR-646 expression in osteosarcoma cells and that ARL2 is a target gene of miR-646. It was demonstrated that miR-646 decreases and that ARL2 is overexpressed in osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) compared to hFOB1.19 cells. Furthermore, we showed that miR-646 is downregulated in osteosarcoma specimens compared to non-tumor specimens. We demonstrated that the level of circ_0000527 is negatively correlated with miR-646 expression in the osteosarcoma specimens. We also showed that the ectopic expression of circ_0000527 promotes cell growth and cell cycle progression by modulating miR-646 expression and that the overexpression of circ_0000527 induces ARL2 expression in MG-63 cells. A previous study showed that microRNA-497-5p suppresses the tumor cell growth of osteosarcoma by targeting ADP ribosylation factor-like protein 2. We showed that the ectopic expression of circ_0000527 promotes cell growth, cell cycle progression, invasion and secretion of inflammatory mediators by modulating ARL2 expression.
In summary, this study revealed that circ_0000527 is overexpressed in osteosarcoma cells and specimens and that ectopic expression of circ_0000527 promotes cell growth, cell cycle progression, invasion and the secretion of inflammatory mediators by modulating ARL2. Therefore, the circ_0000527/miR-646/ARL2 axis may be a potential treatment target for osteosarcoma.
MATERIALS AND METHODS
Cell culture, transfection and clinical samples
Osteosarcoma cells (SAOS-2, HOS, MG-63 and U2OS) and hFOB1.19 cells were acquired from the American type culture collection (ATCC) and cultured in DMEM (Invitrogen Inc., USA) supplemented with streptomycin, penicillin and FBS. miR-646, pcDNA circ_0000527 and their controls were synthesized at Shanghai GenePharma. Cell transfection was performed with Lipofectamine 3000 (Invitrogen Inc., USA). Osteosarcoma surgical specimens and control specimens were obtained from Nanyang First People’s Hospital following the criteria approved by the institutional review board. Informed consent was gained from each patient.
qRT-PCR
Total RNA from cells cultured from the osteosarcoma specimens was collected using a TRIzol kit (Invitrogen Inc, USA). The relative expression of circ_0000527, miR-646 and ARL2 was detected by a qRT-PCR assay using a SYBR Green kit on a Bio-Rad CFX96 PCR system (VisonBio Scientific). U6 snRNA and GAPDH were used as the controls for the miRNA and mRNA, respectively. The method of 2-DDCt was utilized for analysis of the PCR results.
CCK-8 assay, cell cycle analysis and ELISA
The cells were cultured in 96-well dishes for 0, 24, 48 and 72 hours. At the desired time point, 10 ul of CCK-8 solution from the kit was added into each well. After 4 hours, the OD at 450 nM was assessed. Cytokines in the supernatant were assessed by ELISA. IL-1β, IL-6, IL-8 and TNF-α kits (R&D Systems, USA) were used to determine the IL-1β, IL-6, IL-8 and TNF-α concentrations, respectively. For the cell cycle analysis, the cells were stained with propidium iodide (PI) for half an hour. The cell cycle was assessed by flow cytometry.
RIP assay
The Magna RIP RBP Immunoprecipitation Kit (Millipore) was used for the RIP assay. The cells were resuspended in the lysis buffer containing RNase and protease inhibitors (Epicentre). The cell lysate was then incubated with beads coated with an AGO2 antibody or a control IgG. After incubation in proteinase K, the immunoprecipitated RNA was isolated and assessed by qRT-PCR.
Dual-luciferase reporter gene assay
The wt and mut constructs of circ_0000527 and the ARL2 gene were amplified with RT-PCR. The cells were cultured in 24-well dishes and co-transfected with the miR-646 mimic or miR-NC and pGL3-circ_0000527, ARL2-3’UTR-WT, pGL3-circ_0000527 or ARL2-3’UTR-MUT and pRL-TK by Lipofectamine 3000 (Invitrogen Inc, USA). After 48 hours, the firefly and Renilla luciferase values were detected using a dual-luciferase kit (Promega, USA). Renilla luciferase was used as the control.
Statistical analysis
All results are shown as the mean ± standard deviation. The significant difference between two groups was determined with a Student’s t test. P <0.05 was set as statistically significant.
Footnotes
AUTHOR CONTRIBUTIONS: Xiangkun Wu, Lihua Yan, Yongxi Liu, Lilin Shang conducted experiments and collected and analyzed data. Xiangkun Wu and Yongxi Liui write and revised this manuscript.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Zhao J, Zhang C, Gao Z, Wu H, Gu R, Jiang R. Long non-coding RNA ASBEL promotes osteosarcoma cell proliferation, migration, and invasion by regulating microRNA-21. J Cell Biochem. 2018; 119:6461–69. 10.1002/jcb.26671 [DOI] [PubMed] [Google Scholar]
- 2.Yang C, Wu K, Wang S, Wei G. Long non-coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR-195-5p. J Cell Biochem. 2018; 119:5646–56. 10.1002/jcb.26743 [DOI] [PubMed] [Google Scholar]
- 3.Xu R, Feng F, Yu X, Liu Z, Lao L. LncRNA SNHG4 promotes tumour growth by sponging miR-224-3p and predicts poor survival and recurrence in human osteosarcoma. Cell Prolif. 2018; 51:e12515. 10.1111/cpr.12515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Shen J, Chan MT, Wu WK. The long non-coding RNA SPRY4-IT1: an emerging player in tumorigenesis and osteosarcoma. Cell Prolif. 2018; 51:e12446. 10.1111/cpr.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ba Z, Gu L, Hao S, Wang X, Cheng Z, Nie G. Downregulation of lncRNA CASC2 facilitates osteosarcoma growth and invasion through miR-181a. Cell Prolif. 2018; 51:e12409. 10.1111/cpr.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan J, Lang J, Liu C, Zhou K, Chen L, Liu Y. The expression and function of miRNA-451 in osteosarcoma. Med Oncol. 2015; 32:324. 10.1007/s12032-014-0324-x [DOI] [PubMed] [Google Scholar]
- 7.Li H, Liu H, Pei J, Wang H, Lv H. miR-542-3p overexpression is associated with enhanced osteosarcoma cell proliferation and migration ability by targeting Van Gogh-like 2. Mol Med Rep. 2015; 11:851–56. 10.3892/mmr.2014.2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du JY, Wang LF, Wang Q, Yu LD. miR-26b inhibits proliferation, migration, invasion and apoptosis induction via the downregulation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 driven glycolysis in osteosarcoma cells. Oncol Rep. 2015; 33:1890–98. 10.3892/or.2015.3797 [DOI] [PubMed] [Google Scholar]
- 9.Delebinski CI, Georgi S, Kleinsimon S, Twardziok M, Kopp B, Melzig MF, Seifert G. Analysis of proliferation and apoptotic induction by 20 steroid glycosides in 143B osteosarcoma cells in vitro. Cell Prolif. 2015; 48:600–10. 10.1111/cpr.12208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K, Zhang Y, Ren K, Zhao G, Yan K, Ma B. MicroRNA-101 inhibits the metastasis of osteosarcoma cells by downregulation of EZH2 expression. Oncol Rep. 2014; 32:2143–49. 10.3892/or.2014.3459 [DOI] [PubMed] [Google Scholar]
- 11.Xu M, Jin H, Xu CX, Bi WZ, Wang Y. MiR-34c inhibits osteosarcoma metastasis and chemoresistance. Med Oncol. 2014; 31:972. 10.1007/s12032-014-0972-x [DOI] [PubMed] [Google Scholar]
- 12.Wang XH, Cai P, Wang MH, Wang Z. microRNA-25 promotes osteosarcoma cell proliferation by targeting the cell-cycle inhibitor p27. Mol Med Rep. 2014; 10:855–59. 10.3892/mmr.2014.2260 [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Xu G, Shen F, Kang Y. miR-132 targeting cyclin E1 suppresses cell proliferation in osteosarcoma cells. Tumour Biol. 2014; 35:4859–65. 10.1007/s13277-014-1637-2 [DOI] [PubMed] [Google Scholar]
- 14.Tian Z, Guo B, Yu M, Wang C, Zhang H, Liang Q, Jiang K, Cao L. Upregulation of micro-ribonucleic acid-128 cooperating with downregulation of PTEN confers metastatic potential and unfavorable prognosis in patients with primary osteosarcoma. Onco Targets Ther. 2014; 7:1601–08. 10.2147/OTT.S67217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Z, Zhang T, Hong H, Liu Q, Zhang H. miR-202 suppresses proliferation and induces apoptosis of osteosarcoma cells by downregulating Gli2. Mol Cell Biochem. 2014; 397:277–83. 10.1007/s11010-014-2195-z [DOI] [PubMed] [Google Scholar]
- 16.Pan W, Wang H, Jianwei R, Ye Z. MicroRNA-27a promotes proliferation, migration and invasion by targeting MAP2K4 in human osteosarcoma cells. Cell Physiol Biochem. 2014; 33:402–12. 10.1159/000356679 [DOI] [PubMed] [Google Scholar]
- 17.Liang M, Huang G, Liu Z, Wang Q, Yu Z, Liu Z, Lin H, Li M, Zhou X, Zheng Y. Elevated levels of hsa_circ_006100 in gastric cancer promote cell growth and metastasis via miR-195/GPRC5A signalling. Cell Prolif. 2019; 52:e12661. 10.1111/cpr.12661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Song S, Lin S, Zhang M, Du Y, Zhang D, Xu W, Wang H. circ-SERPINE2 promotes the development of gastric carcinoma by sponging miR-375 and modulating YWHAZ. Cell Prolif. 2019; 52:e12648. 10.1111/cpr.12648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu P, Li X, Guo X, Chen J, Li C, Chen M, Liu L, Zhang X, Zu X. Circular RNA DOCK1 promotes bladder carcinoma progression via modulating circDOCK1/hsa-miR-132-3p/Sox5 signalling pathway. Cell Prolif. 2019; 52:e12614. 10.1111/cpr.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Chen Z, Feng Y, Hu G, Jiang Y. CircMMP11 acts as a ce-circRNA in breast cancer progression by regulating miR-1204. Am J Transl Res. 2020; 12:2585–99. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang ZW, Pang B, Chen YC, Peng AQ. TMPRSS3 regulates cell viability and apoptosis processes of HEI-OC1 cells via regulation of the circ-Slc4a2, miR-182 and Akt cascade. J Gene Med. 2019; 21:e3118. 10.1002/jgm.3118 [DOI] [PubMed] [Google Scholar]
- 22.Zheng S, Qian Z, Jiang F, Ge D, Tang J, Chen H, Yang J, Yao Y, Yan J, Zhao L, Li H, Yang L. CircRNA LRP6 promotes the development of osteosarcoma via negatively regulating KLF2 and APC levels. Am J Transl Res. 2019; 11:4126–38. [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, Ma K, Sun M, Shi S. Identification of the tumor-suppressive function of circular RNA ITCH in glioma cells through sponging miR-214 and promoting linear ITCH expression. Am J Transl Res. 2018; 10:1373–86. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Ma HB, Yao YN, Yu JJ, Chen XX, Li HF. Extensive profiling of circular RNAs and the potential regulatory role of circRNA-000284 in cell proliferation and invasion of cervical cancer via sponging miR-506. Am J Transl Res. 2018; 10:592–604. [PMC free article] [PubMed] [Google Scholar]
- 25.Pan J, Bao X, Zhu K. Circular RNA_LARP4 inhibits cell proliferation and invasion of nasopharyngeal carcinoma by repressing ROCK1. Eur Rev Med Pharmacol Sci. 2019; 23:9915–22. 10.26355/eurrev_201911_19557 [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Wang S, Qin T, Wang W. Circular RNA (circ-0075804) promotes the proliferation of retinoblastoma via combining heterogeneous nuclear ribonucleoprotein K (HNRNPK) to improve the stability of E2F transcription factor 3 E2F3. J Cell Biochem. 2020; 121:3516–25. 10.1002/jcb.29631 [DOI] [PubMed] [Google Scholar]
- 27.Ding Y, Dong Y, Lu H, Luo X, Fu J, Xiu B, Liang A, Zhang W. Circular RNA profile of acute myeloid leukaemia indicates circular RNA annexin A2 as a potential biomarker and therapeutic target for acute myeloid leukaemia. Am J Transl Res. 2020; 12:1683–99. [PMC free article] [PubMed] [Google Scholar]
- 28.Long MY, Chen JW, Zhu Y, Luo DY, Lin SJ, Peng XZ, Tan LP, Li HH. Comprehensive circular RNA profiling reveals the regulatory role of circRNA_0007694 in papillary thyroid carcinoma. Am J Transl Res. 2020; 12:1362–78. [PMC free article] [PubMed] [Google Scholar]
- 29.Tu FL, Guo XQ, Wu HX, He ZY, Wang F, Sun AJ, Dai XD. circ-0001313/miRNA-510-5p/AKT2 axis promotes the development and progression of colon cancer. Am J Transl Res. 2020; 12:281–91. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Wu J, Li Y, Jiang Y, Wang L, Chen Y, Lv Y, Zou Y, Ding X. Circ_0000527 promotes the progression of retinoblastoma by regulating miR-646/LRP6 axis. Cancer Cell Int. 2020; 20:301. 10.1186/s12935-020-01396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Zheng X, Liu H. Hsa_circ_0102272 serves as a prognostic biomarker and regulates proliferation, migration and apoptosis in thyroid cancer. J Gene Med. 2020; 22:e3209. 10.1002/jgm.3209 [DOI] [PubMed] [Google Scholar]
- 32.Cheng H, Wu B, Wang L, Hu T, Deng Z, Li D. Insights into the expression profiles and functions of circRNAs in a newborn hyperoxia-induced rat bronchopulmonary dysplasia model. J Gene Med. 2020; 22:e3163. 10.1002/jgm.3163 [DOI] [PubMed] [Google Scholar]
- 33.Xiong Z, Zhou C, Wang L, Zhu R, Zhong L, Wan D, Wang Q. Circular RNA SMO sponges miR-338-3p to promote the growth of glioma by enhancing the expression of SMO. Aging (Albany NY). 2019; 11:12345–60. 10.18632/aging.102576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Z, Lin J, Wu D, He X, Wang W, Hu X, Zhang L, Wang M. Hsa_circ_0006948 enhances cancer progression and epithelial-mesenchymal transition through the miR-490-3p/HMGA2 axis in esophageal squamous cell carcinoma. Aging (Albany NY). 2019; 11:11937–54. 10.18632/aging.102519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao W, Zheng S, Zou Y, Yang A, Xie X, Tang H, Xie X. CircAHNAK1 inhibits proliferation and metastasis of triple-negative breast cancer by modulating miR-421 and RASA1. Aging (Albany NY). 2019; 11:12043–56. 10.18632/aging.102539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Xue N, Guo Y, Niu K, Gao L, Zhang S, Gu H, Wang X, Zhao D, Fan R. CircRNA_100367 regulated the radiation sensitivity of esophageal squamous cell carcinomas through miR-217/Wnt3 pathway. Aging (Albany NY). 2019; 11:12412–27. 10.18632/aging.102580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mi B, Xiong Y, Chen L, Yan C, Endo Y, Liu Y, Liu J, Hu L, Hu Y, Sun Y, Cao F, Zhou W, Liu G. CircRNA AFF4 promotes osteoblast cells proliferation and inhibits apoptosis via the mir-7223-5p/PIK3R1 axis. Aging (Albany NY). 2019; 11:11988–2001. 10.18632/aging.102524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding S, Zhang G, Gao Y, Chen S, Cao C. Circular RNA hsa_circ_0005909 modulates osteosarcoma progression via the miR-936/HMGB1 axis. Cancer Cell Int. 2020; 20:305. 10.1186/s12935-020-01399-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin E, Liu S, Xiang W, Zhang H, Xie C. CircEIF4G2 promotes tumorigenesis and progression of osteosarcoma by sponging miR-218. Biomed Res Int. 2020; 2020:8386936. 10.1155/2020/8386936 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Li L, Kong XA, Zang M, Dong J, Feng Y, Gui B, Hu Y. Hsa_circ_0003732 promotes osteosarcoma cells proliferation via miR-545/CCNA2 axis. Biosci Rep. 2020; 40:BSR20191552. 10.1042/BSR20191552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H, Li W, Zhu S, Zhang D, Zhang M. Circular RNA circUBAP2 regulates proliferation and invasion of osteosarcoma cells through miR-641/YAP1 axis. Cancer Cell Int. 2020; 20:223. 10.1186/s12935-020-01318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Yao T, Huang K, Liu G, Huang Y, Gao J, Ye H, Shen S, Ma J. Circular RNA circTUBGCP3 is up-regulated and promotes cell proliferation, migration and survivability via sponge mir-30b in osteosarcoma. Onco Targets Ther. 2020; 13:3729–37. 10.2147/OTT.S245366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen NN, Chao DL, Li XG. Circular RNA has_circ_0000527 participates in proliferation, invasion and migration of retinoblastoma cells via miR-646/BCL-2 axis. Cell Biochem Funct. 2020; 38:1036–46. 10.1002/cbf.3535 [DOI] [PubMed] [Google Scholar]
- 44.Wang W, Wang Z, Chen S, Zang X, Miao J. Interleukin-1β/nuclear factor-κB signaling promotes osteosarcoma cell growth through the microRNA-181b/phosphatase and tensin homolog axis. J Cell Biochem. 2019; 120:1763–72. 10.1002/jcb.27477 [DOI] [PubMed] [Google Scholar]
- 45.Dai X, Guo X, Liu J, Cheng A, Peng X, Zha L, Wang Z. Circular RNA circGRAMD1B inhibits gastric cancer progression by sponging miR-130a-3p and regulating PTEN and p21 expression. Aging (Albany NY). 2019; 11:9689–708. 10.18632/aging.102414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu W, Wu Z, Xia Y, Qin S, Li Y, Wu J, Liang J, Wang L, Zhu H, Fan L, Fu J, Xu W, Jin H, Li J. Downregulation of circ_0132266 in chronic lymphocytic leukemia promoted cell viability through miR-337-3p/PML axis. Aging (Albany NY). 2019; 11:3561–73. 10.18632/aging.101997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Zhou H, Yuan K, Xie R, Chen C. Overexpression of hsa_circ_0136666 predicts poor prognosis and initiates osteosarcoma tumorigenesis through miR-593-3p/ZEB2 pathway. Aging (Albany NY). 2020; 12:10488–96. 10.18632/aging.103273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang Y, Long F. Circular RNA circ_0000337 contributes to osteosarcoma via the miR-4458/BACH1 pathway. Cancer Biomark. 2020; 28:411–19. 10.3233/CBM-190647 [DOI] [PubMed] [Google Scholar]
- 49.Zhang PR, Ren J, Wan JS, Sun R, Li Y. Circular RNA hsa_circ_0002052 promotes osteosarcoma via modulating miR-382/STX6 axis. Hum Cell. 2020; 33:810–18. 10.1007/s13577-020-00335-9 [DOI] [PubMed] [Google Scholar]