Abstract
Background
Studies on the prognostic value of the soluble programmed death ligand 1 (sPD-L1) in cancer patients have not yielded consistent results.
Objective
This meta-analysis was performed to assess the association between sPD-L1 and the prognosis of cancer patients.
Methods
Published articles in Pubmed, EMBASE, and Cochrane clinical trial databases were searched from the inception to September 2020. Overall survival (OS), progression-free survival (PFS), recurrence-free survival (RFS), and disease-free survival (DFS) data were evaluated using a hazard ratio (HR) at 95% confidence interval (95% CI).
Results
A total 31 studies involving 17 tumors and 3,780 patients were included. The overexpression of sPD-L1 was associated with shorter OS (HR 1.85, 95% CI 1.59–2.15, I2 = 33%). High sPD-L1 had worse PFS (HR 2.40, 95% CI 1.55–3.72, I2 = 83%), and worse DFS (HR 2.92, 95% CI 2.02–4.29, I2 = 40%), without significant statistical difference in RFS (HR 2.08, 95% CI 0.99–4.40, I2 = 0%).
Conclusions
High sPD-L1 levels were associated with worse survival prognosis in cancer patients. The sPD-L1 may be a potential prognostic, non-invasive, and dynamic monitoring biomarker for cancers in the future.
Keywords: soluble programmed death ligand 1, cancers, prognosis, survival, meta-analysis
Introduction
Immune checkpoint inhibitors such as anti-PD-1/PD-L1 have remarkable clinical benefits in a variety of tumors (1, 2). The expression of programmed death ligand 1 (PD-L1) is often observed in a variety of cancers. The combination of PD-L1 and its receptor PD-1 on activated T cells suppresses antitumor immunity by counteracting T cell activation signals. Antibody-based PD-1/PD-L1 inhibitors can induce durable tumor remission of various advanced cancer types.
PD-L1 exists in two forms; membrane-bound and soluble form (3). Studies have shown that soluble PD-L1 (sPD-L1) may be derived from the shedding of tumor cells or the release of immune cells in tumor tissues (3, 4). Evidence shows that the surfaces of exosomes secreted by tumor cells have biologically active PD-L1, which can suppress immune responses (5). The circulatory cell expressing PD-L1 still retains its biological activity, and it can specifically bind the PD-1 receptor of the T cells in peripheral blood, thereby activating the PD-1/PD-L1 pathway and establishing systemic immunosuppression (6, 7). Moreover, sPD-L1 is a prognostic marker of tumor treatment (8).
Studies on the relationship between sPD-L1 and the prognosis of cancer patients have yielded contrasting results. In NSCLC, studies by He et al. showed that patients with high sPD-L1 have a reduced risk of death (9). However, data from Okuma et al. showed that lung cancer patients with high sPD-L1 have an increased risk of death (10). In gastric cancer and nasopharyngeal carcinoma, there is no correlation between soluble PD-L1 levels and overall survival (4, 11). This systematic review and meta-analysis aimed at evaluating the value and clinical significance of sPD-L1 in the survival prognosis of cancer patients.
Materials and Methods
Search Strategy and Inclusion Criteria
Relevant articles published in various databases were searched since the inception of the databases to September 2020. The Pubmed, EMBASE, and Cochrane clinical trial databases were searched for the meta-analysis. The Clinicaltrial.gov, American Society of Clinical Oncology (ASCO) and ESMO databases were also searched. Search terms were composed of various combinations of “soluble PD-L1”, “sPD-L1”, “serum PD-L1”, “plasma PD-L1”, “circulatory PD-L1”, “blood PD-L1”, “programmed death ligand 1”, “B7-H1” and “cancer”, “neoplasm”, “carcinoma”, “lymphoma”, “sarcomas”. The language of the article was not restricted.
The inclusion criteria for various studies were: i. The included patients were all pathologically diagnosed tumor patients; ii. With analyzable soluble PD-L1 data; iii. With sufficient clinical features and data that could be combined to analyze soluble PD- L1 and survival prognosis; iv. Had their sPD-L1 levels detected by enzyme-linked immunosorbent assay (ELISA). The exclusion criteria were: i. Case reports, reviews, and tissue immunohistochemical (IHC) detection for PD-L1; ii. Animal experiments; iii. No direct analysis of the relationship between soluble PD-L1 and survival prognosis; iv. Incomplete data or non-original research.
Data Extraction and Quality Assessment
First, the outcomes of each study were extracted, including overall survival (OS), progression-free survival (PFS), recurrence-free survival (RFS), and disease-free survival (DFS) prognosis data. Next, the baseline data of the studies were extracted and analyzed; publication years, study type, patient characteristics, treatment methods, and the cut-off value of sPD-L1, etc. Based on the purpose of our research, OS was the primary outcome of interest. All data screening and extraction processes were independently performed by two investigators.
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to assess the quality of the study. The NOS consists of three parts: selection (4 points), comparability (2 points), and outcome evaluation (3 points). Table 1 shows the NOS scores of the included studies. All processes were independently performed by two reviewers and in case of disagreements, they were discussed to reach a consensus. Studies with an NOS score ≥6 were considered high-quality.
Table 1.
Characteristics of included studies.
| Study/year | Cancer Type | Country/region | Study Type | Included Period | No of Samples | Median Age | Primary Outcome | |
|---|---|---|---|---|---|---|---|---|
| Lu et al., 2020 (11) | Nasopharyngeal Carcinoma | China | R | 2012–2015 | 219 | 48 | OS/DFS/RFS | |
| Ha et al., 2016 (12) | Cholangiocarcinoma | Korea | R | 2004–2009 | 158 | 59.6 | OS | |
| Meyo et al., 2020 (13) | Lung carcinoma | France | R | 2015–2018 | 51 | 66 | OS/PFS | |
| Jin et al., 2018 (14) | SCLC | China | R | 2010–2016 | 250 | 64.5 | OS | |
| He et al., 2020 (9) | NSCLC | China | R | 2008–2009 | 88 | 59 | OS | |
| Zhao et al., 2017 (15) | NSCLC | China | R | 2009–2013 | 126 | 65 | OS | |
| Okuma et al., 2017 (10) | Lung carcinoma | Japan | P | 2014–2016 | 96 | 68.5 | OS | |
| Okuma et al., 2018 (16) | NSCLC | Japan | P | 2016–2017 | 39 | 69 | OS/PFS | |
| Bai et al., 2018 (17) | NSCLC | Netherlands | R | After 2012 | 102 | 61.5 | OS/PFS | |
| Lee 2018 (18) | Hepatocellular carcinoma | Korea | R | NA | 78 | NA | OS/RFS | |
| Ma 2019 (19) | Hepatocellular carcinoma | China | NA | 2012–2013 | 114 | NA | OS/RFS | |
| Chang 2019 (20) | Hepatocellular carcinoma | China | R | 2008–2014 | 120 | NA | OS/DFS/RFS | |
| Finkelmeier et al., 2016 (21) | Hepatocellular carcinoma | Germany | P | 2009–2015 | 215 | 64 | OS | |
| Aghajani et al., 2019 (22) | Thyroid carcinoma | Australia | R | 2012–2017 | 101 | 47 | DFS | |
| Chiarucci et al., 2020 (23) | Mesothelioma | Italy | R | NA | 40 | 66 | OS | |
| Omura et al., 2020 (24) | Colorectal carcinoma | Japan | R | 2013–2015 | 131 | 69 | OS/DFS | |
| Tominaga et al., 2019 (25) | Rectal carcinoma | Japan | P | 2013–2017 | 117 | 61 | DFS | |
| Wang et al., 2016 (26) | NK/T cell lymphoma | China | P | 2008–2015 | 97 | 42 | OS/PFS | |
| Nagato et al., 2017 (27) | NK/T cell lymphoma | Japan | R | 2000–2014 | 17 | 52 | OS | |
| Shen et al., 2019 (28) | Peripheral T-cell lymphoma | China | P | 2016–2018 | 80 | 46.5 | OS/PFS | |
| Rossille et al., 2014 (29) | Large B-Cell lymphoma | France | R | 2005–2010 | 283 | NA | OS | |
| Rossille 2017 (30) | Large B-Cell lymphoma | American | P | NA | 225 | NA | OS | |
| Guo et al., 2018 (31) | Hodgkin lymphoma | China | R | 2005–2015 | 108 | 34.6 | OS/PFS | |
| Buderath et al., 2019 (32) | Ovarian carcinoma | Germany | R | 2007–2014 | 83 | 68 | OS/PFS | |
| Asanuma et al., 2020 (33) | Soft tissue sarcomas | Japan | R | 2009–2016 | 135 | 63.4 | OS/PFS/RFS | |
| Frigola et al., 2011 (7) | Renal cell carcinoma | American | R | 2003–2007 | 172 | 52 | OS | |
| Cheng et al., 2019 (34) | Esophageal carcinoma | China | P | NA | 161 | NA | OS | |
| Shigemori 2019 (35) | Gastric carcinoma | Japan | R | 2008–2014 | 180 | 70 | OS/DFS | |
| Takahashi et al., 2016 (4) | Gastric carcinoma | Japan | R | 2011–2015 | 75 | 67 | OS/PFS | |
| Bian et al., 2019 (36) | Pancreatic adenocarcinoma | France | R | 2012–2016 | 59 | 68 | OS | |
| Park 2019 (37) | Pancreatic adenocarcinoma | Korea | P | 2013–2015 | 60 | NA | OS/PFS | |
| Follow-up (Months) | Cut-off | Cut-off Selection | Surgery | Immunotherapy | Chemotherapy | Stage/T stage | NOS score | Conference summary |
| 50 | 93.7 pg/ml | X-Tile | No surgery | No | NA | I–IV | 8 | No |
| 95.3 | 0.94 ng/ml | Minimum P value approach | No surgery | No | NA | Advanced | 8 | No |
| 26.8 | 0.156 ng/ml | Lower limit of quantification | No surgery | Nivolumab | Yes | Advanced | 7 | No |
| NA | 7.1 ng/ml | ROC | No surgery | No | Yes | Advanced | 8 | No |
| 67 | NA | NA | Surgery | No | No | Ia–IIIb | 7 | No |
| 25 | 96.5 pg/ml | ROC | No surgery | No | NA | IIIb | 8 | No |
| NA | 7.32 ng/ml | ROC | No surgery | NA | NA | IIIb–IV | 7 | No |
| NA | 3.35 ng/ml | ROC | No surgery | Nivolumab | Yes | IV | 6 | No |
| NA | NA | NA | NA | No | NA | Advanced | NA | Yes |
| 16.1 | 19.2 pg/ml | NA | Surgery | No | NA | NA | NA | Yes |
| NA | NA | NA | NA | No | NA | NA | NA | Yes |
| NA | 11.2 µg/ml | X-Tile | Surgery | No | NA | NA | 8 | No |
| 9.93 | 0.8 ng/ml | Median value | Mixed | No | NA | NA | 8 | No |
| 16 | 0.44 ng/ml | ROC | Surgery | No | No | I–IVb | 8 | No |
| NA | 0.07 ng/ml | ROC | No surgery | Multiple therapy* | No | III–IV | 6 | No |
| NA | 0.08 ng/ml | ROC | Surgery | No | NA | I–III | 8 | No |
| 33.7 | 0.16 ng/ml | Median value | Surgery | No | Yes | II–III | 8 | No |
| 38 | 3.23 ng/ml | ROC | No surgery | No | Yes | I–II | 7 | No |
| 90 | 850 pg/ml | ROC | No surgery | No | Yes | I–IV | 6 | No |
| 20 | 176.30 pg/ml | ROC | No surgery | No | Yes | I–IV | 7 | No |
| 41.4 | 1.52 ng/ml | MaxStat test | No surgery | No | Yes | III–IV | 8 | No |
| NA | 1652 pg/ml | Median value | No surgery | No | Yes | I–IV | 8 | No |
| 47 | 25.16 ng/ml | ROC | No surgery | No | Yes | I–IV | 8 | No |
| NA | 6.4 pg/ml | ROC | Surgery | No | NA | II–IV | 7 | No |
| 42.9 | 44.26 pg/ml | ROC | Surgery | No | NA | I–III | 7 | No |
| 45.6 | NA | NA | Surgery | No | NA | I–IV | 8 | No |
| NA | NA | Median Value | Surgery | No | Yes | Advanced | NA | Yes |
| NA | 0.507 ng/ml | ROC | Surgery | No | NA | I–IV | 8 | No |
| NA | 1.081 ng/ml | ROC | No surgery | No | Yes | IV | 7 | No |
| NA | 8.6 ng/ml | ROC | Mixed | No | Yes | Tx-T4 | 7 | No |
| 11.4 | 4.6 ng/ml | ROC | No surgery | No | Yes | Advanced | 6 | No |
P, prospctive; R, retrospctive; OS, overal survival; PFS, progression-free survival; RFS, recurrence-free survival; DFS, disease-free survival; SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; ROC, receiver operating characteristic curve; NOS, Newcastle–Ottawa quality assessment scale; *: tezolizumab/durvalumab/ipilimumab/tremelimumab/pembrolizumab/nivolumab.
Statistical Analysis
The 95% confidence interval (CI) of all results was calculated. All outcomes were directly extracted from the studies. Time-to-event endpoints (OS, PFS, DFS, and RFS) were pooled with the use of hazard ratio (HR). Heterogeneity was assessed using the χ2-based Q test and I2 statistics. I2 >75% indicated considerable heterogeneity (38). The random-effects model was applied for pooled analysis. The RevMan software (version 5.3) from Cochrane library was used for meta-analysis. Publication bias was evaluated by funnel plots. Sensitivity analysis was performed by the one by one elimination method of individual studies to assess the reliability of the results.
Results
Characteristics of the Included Studies
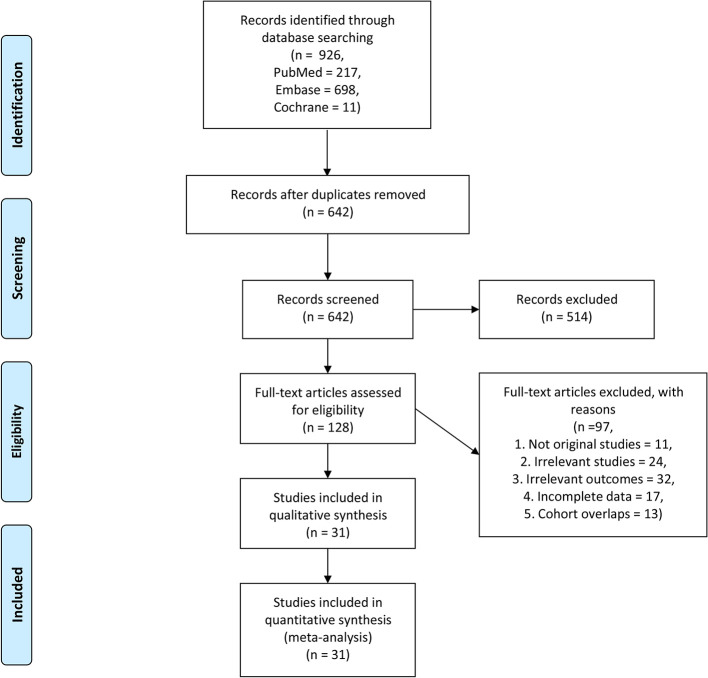
We initially identified 926 articles from the searched databases, and after excluding duplicates, 642 articles remained. After excluding 514 records, the full text of 128 articles were screened. After full-text screening, 97 studies were excluded due to the following reasons; not original studies (n = 11), irrelevant studies (n = 24), irrelevant outcomes (n = 32), incomplete data (n = 17), and the cohort overlaps (n = 13). Finally, 31 studies involving 17 tumors and a total of 3,780 patients met our inclusion criteria ( Figure 1 ). The tumor types included in the study were; Nasopharyngeal Carcinoma (11), Cholangiocarcinoma (12), lung cancer (9, 10, 13–17), Hepatocellular carcinoma (20, 21, 39, 40), Thyroid carcinoma (22), Mesothelioma (23), Colorectal carcinoma (24, 25), NK/T cell lymphoma (26, 27), Peripheral T-cell lymphoma (28), Large B-Cell lymphoma (29, 35), Hodgkin lymphoma (31), Ovarian carcinoma (32), Soft tissue sarcomas (33), Renal cell carcinoma (7), Esophageal carcinoma (34), Gastric carcinoma (4, 35), and Pancreatic adenocarcinoma (36, 41).
Figure 1.
Flow chart for the study selection.
The main characteristics of the 31 studies are summarized in Table 1 . The NOS scores of the included studies were all greater than or equal to 6 points, except for several conference abstract articles ( Table 1 ).
Survival Analysis
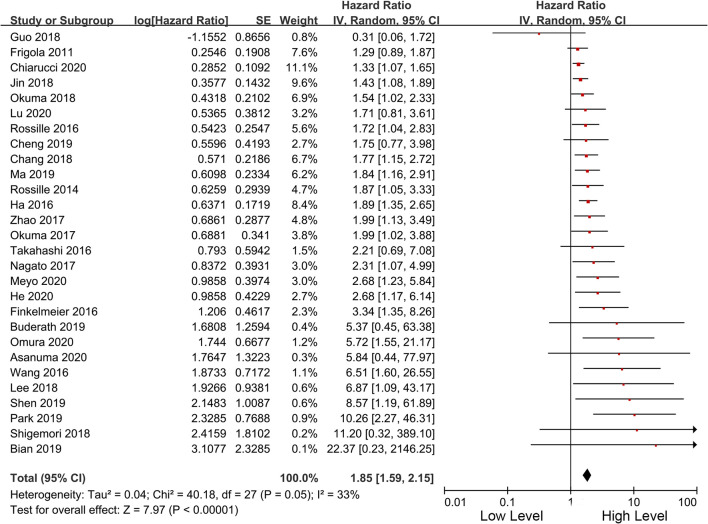
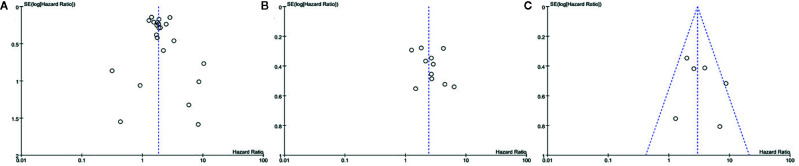
Among the included studies, 28 studies have OS as their primary outcome. OS data from these 28 studies were pooled, and the results showed that overexpression of sPD-L1 was associated with shorter OS (HR 1.85, 95% CI 1.59–2.15, I2 = 33%) ( Figure 2 ). The funnel plot showed that there was no significant publication bias ( Figure 3 ). The publication bias analysis of RFS was not performed due to the limited number of included studies. Sensitivity analysis showed that the results were stable and reliable.
Figure 2.
Forest plot of the overall survival in patients of high and low sPD-L1.
Figure 3.
Funnel plot of the (A) overall survival, (B) PFS, and (C) DFS in patients of high and low sPD-L1.
In terms of secondary outcomes, PFS, RFS, and DFS were also pooled for analysis. The pooled data of 11 studies showed that high sPD-L1 is correlated with worse PFS (HR 2.40, 95% CI 1.55–3.72, I2 = 83%). Besides, six studies included DFS data. High sPD-L1 was correlated with worse DFS (HR 2.92, 95% CI 2.02–4.29, I2 = 40%). Moreover, only two studies reported RFS, and sPDL1 was insignificantly correlated with a worse RFS (HR 2.08, 95% CI 0.99–4.40, I2 = 0%) ( Table 2 ).
Table 2.
Analyses of secondary outcomes.
| Secondaryoutcomes | No. of studies | Pooled HR (95%CI) | P-value | Heterogeneity | ||
|---|---|---|---|---|---|---|
| Model | I2 | PQ | ||||
| PFS | 11 | 2.40 (1.55–3.72) | <0.001 | Random | 83 | <0.001 |
| RFS | 2 | 2.08 (0.99–4.40) | 0.050 | Random | 0 | 0.670 |
| DFS | 6 | 2.92 (2.02–4.29) | <0.001 | Random | 40 | 0.140 |
CI, confidence interval; HR, hazard ratio; PFS, progression-free survival; RFS, recurrence-free survival; DFS, disease-free survival.
Subgroup Analysis of OS
In the included 28 studies with OS data, there were different observation types, treatment methods, cut-off values of sPD-L1 and survival analysis methods. Therefore, we performed a subgroup analysis of these differences. The results showed that high sPD-L1 in both retrospective (HR 1.70, 95% CI 1.47–1.96, I2 = 21%) and prospective studies (HR 2.44, 95% CI 1.70–3.51, I2 = 32%) was correlated with worse OS. Studies were stratified according to whether surgery was performed, and the results showed that surgery did not affect the correlation between sPD-L1 and OS (surgery: HR 2.06, 95% CI 1.43–2.96, I2 = 31%; non-surgery: HR 1.91, 95% CI 1.57–2.32, I2 = 44%) ( Table 3 ). The subgroup analysis based on immunotherapy revealed similar results.
Table 3.
Results of subgroup analysis of pooled hazard ratios of OS of patients.
| Stratified analysis | No. of studies | Pooled HR (95% CI) | P‐value | Heterogeneity | |
|---|---|---|---|---|---|
| I2 (%) | PQ | ||||
| Study type | |||||
| Prospective | 8 | 2.44 (1.70–3.51) | <0.001 | 32 | 0.17 |
| Retrospective | 20 | 1.70 (1.47–1.96) | <0.001 | 21 | 0.19 |
| Treatment | |||||
| Surgery | 9 | 2.06 (1.43–2.96) | <0.001 | 31 | 0.17 |
| Non surgery | 18 | 1.91 (1.57–2.32) | <0.001 | 44 | 0.02 |
| Cut-off value | |||||
| <1 ng/ml | 14 | 2.17 (1.62–6.89) | <0.001 | 45 | 0.05 |
| 1–5 ng/ml | 6 | 2.12 (1.42–3.17) | <0.001 | 44 | 0.11 |
| >5ng/ml | 6 | 1.53 (1.17–1.99) | 0.002 | 21 | 0.28 |
| Analysis | |||||
| Multivariate | 22 | 1.83 (1.55–2.18) | <0.001 | 30 | 0.10 |
| Univariate | 6 | 2.07 (1.40–3.04) | <0.001 | 45 | 0.09 |
| Immunotherapy | |||||
| No | 25 | 1.95 (1.63–2.34) | <0.001 | 30 | 0.09 |
| Yes | 3 | 1.51 (1.14–1.99) | 0.004 | 44 | 0.22 |
| Detection of sPD-L1 | |||||
| Pre-treatment | 21 | 1.90 (1.56–2.32) | <0.001 | 38 | 0.04 |
| On-treatment | 3 | 2.19 (1.35–3.56) | 0.002 | 22 | 0.28 |
| Post-treatment | 1 | 6.51 (1.60–26.55) | 0.009 | – | – |
CI, confidence interval; HR, hazard ratio; OS, overall survival.
As an important indicator, the cut-off value of sPD-L1 was divided into three levels of less than 1, 1–5, and greater than 5 ng/ml for subgroup analysis. In the three levels, high sPD-L1 was consistently correlated with worse OS (<1 ng/ml: HR 2.17, 95% CI 1.62–6.89, I2 = 45%; 1–5 ng/ml: HR 2.12, 95% CI 1.42–3.17, I2 = 44%, and >5 ng/ml: HR 1.53, 95% CI 1.17–1.99, I2 = 21%). We also performed subgroup analyses based on cut-off points (0.5 and 1 ng/ml, 1 and 10 ng/ml, and others). We found that the significant association between sPD-L1 and survival did not change with the change in cutoff points. Furthermore, sPD-L1 was associated with worse OS in both univariate (HR 2.07, 95% CI 1.40–3.04, I2 = 45%) and multivariate analysis (HR 1.83, 95% CI 1.55–2.18, I2 = 30%) ( Table 3 ). We further performed subgroup analysis based on the time point of the detection of sPD-L1. Majority of the studies used the pre-treatment sPD-L1. They assessed the level of sPD-L1 at baseline when patients were initially diagnosed. The high level of sPD-L1 represented a worse OS in all three subgroups. Additionally, we performed subgroup analysis according to the specific cancer types. As presented in Figure 4 , sPD-L1 was correlated with a worse OS in a majority of the cancers. Insignificant results were only obtained in the subgroups with less included studies (nasopharyngeal cancer, ovarian carcinoma, soft tissue sarcomas, renal cell carcinoma and esophageal carcinoma, gastric carcinoma).
Figure 4.
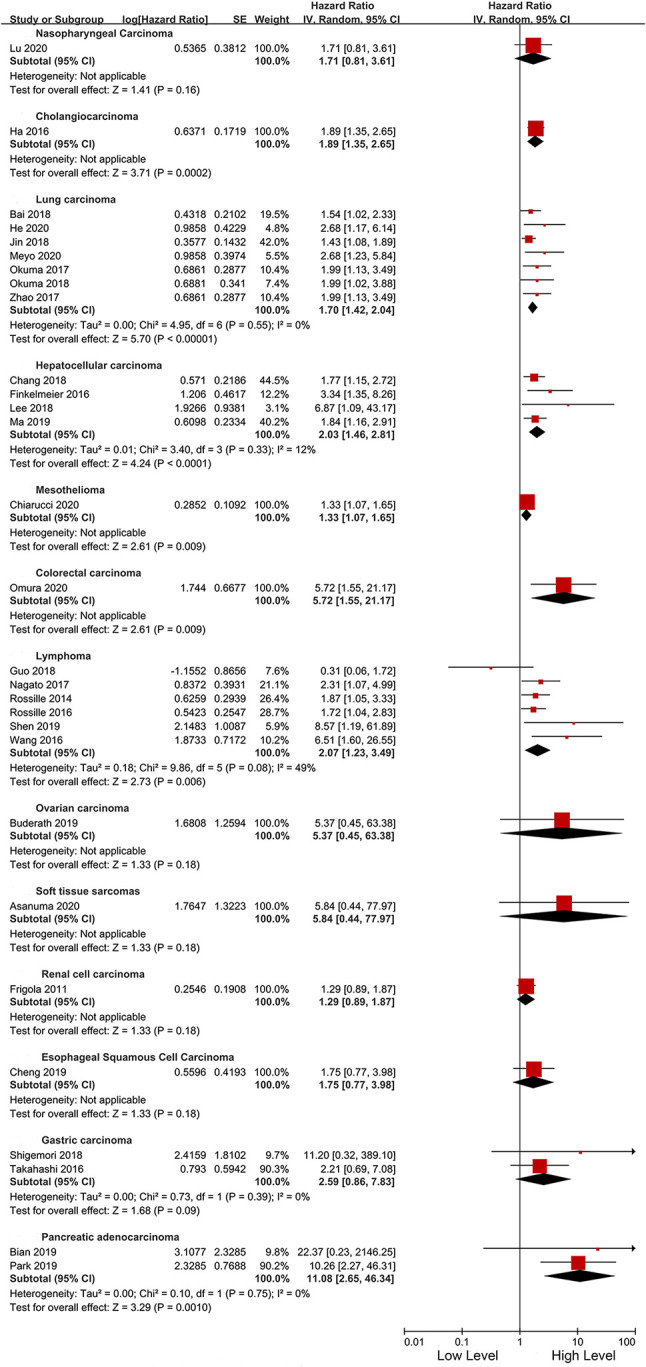
Forest plot of subgroup analysis in different types cancers.
Discussion
This meta-analysis summarizes the correlation between the level of sPD-L1 and the prognosis of various cancer patients. The main significance of sPD-L1 detection is to establish a minimally invasive, easy-to-clinical, and predictive immune biomarker for cancer patients.
PD-L1 can be divided into membrane-bound PD-L1 (mPD-L1) and sPD-L1. It is believed that sPD-L1 is mainly produced by the proteolysis of mPD-L1. The immunosuppressive correlation and prognostic values of mPD-L1/sPD-L1 have not been established. Currently, IHC detection is commonly used in clinical practice to detect mPD-L1. Previous studies have explored the relationships between mPD-L1 expression levels in tumor tissues and clinical prognosis. However, these studies are limited by difficulties in sampling tumor tissues, as well as by the inability to dynamically detect changes in patients during treatment. In addition, the relationships between the levels of PD-L1 as detected by tissue IHC and the prognosis of cancer patients are not clinically binding (42, 43). Uncertainty in sampling tumor tissues, IHC staining conditions and antibody complexity, evaluator standards as well as positive cut-off values may all affect the assessment of PD-L1 levels. The advantage of sPD-L1 over mPD-L1 is that sPD-L1 can be ubiquitously present in plasma and serous effusions, and its detection is relatively convenient, repeatable and objective. A study tracked and monitored the levels of PD-L1 in plasma exosomes of patients with melanoma, and showed that all patients had exosomal-PD-L1, while only 67% of tumor biopsy specimens were PD-L1 positive (44).
We found that high sPD-L1 levels can be used as a prognostic biomarkers for poor treatment outcomes. Secondly, it has been proved that the sPD-L1 still retains its biological activity. We can further postulate that sPD-L1 in peripheral blood can be able to specifically bind T cells in peripheral blood, thereby, inhibiting T cell activity and inducing systemic immune suppression (33). When PD-L1 positive exosomes were co-cultured with activated T cells, a significant decrease in CD69 on the surface of T cells could be observed (45). The sPD-L1 can not only exert an immunosuppressive effect in the local tumor microenvironment but can also act on the distal end of the body. sPD-L1 in the blood can effectively suppress the secretion of IFN-γ by T cells, and can participate in systemic anti-tumor immune regulation by targeting T lymphocytes in secondary lymphoid organs (44, 46).
Subgroup analyses were performed based on the treatment or cut-off value of the patient. In the subgroup analysis of patients receiving immunotherapy, patients with high sPD-L1 levels were correlated with a worse OS. An NSCLC study showed that the death risk in patients with high levels of sPD-L1 was 2.68 (95%CI 1.36–5.28) times the risk in patients with low sPD-L1 levels (14). Given that sPD-L1 can be derived from tumor tissue cells, the level of sPD-L1 can reflect tumor regression to a certain extent. This implies that sPD-L1 has the potential to be used as a biomarker for predicting the prognosis of immunotherapy. Due to the high variations in sPD-L1 between various tumors and the limited number of samples, the median level of the sPD-L1 and the analyzed cut-off values are quite different. We divided sPD-L1 into three intervals for subgroup analysis, and the results consistently showed that sPD-L1 was associated with poor prognosis. The sampling error of patients and the differences between multiple tumors make it difficult for us to accurately analyze the relationship between the specific level of sPD-L1 and prognosis, however, it can still reflect the prognostic differences between patients with high and low sPD-L1. More stringent cut-off values require further exploration of large sample RCT experiments. Additionally, we also found that sPD-L1 can predict prognosis in patients subjected to, and those not subjected to surgical procedures. In NSCLC patients after radical surgery, sPD-L1 with a median value of 3.84 ng/ml could still be detected (9). This suggests that there is no release of primary foci and that there is still some detectable sPD-L1 in peripheral blood. Moreover, it implies that sPD-L1 can be derived from antigen-presenting cells in the blood. This indicates that in patients with radically resected tumors, the detection of sPD-L1 can be used to monitor immune checkpoint suppression, predict the efficacy of immunotherapy, and predict survival prognosis. This is the ability that tumor tissue IHC does not have in PD-L1 detection.
sPD-L1 can be used as a good indicator for the survival of cancer patients. Several studies have evaluated the predictive role of other immune-related biomarkers (such as soluble PD-L1, Vascular Endothelial Growth Factor A, soluble CD40L, CTLA-4, and soluble CD44) in different types of cancers (47). Meyo et al. proposed a composite biomarker using sPD-L1 and other immune-related biomarkers to predict nivolumab efficacy in NSCLC patients (13). The combination of inflammatory status indicators such as neutrophil to lymphocyte ratio with sPD-L1 has also been evaluated (12). The peripheral cytokine can be combined with sPD-L1 to predict treatment benefits and prognosis (48). The prognostic values of biomarkers that play a role in the expression of PD-L1 (such as STAT3) and their combination with sPD-L1 have also been explored (49). More combinations of sPD-L1 and other plasma indicators should be evaluated for cancer patients’ prognosis. The plasma indicators might improve the predictive performance of sPD-L1.
Two systematic reviews and meta-analysis have evaluated the relationship between sPD-L1 and tumor prognosis. The review by Ding et al. in 2017 included eight articles with a total of 1,102 cancer patients (50). Wei et al. also published a review article in 2017 that included eight articles and a total of 1,040 patients with solid tumors (51). They all found worse prognostic outcomes for patients with highly expressed sPD-L1. In recent years, a studies sPD-L1 have been published, and their results are not consistent with the above two reviews. Therefore, this review, we increased the number of studies to 31 involving 17 tumors. Compared to the previous reviews, the results of this study show that sPD-L1 is associated with worse prognostic outcomes among tumor patients receiving immunotherapy. This is different from the well-known high response rate of immunotherapy in patients with positive mPD-L1 expression (52), which also shows the different functions and significance of sPD-L1 and mPD-L1 in tumors.
This study has certain limitations. First, the studies included in this meta-analysis were observational studies, due to the lack of relevant randomized controlled trials (RCTs). Higher quality RCTs are needed to further evaluate the prognostic value of sPD-L1. Second, the number of patients with each tumor included in this study was small, and a larger sample population is needed for verification. Third, different tumor types have different molecular signatures including immune checkpoint regulators, therefore, combining them leads to inaccurate inferences and misleading clinical applications. Finally, the cut-off values of sPD-L1 in different studies were significantly different, leading to limitations in clinical applications. In future, more studies should aim at accurately establishing the correlation between sPD-L1 expression level and prognosis. However, this study shows the prognostic value of sPD-L1 in a variety of tumors and immunotherapy, indicating that sPD-L1 can potentially serve as an innovative biomarker for predicting the prognosis of cancer patients.
In conclusion, this meta-analysis revealed that high sPD-L1 levels were associated with worse survival outcomes (including OS, DFS, and PFS) in cancer patients. And among patients who had received immunotherapy, patients with high sPD-L1 levels had worse OS. The sPD-L1 may be a potential prognostic, non-invasive, and dynamic monitoring biomarker for cancers.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
PH, HL: protocol/project development. PH, HL, YW: data collection and management. HL, WH: data analysis. HL, WH, YZ: manuscript writing/editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Carlisle JW, Steuer CE, Owonikoko TK, Saba NF. An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin (2020). 10.3322/caac.21630 [DOI] [PubMed] [Google Scholar]
- 2. Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol (2020). 10.1038/s41571-020-0413-z [DOI] [PubMed] [Google Scholar]
- 3. Abu Hejleh T, Furqan M, Ballas Z, Clamon G. The clinical significance of soluble PD-1 and PD-L1 in lung cancer. Crit Rev Oncol Hematol (2019) 143:148–52. 10.1016/j.critrevonc.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 4. Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol (2016) 142(8):1727–38. 10.1007/s00432-016-2184-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xie F, Xu M, Lu J, Mao L, Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer (2019) 18(1):146. 10.1186/s12943-019-1074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, et al. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine (2011) 56(2):231–8. 10.1016/j.cyto.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 7. Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res (2011) 17(7):1915–23. 10.1158/1078-0432.CCR-10-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia Y, Li X, Zhao C, Ren S, Su C, Gao G, et al. Soluble PD-L1 as a Predictor of the Response to EGFR-TKIs in Non-small Cell Lung Cancer Patients With EGFR Mutations. Front Oncol (2020) 10:1455. 10.3389/fonc.2020.01455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He J, Pan Y, Guo Y, Li B, Tang Y. Study on the Expression Levels and Clinical Significance of PD-1 and PD-L1 in Plasma of NSCLC Patients. J Immunother (Hagerstown Md 1997) (2020) 43(5):156–64. 10.1097/CJI.0000000000000315 [DOI] [PubMed] [Google Scholar]
- 10. Okuma Y, Hosomi Y, Nakahara Y, Watanabe K, Sagawa Y, Homma S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer (Amsterdam Netherlands) (2017) 104:1–6. 10.1016/j.lungcan.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 11. Lu T, Chen Y, Li J, Guo Q, Lin W, Zheng Y, et al. High Soluble Programmed Death-Ligand 1 Predicts Poor Prognosis in Patients with Nasopharyngeal Carcinoma. Onco Targets Ther (2020) 13:1757–65. 10.2147/OTT.S242517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ha H, Nam AR, Bang JH, Park J-E, Kim T-Y, Lee K-H, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget (2016) 7(47):76604–12. 10.18632/oncotarget.12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tiako Meyo M, Jouinot A, Giroux-Leprieur E, Fabre E, Wislez M, Alifano M, et al. Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study. Cancers (2020) 12(2):473. 10.3390/cancers12020473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin J, Si J, Liu Y, Wang H, Ni R, Wang J. Elevated serum soluble programmed cell death ligand 1 concentration as a potential marker for poor prognosis in small cell lung cancer patients with chemotherapy. Respir Res (2018) 19(1):197. 10.1186/s12931-018-0885-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao J, Zhang P, Wang J, Xi Q, Zhao X, Ji M, et al. Plasma levels of soluble programmed death ligand-1 may be associated with overall survival in nonsmall cell lung cancer patients receiving thoracic radiotherapy. Med (Baltimore) (2017) 96(7):e6102. 10.1097/MD.0000000000006102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okuma Y, Wakui H, Utsumi H, Sagawa Y, Hosomi Y, Kuwano K, et al. Soluble Programmed Cell Death Ligand 1 as a Novel Biomarker for Nivolumab Therapy for Non-Small-cell Lung Cancer. Clin Lung Cancer (2018) 19(5):410–17.e411. 10.1016/j.cllc.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 17. Bai G, Xu Y, Wang M. Relationship of the level of soluble PD-L1 and its relevant proteins in peripheral blood with the prognosis of patients with NSCLC. Ann Oncol (2018) 29:ix143–4. 10.1093/annonc/mdy446.002 [DOI] [Google Scholar]
- 18. Lee HW, Cho KJ, Shin SY, Kim BK, Kim SU, Park JY, et al. PD-1 and PD-L1 levels do not predict prognosis in patients with hepatocellular carcinoma. Liver Cancer (2018) 7Supplement 1 (121). [Google Scholar]
- 19. Ma X, Yang W, Wang H, Wang B, Shen M, Zhou Y, et al. Soluble programmed death-ligand 1 indicate poor prognosis in hepatocellular carcinoma patients undergoing transcatheter arterial chemoembolization. Annals Oncology (2019) 30Supplement 5 (v283–v284). [Google Scholar]
- 20. Chang B, Huang T, Wei H, Shen L, Zhu D, He W, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother (2019) 68(3):353–63. 10.1007/s00262-018-2271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finkelmeier F, Canli Ö, Tal A, Pleli T, Trojan J, Schmidt M, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer (2016) 59:152–9. 10.1016/j.ejca.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 22. Aghajani MJ, Roberts TL, Yang T, McCafferty CE, Caixeiro NJ, DeSouza P, et al. Elevated levels of soluble PD-L1 are associated with reduced recurrence in papillary thyroid cancer. Endocr Connect (2019) 8(7):1040–51. 10.1530/EC-19-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chiarucci C, Cannito S, Daffinà MG, Amato G, Giacobini G, Cutaia O, et al. Circulating Levels of PD-L1 in Mesothelioma Patients from the NIBIT-MESO-1 Study: Correlation with Survival. Cancers (Basel) (2020) 12(2):361. 10.3390/cancers12020361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Omura Y, Toiyama Y, Okugawa Y, Yin C, Shigemori T, Kusunoki K, et al. Prognostic impacts of tumoral expression and serum levels of PD-L1 and CTLA-4 in colorectal cancer patients. Cancer Immunol Immunother (2020) 69:2533–46. 10.1007/s00262-020-02645-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tominaga T, Akiyoshi T, Yamamoto N, Taguchi S, Mori S, Nagasaki T. Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS One (2019) 14(2):e0212978. 10.1371/journal.pone.0212978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang H, Wang L, Liu W-J, Xia Z-J, Huang H-Q, Jiang W-Q, et al. High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget (2016) 7(22):33035–45. 10.18632/oncotarget.8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother (2017) 66(7):877–90. 10.1007/s00262-017-1987-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen H, Ji Y, Zhou D, Zhang Y, Wang W, Sun J, et al. Soluble programmed death-ligand 1 are highly expressed in peripheral T-cell lymphoma: a biomarker for prognosis. Hematology (2019) 24(1):392–8. 10.1080/16078454.2019.1590965 [DOI] [PubMed] [Google Scholar]
- 29. Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia (2014) 28(12):2367–75. 10.1038/leu.2014.137 [DOI] [PubMed] [Google Scholar]
- 30. Rossille D, Azzaoui I, Feldman AL, Maurer MJ, Labouré G, Parrens C, et al. Soluble programmed death-ligand 1 as a prognostic biomarker for overall survival in patients with diffuse large B-cell lymphoma: a replication study and combined analysis of 508 patients. Leukemia (2017) 31(4):988–91. 10.1038/leu.2016.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo X, Wang J, Jin J, Chen H, Zhen Z, Jiang W, et al. High Serum Level of Soluble Programmed Death Ligand 1 is Associated With a Poor Prognosis in Hodgkin Lymphoma. Transl Oncol (2018) 11(3):779–85. 10.1016/j.tranon.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buderath P, Schwich E, Jensen C, Horn PA, Kimmig R, Kasimir-Bauer S, et al. Soluble Programmed Death Receptor Ligands sPD-L1 and sPD-L2 as Liquid Biopsy Markers for Prognosis and Platinum Response in Epithelial Ovarian Cancer. Front Oncol (2019) 9:1015. 10.3389/fonc.2019.01015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Asanuma K, Nakamura T, Hayashi A, Okamoto T, Iino T, Asanuma Y, et al. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci Rep (2020) 10(1):9077. 10.1038/s41598-020-65895-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng JCH, Hsu FM, Lee JM, Huang PM, Guo JC, Hsu CH. A Prospective Study on Serum PD-L1, TGF-β1, and VEGF-A as Immune-integrated Biomarkers for Locally Advanced Esophageal Squamous Cell Carcinoma. Int J Radiat Oncol Biology Physics (2019) 105(1):S132. 10.1016/j.ijrobp.2019.06.118 [DOI] [Google Scholar]
- 35. Shigemori T, Toiyama Y, Okugawa Y, Yamamoto A, Yin C, Narumi A, et al. Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD-L1 Expression. Ann Surg Oncol (2019) 26(3):876–83. 10.1245/s10434-018-07112-x [DOI] [PubMed] [Google Scholar]
- 36. Bian B, Fanale D, Dusetti N, Roque J, Pastor S, Chretien A-S, et al. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology (2019) 8(4):e1561120. 10.1080/2162402X.2018.1561120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park H, Bang J-H, Nam A-R, Park JE, Jin MH, Bang Y-J, et al. Prognostic implications of soluble programmed death-ligand 1 and its dynamics during chemotherapy in unresectable pancreatic cancer. Sci Rep (2019) 9(1):11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials (2015) 45(Pt A):139–45. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han SH. PD-1 and PD-L1 levels do not predict prognosis in patients with hepatocellular carcinoma. Liver Cancer (2018) 7(Supplement 1):121. [Google Scholar]
- 40. Hu B, Gong Z, Zhang X, Pan B, Zhou J, Fan J, et al. Soluble programmed death-ligand 1 indicate poor prognosis in hepatocellular carcinoma patients undergoing transcatheter arterial chemoembolization. Ann Oncol (2019) 30(Supplement 5):v283–4. 10.1093/annonc/mdz247.065 [DOI] [Google Scholar]
- 41. Chu HH, Kim JH, Kim PN, Kim SY, Lim Y-S, Park SH, et al. Surgical resection versus radiofrequency ablation very early-stage HCC (≤2 cm Single HCC): A propensity score analysis. Liver Int (2019) 39(12):2397–407. 10.1111/liv.14258 [DOI] [PubMed] [Google Scholar]
- 42. Stovgaard ES, Dyhl-Polk A, Roslind A, Balslev E, Nielsen D. PD-L1 expression in breast cancer: expression in subtypes and prognostic significance: a systematic review. Breast Cancer Res Treat (2019) 174(3):571–84. 10.1007/s10549-019-05130-1 [DOI] [PubMed] [Google Scholar]
- 43. Tsao M-S, Le Teuff G, Shepherd FA, Landais C, Hainaut P, Filipits M, et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol (2017) 28(4):882–9. 10.1093/annonc/mdx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cordonnier M, Nardin C, Chanteloup G, Derangere G, Algros M-P, Arnould L, et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracellular Vesicles (2020) 9(1):1710899. 10.1080/20013078.2019.1710899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Theodoraki M-N, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin Cancer Res (2018) 24(4):896–905. 10.1158/1078-0432.CCR-17-2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J ImmunoTherapy Cancer (2018) 6(1):132. 10.1186/s40425-018-0449-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Omura Y, Toiyama Y, Okugawa Y, Yin C, Shigemori T, Kusunoki K, et al. Prognostic impacts of tumoral expression and serum levels of PD-L1 and CTLA-4 in colorectal cancer patients. Cancer Immunol Immunother CII (2020) 69(12):2533–46. 10.1007/s00262-020-02645-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ji S, Chen H, Yang K, Zhang G, Mao B, Hu Y, et al. Peripheral cytokine levels as predictive biomarkers of benefit from immune checkpoint inhibitors in cancer therapy. Biomed Pharmacother = Biomed Pharmacother (2020) 129:110457. 10.2139/ssrn.3578741 [DOI] [PubMed] [Google Scholar]
- 49. Fei Y, Yu J, Li Y, Li L, Zhou S, Zhang T, et al. Plasma soluble PD-L1 and STAT3 predict the prognosis in diffuse large B cell lymphoma patients. J Cancer (2020) 11(23):7001–8. 10.7150/jca.47816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ding Y, Sun C, Li J, Hu L, Li M, Lui J, et al. The Prognostic Significance of Soluble Programmed Death Ligand 1 Expression in Cancers: A Systematic Review and Meta-analysis. Scand J Immunol (2017) 86(5):361–7. 10.1111/sji.12596 [DOI] [PubMed] [Google Scholar]
- 51. Wei W, Xu B, Wang Y, Wu C, Jiang J, Wu C. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors: A meta-analysis. Med (Baltimore) (2018) 97(3):e9617. 10.1097/MD.0000000000009617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zou Y, Zou X, Zheng S, Tang H, Zhang L, Liu P, et al. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta-analysis. Ther Adv Med Oncol (2020) 121758835920940928. 10.1177/1758835920940928 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.