Abstract
Purpose:
We evaluated 18F-FDOPA positron emission tomography (PET) and magnetic resonance imaging (MRI) characteristics in association with the molecular status and overall survival (OS) in a large number of low-grade gliomas (LGGs).
Methods:
Eighty-six patients, who underwent 18F-FDOPA PET and MRI, and were diagnosed with new or recurrent LGGs were retrospectively evaluated with respect to their isocitrate dehydrogenase (IDH) and 1p19q status (10 IDH wild-type, 57 mutant, 19 unknown; 1p19q status in IDH mutant: 20 non-codeleted, 37 codeleted). After segmentation of the hyperintense area on fluid-attenuated inversion recovery image (FLAIRROI), the following were calculated: normalized maximum standardized uptake value (nSUVmax) of 18F-FDOPA relative to the striatum, 18F-FDOPA hypermetabolic volume (tumor-to-striatum ratios > 1), FLAIRROI volume, relative cerebral blood volume, and apparent diffusion coefficient within FLAIRROI. Receiver-operator characteristic and Cox regression analyses were performed.
Results:
PET and MRI metrics combined with age predicted the IDH mutation and 1p19q codeletion statuses with sensitivities of 73% and 76%, specificities of 100% and 94%, respectively. Significant correlations were found between OS and the IDH mutation status (hazard ratio [HR] = 4.939), nSUVmax (HR = 2.827), 18F-FDOPA hypermetabolic volume (HR = 1.048), and FLAIRROI volume (HR = 1.006). The nSUVmax (HR = 151.6) for newly diagnosed LGGs, and the 18F-FDOPA hypermetabolic volume (HR = 1.038) for recurrent LGGs demonstrated significant association with OS.
Conclusion:
Combining 18F-FDOPA PET and MRI with age proved useful for predicting the molecular status in patients with LGGs, while the nSUVmax and 18F-FDOPA hypermetabolic volume may be useful for prognostication.
Keywords: 18F-FDOPA PET, low-grade glioma, molecular biomarker, overall survival
Introduction
For clinical evaluation of low-grade gliomas (LGGs), magnetic resonance imaging (MRI) is the primary imaging modality owing to its high spatial resolution, high contrast within soft tissues, and no ionizing radiation. Numerous studies have reported the impact of MRI characteristics on tumor classification and prognosis; however, due to lack of contrast enhancement, LGGs cannot be fully evaluated by MRI alone. Meanwhile, radiolabeled amino acids positron emission tomography (PET), such as 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (18F-FDOPA), O-(2-[18F] fluoroethyl)-L-tyrosine (18F-FET), and [11C] methyl-L-methionine (11C-MET), is often employed in neuro-oncological practice to identify metabolically active tissue.1 18F-FDOPA and 18F-FET PET have improved distribution and efficiency owing to the relatively long half-lives of fluorinated tracers compared to carbon tracers.2 They have been used for the evaluation of LGGs with regard to tumor classification,3 preoperative biopsy guidance,4 and prognostication.5 Because amino acids PET provides metabolic information to complement MRI, a combination of the techniques may yield more accurate observations for differentiating subtypes of LGGs and predicting prognosis than either technique alone.
Villani et al.5 reported that hyper 18F-FDOPA uptake was an independent predictor of progression-free survival; however, they did not evaluate the overall survival (OS). Patel et al.6 demonstrated that the combination of 18F-FDOPA and MRI characteristics is capable of predicting the degree of malignancy and OS among 45 gliomas, including 16 LGGs. The ability of 18F-FDOPA and MRI to predict OS in a large cohort of patients with LGGs has yet to be evaluated. In 2016, the World Health Organization (WHO) glioma classification was modified to include molecular subtypes including isocitrate dehydrogenase (IDH) gene mutations or chromosomal 1p19q codeletion.7 These molecular biomarkers have become essential for brain tumor classification, treatment decisions, and predicting prognosis for LGGs; however, the features of LGGs with different molecular subtypes are still debated.3, 8 Hence, there is a demand for non-invasive imaging biomarkers that can identify the molecular status and predict OS for LGGs.
The purpose of the current study was to evaluate characteristics of LGGs using 18F-FDOPA PET and multi-parametric MRI in a large patient cohort and determine associations between imaging metrics and their molecular status or OS.
Materials and methods
Patient Selection
Eighty-six patients with histologically confirmed LGGs with WHO grade II, who underwent 18F-FDOPA PET and MRI scans between 2007 and 2019, were retrospectively included. Selected MRI scans were performed within two months of the corresponding PET scans. MR perfusion imaging for 55 subjects and diffusion-weighted imaging (DWI) for 83 subjects, as well as conventional sequences, were obtained. All patients were diagnosed with new or recurrent LGGs according to the WHO 2007 or 2016 classification. When available, IDH1 mutational status, 1p19q codeletion status, and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status were obtained.9 For newly diagnosed LGGs, no patients underwent stereotactic biopsy prior to 18F-FDOPA PET or MRI, and the median date between PET scan and surgery/biopsy was 18 days (range 1–505). OS was measured from the time of the PET scan until death or the censored date (median term 1272 days). This Health Insurance Portability and Accountability Act–compliant study has been approved by the institutional review board, and all subjects signed an informed consent form. The patient cohort was partly overlapped with a previous study.10
18F-FDOPA PET Image Acquisition
A 18F-FDOPA PET scan was performed with a full-ring PET/CT scanner (ECAT-HR; CTI/MIMVista) on the subjects, after they fasted for more than four hours. Following previously established procedures, 18F-FDOPA was synthesized and injected intravenously.11 CT images were acquired prior to the PET scan for attenuation correction. Three-dimensional 18F-FDOPA emission data were acquired for a total of 30 minutes and integrated between 10 and 30 minutes following the injection to obtain 20-minute static 18F-FDOPA images. PET images were reconstructed using an ordered-subset expectation maximization iterative reconstruction algorithm, consisting of six iterations with eight subsets.12 Finally, a Gaussian filter with a full width at half maximum of 4 mm was applied. The resulting voxel size was 1.34 × 1.34 × 3 mm for the 18F-FDOPA PET images. Standardized uptake value (SUV) maps of 18F-FDOPA were calculated based on the radioactive activity divided by the decay-corrected injected dose per body mass.13 The resulting SUV maps were subsequently normalized relative to the median value of the striatum (nSUV).14
MRI Acquisition
Anatomical MRI at least consisted of standard T1-weighted pre- and post-contrast images (2D axial turbo spin echo with 3-mm slice thickness and no interslice gap or 3D inversion prepared gradient echo images with 1–1.5 mm isotropic voxel size) and T2-weighted fluid-attenuated inversion recovery (FLAIR) images acquired at 3-mm slice thickness with no interslice gap on a 1.5-T or 3-T clinical MRI scanner.
For the dynamic susceptibility contrast perfusion MRI, a total dose of 0.1 mmol/kg of Gd-DTPA or Gd-BTDO3A (Magnevist or Gadavist; Bayer HealthCare Pharmaceuticals, Wayne, NJ) was administered. Relative cerebral blood volume (rCBV) maps were calculated using previously established procedures.15 Normalized rCBV maps were then computed by dividing the rCBV map by the median rCBV value of regions of interest (ROIs), placed on the contralateral normal-appearing white matter.
DWI was performed by a single-shot echo-planar imaging sequence in the axial plane with b = 1000 s/mm2, slice thickness = 3 mm, and no interslice gap. Apparent diffusion coefficient (ADC) maps were calculated from the acquired images with b = 1000 s/mm2 and b = 0 s/mm2. In the case where DWI was not obtained, diffusion tensor imaging was used, acquired from 12–64 equidistant diffusion-sensitizing directions with b = 1000 s/mm2 with a single b = 0 s/mm2 image with slice thickness = 2–3 mm, and no interslice gap. Mean diffusivity maps were employed as estimates of the ADC using FSL software (dtifit; FMRIB, Oxford, UK; http://www.fmrib.ox.ac.uk/fsl/).
Postprocessing Analysis
All PET and MR images were registered to the corresponding post-contrast T1-weighted images using a six-degrees of freedom rigid transformation and a mutual information cost function using FSL (flirt). A single ROI as a FLAIRROI was semi-automatically segmented based on the regions of hyperintensity on the T2-weighted FLAIR images by a board-certificated neuroradiologist (H.T. with 13 years of clinical experience) using Analysis of Functional NeuroImages software (NIMH Scientific and Statistical Computing Core; Bethesda, MD; https://afni.nimh.nih.gov ).16 The maximum nSUV (nSUVmax) was quantified within the FLAIRROI. 18F-FDOPA hypermetabolic volume, including the voxel with nSUV > 1 within FLAIRROI, and FLAIRROI volume were calculated in milliliters. The median rCBV was calculated along with ADC low (ADClow), which is defined as the lower mean of a double Gaussian mixed model fitted to the histogram of ADC values within the FLAIRROI.17
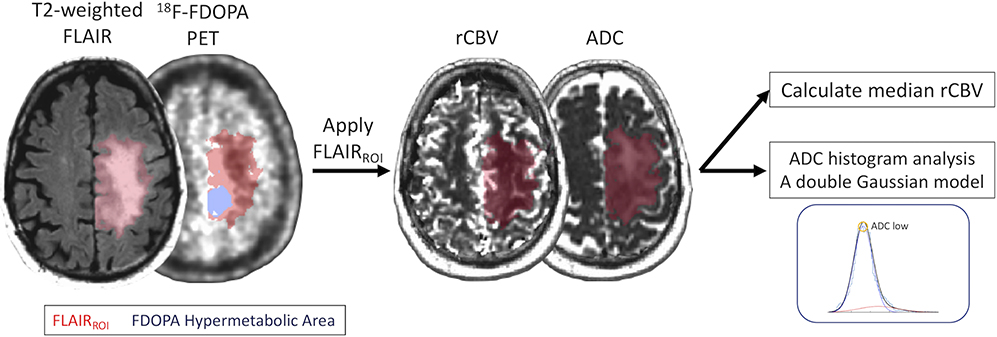
Figure 1 illustrates an example of segmentations of FLAIRROI and the 18F-FDOPA hypermetabolic region in a newly diagnosed 57-year-old male low-grade diffuse glioma patient.
Figure 1.
Postprocessing and segmentation example. A 57-year-old man with newly diagnosed astrocytic diffuse glioma (WHO grade II, IDH1 mutant, 1p19q non-codeleted, and MGMT methylated status). ROIs of the FLAIR hyperintense region (FLAIRROI, red area) and 18F-FDOPA hypermetabolic area (nSUV > 1, blue area) within FLAIRROI are shown. nSUVmax and volumes for each ROI are calculated. FLAIRROI is copied and pasted on rCBV and ADC maps, and median rCBV and ADClow are calculated.
Statistical Analyses
The Shapiro-Wilk test was used to test for normality of the data. The student’s t-test for normally-distributed data and Mann-Whitney U test for non-normally-distributed data were performed.
Within the FLAIRROI, the evaluation included pairwise Spearman’s correlation between the nSUVmax, 18F-FDOPA hypermetabolic volume, FLAIRROI volume, median rCBV, and ADClow, and the comparison of these values with regard to different molecular status (IDH mutation, 1p19q codeletion, and MGMT methylation status) and patient status (newly diagnosed and recurrent). The Kaplan-Meier curves and log-rank test were employed to compare OS for different molecular status. A multiple logistic regression model, integrating known clinical information such as age and MR-PET metrics, was used to predict the molecular status. Receiver operating characteristic (ROC) curves were used to determine whether a combination of clinical and MR-PET imaging information can discriminate between different molecular statuses. Area under the curve (AUC), along with the sensitivity and specificity of differentiation, was evaluated as a measure of model performance. Leave-one-out cross-validation was used to evaluate the accuracy of the multivariate logistic regression model.
Cox univariate regression analyses were conducted to investigate the association between OS and predictor variables including clinical information (sex, age, and molecular status) and imaging metrics (nSUVmax, 18F-FDOPA hypermetabolic volume, FLAIRROI volume, median rCBV, and ADClow). For the Cox multivariate regression, the hazard of the nSUVmax, 18F-FDOPA hypermetabolic volume, and FLAIRROI volume controlling for age or molecular status (IDH or 1p19q) were evaluated separately because these three imaging variables were available from all subjects.
Additionally, subjects were stratified by molecular status and patient status, and the imaging metrics and their association with OS were evaluated for each subgroup analysis.
Statistical analysis was performed using R software (version 3.5.2; http://www.r-project.org/) and GraphPad Prism (version 8.3; GraphPad Software, La Jolla, CA). Statistical significance was defined as P < 0.05, and no correction for multiple comparisons was performed.
Results
Table 1 summarizes the patient demographics and molecular information, and Supplemental Table 1 describes this in more detail. The current study included 86 LGG patients (34 females) with a mean age of 43 years. Twenty-nine patients were newly diagnosed, while 57 patients had recurrent status. Ten gliomas were IDH wild-type (IDHwt), 57 were IDH mutant (IDHm), and 19 did not have confirmed IDH status. Among the IDHm gliomas, 20 were 1p19q non-codeleted (IDHm-non-codel), and 37 were 1p19q codeleted (IDHm-codel).
Table 1.
Patient demographics and molecular information
| No. of patients | 86 | |
| Female | 34 (39.5%) | |
| Age ± standard deviation (year) | 43.8 ± 12.6 | |
| IDH mutation and 1p19q codeletion status | IDH wild-type | 10 (11.6%) |
| IDH mutant 1p19q non-codeleted | 20 (23.2%) | |
| IDH mutant 1p19q codeleted | 37 (43.0%) | |
| Unknown | 19 (22.1%) | |
| MGMT-promoter methylation status | Unmethylated | 17 (19.8%) |
| Methylated | 25 (29.1%) | |
| Unknown | 44 (51.2%) | |
| Patient status | Newly diagnosed | 29 (33.7%) |
| Recurrent | 57 (66.3%) |
IDH = isocitrate dehydrogenase; MGMT = O6-methylguanine-DNA methyltransferase
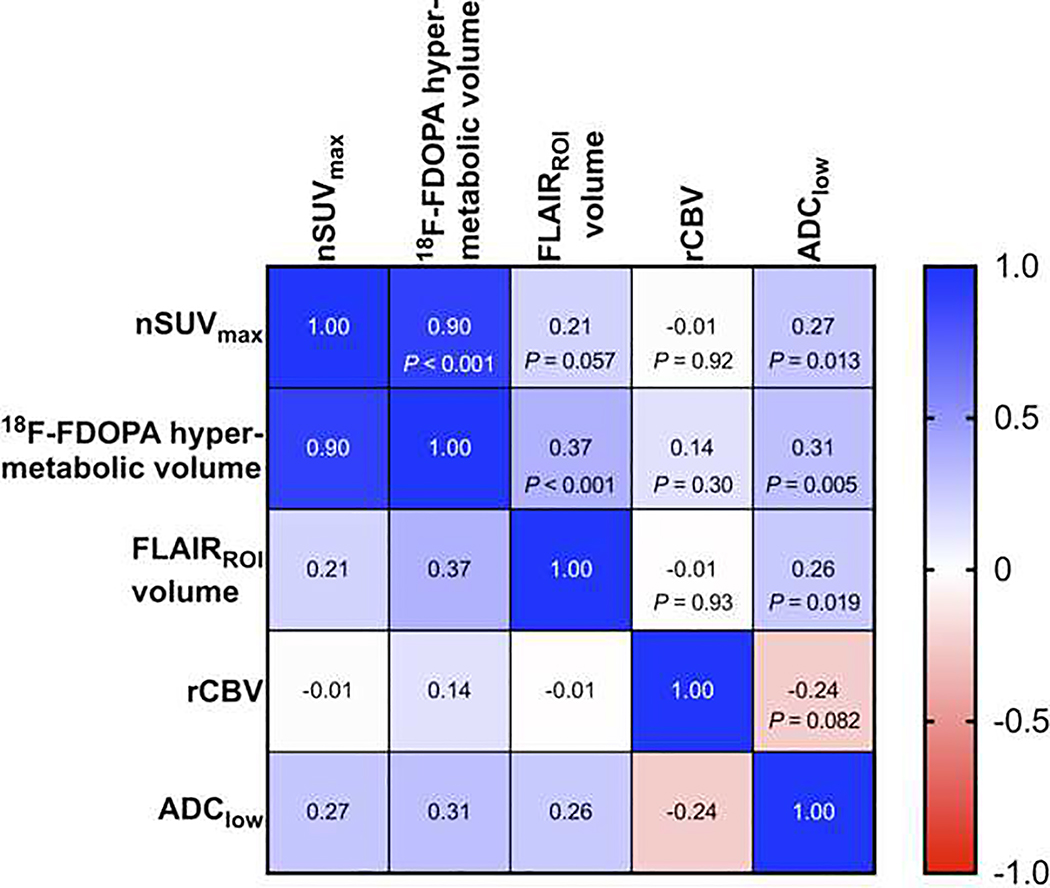
The pairwise Spearman’s correlation analysis (Figure 2) between the nSUVmax, 18F-FDOPA hypermetabolic volume, FLAIRROI volume, median rCBV, and ADClow demonstrated a strong correlation between the nSUVmax and 18F-FDOPA hypermetabolic volume (rs = 0.90), whereas the other pairs had weak or no correlations (−0.24 < rs < 0.37).
Figure 2.
Pairwise Spearman’s correlation matrix between nSUVmax, 18F-FDOPA hypermetabolic volume, FLAIRROI volume, median rCBV, and ADClow.
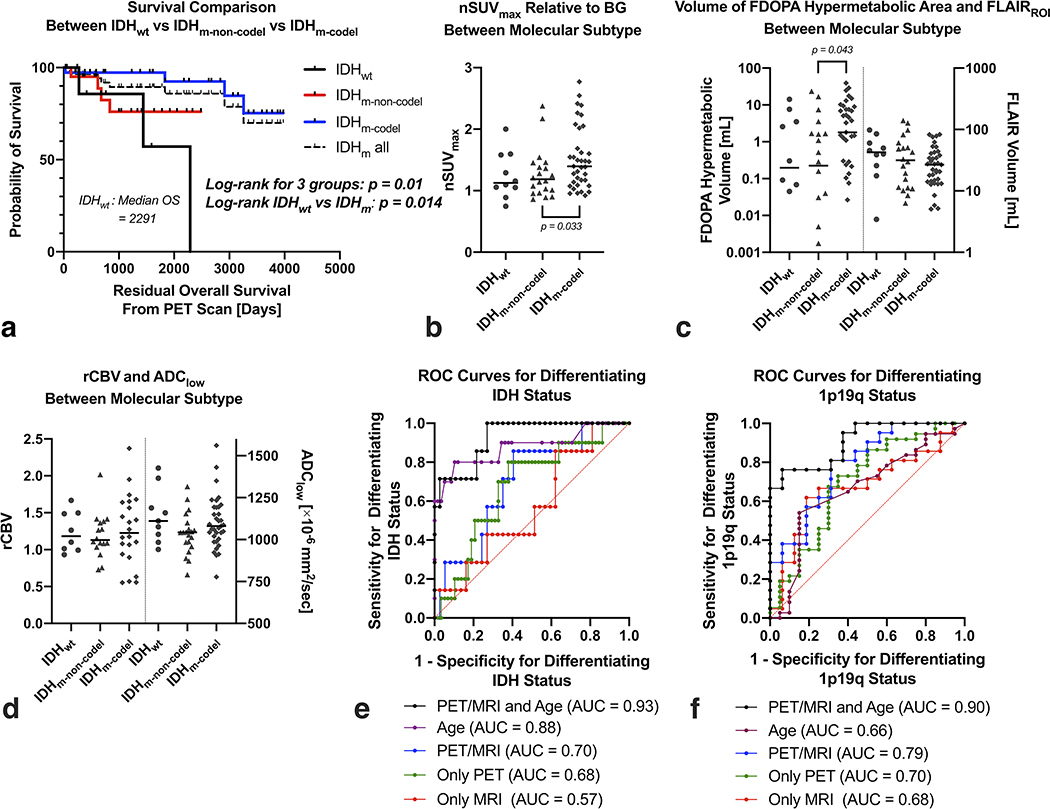
In the evaluation of patients stratified by the molecular status (Figure 3), the Kaplan-Meier curves and log-rank tests showed significant differences in the OS (P = 0.01). The IDHm-codel subtype had the longest OS, followed by the IDHm-non-codel subtype, and the IDHwt subtype had the worst OS. When comparing the imaging metrics, the nSUVmax (P = 0.033) and 18F-FDOPA hypermetabolic volume (P = 0.043) were significantly higher in IDHm-codel than IDHm-non-codel. Other analyses did not yield any significant differences between the different molecular subtypes (all Ps > 0.06). The evaluation between MGMT unmethylated and methylated subtypes was shown in Supplemental Figure 1.
Figure 3.
a) Kaplan-Meier plots showing significant differences in OS between IDHwt, IDHm-non-codel, and IDHm-codel gliomas, or between IDHwt and IDHm. b) The nSUVmax and c) volume of 18F-FDOPA hypermetabolic area and FLAIRROI, and d) median rCBV and ADClow between different molecular statuses. The nSUVmax and 18F-FDOPA hypermetabolic volume are significantly higher in IDHm-codel than IDHm-non-codel. When comparing between IDHwt and IDHm (including IDHm-non-codel and IDHm-codel), no imaging metrics show significant differences. ROC curves for differentiating e) IDH mutation or f) 1p19q codeletion status using patient age and imaging information, only age, both PET and MRI, only PET, or only MRI.
Subsequently, we tested whether the combination of the MR and PET imaging metrics and patient age can be used to predict the molecular status. We created a new metric from a combination of the imaging factors and patient age by incorporating a multiple logistic regression model as follows:
This metric enabled to differentiate the IDH mutation status (AUC, 0.93, sensitivity, 73%, specificity, 100%), with only patient age as a significant factor (odds ratio = 1.34, P = 0.04). Leave-one-out classification accuracy to differentiate IDH mutation was 86% (sensitivity, 97%, specificity, 71%). Similarly, for prediction of the 1p19q codeletion status, a multiple logistic regression analysis was performed as follows:
This metric enabled to differentiate the 1p19q codeletion status (AUC, 0.91, sensitivity, 76%, specificity, 94%), with patient age (odds ratio = 1.27, P = 0.011) and FLAIRROI volume (odds ratio = 0.93, P = 0.019) being significant factors. Leave-one-out classification accuracy to differentiate 1p19q codeletion was 65% (sensitivity, 63%, specificity, 67%). ROC curves to predict IDH or 1p19q status were also evaluated using only the patient age, only PET metrics (i.e., nSUVmax and 18F-FDOPA hypermetabolic volume), only MRI metrics (i.e., FLAIRROI volume, median rCBV, and ADClow), or combined PET and MRI metrics. The AUC of IDH or 1p19q status was higher using both PET and MRI parameters than when using either of the parameters individually; however, the AUC incorporating patient age and MR-PET parameters yielded the highest value.
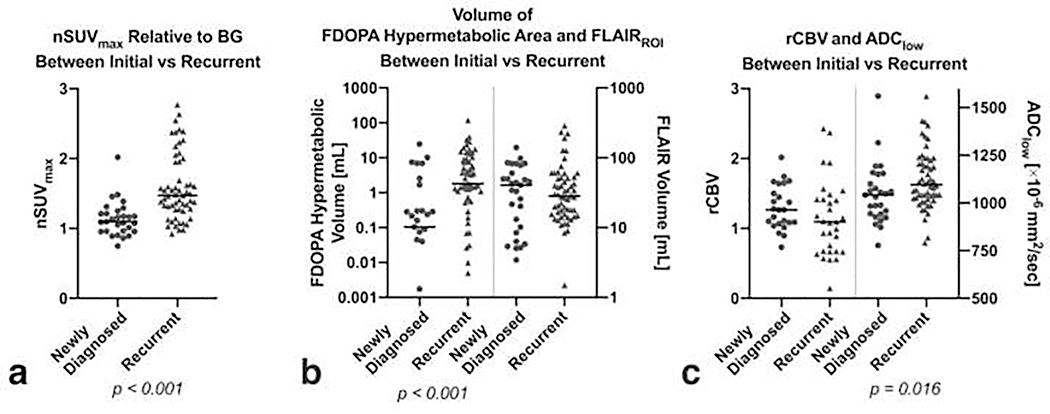
When stratifying the patients into newly diagnosed and recurrent LGGs (Figure 4), the nSUVmax, 18F-FDOPA hypermetabolic volume, and ADClow were significantly higher in the recurrent LGGs than newly diagnosed LGGs (P < 0.001, < 0.001, and 0.016, respectively). Further subgroup analyses, stratified by molecular status (IDHwt, IDHm-non-codel, and IDHm-codel) and patient status (newly diagnosed and recurrent), are shown in Supplemental Figure 2, and exhibited similar trends with higher nSUVmax and 18F-FDOPA hypermetabolic volume in IDHm-codel than IDHm-non-codel in both newly diagnosed and recurrent groups, although they were not significant.
Figure 4.
a) The nSUVmax, b) volume of 18F-FDOPA hypermetabolic area or FLAIRROI, and c) median rCBV and ADClow between newly diagnosed and recurrent LGGs.
The Cox univariate analysis (Table 2) showed a significant increase in hazard associated with the IDH status (hazard ratio [HR] = 4.939, 95% confidence interval [CI] = [1.203–20.28], P = 0.026), nSUVmax (HR = 2.827, CI = [1.202–6.649], P = 0.017), 18F-FDOPA hypermetabolic volume (HR = 1.048, CI = [1.019–1.078], P = 0.001), and FLAIRROI volume (HR = 1.006, CI = [1.000–1.013], P = 0.046). The Cox multivariate regression analysis controlling for age showed a significant increase in the hazard associated with the nSUVmax (HR = 3.208, CI = [1.272–8.090], P = 0.013) and 18F-FDOPA hypermetabolic volume (HR = 1.045, CI = [1.017–1.073], P = 0.001), but not with FLAIR volume (HR = 1.006, CI = [0.999–1.013], P = 0.08). When controlling for IDH status or 1p19q status (for IDHm LGGs), the Cox multivariate regression analysis showed a significant increase in the hazard of OS associated with the nSUVmax (controlling for IDH status: HR = 4.101, CI = [1.244–13.53], P = 0.020; controlling for 1p19q status: HR = 6.169, CI = [1.537–24.76], P = 0.010) and 18F-FDOPA hypermetabolic volume (controlling for IDH status: HR = 1.063, CI = [1.005–1.125], P = 0.032; controlling for 1p19q status: HR = 1.097, CI = [1.021–1.180], P = 0.011), but not with FLAIR volume (controlling for IDH status: HR = 1.002, CI = [0.981–1.024], P = 0.83; controlling for 1p19q: HR = 1.003, CI = [0.981–1.025], P = 0.80). Subgroup analysis stratified by different molecular status or patient status demonstrated that the nSUVmax represented an independent predictor of OS for IDHm-non-codel (HR = 6.100, CI = [1.155–32.22], P = 0.033) and newly diagnosed LGGs (HR = 151.6, CI = [1.289–17830], P = 0.038), while the increase in the 18F-FDOPA hypermetabolic volumes (HR = 1.038, CI = [1.009–1.067], P = 0.009) and FLAIRROI volumes (HR = 1.006, CI = [1.000–1.013], P = 0.049) were independent predictors for recurrent LGGs (Supplemental Table 2).
Table 2.
Cox univariate regression
| Cox univariate regression |
|||
|---|---|---|---|
| Hazard ratio | 95% confidence interval | P value | |
| Sex | 1.579 | 0.551–4.517 | 0.39 |
| Age (year) | 1.03 | 0.996–1.064 | 0.083 |
| IDH status (wild-type vs. mutant) | 4.939 | 1.203–20.28 | 0.026* |
| 1p19q status for IDH mutant (non-codel vs. codel) | 0.234 | 0.042–1.301 | 0.097 |
| MGMT Status (unmethylated vs. methylated) | 0.56 | 0.078–4.001 | 0.56 |
| Patient Status (newly diagnosed vs. recurrent) | 2.231 | 0.640–7.766 | 0.2 |
| nSUVmax | 2.827 | 1.202–6.649 | 0.017* |
| FDOPA hypermetabolic volume (mL) | 1.048 | 1.019–1.078 | 0.001* |
| FLAIRROI volume (mL) | 1.006 | 1.000–1.013 | 0.046* |
| rCBV | 1.05 | 0.214–5.151 | 0.95 |
| ADClow (×10−6 mm2/s) | 1.002 | 0.999–1.005 | 0.17 |
IDH = isocitrate dehydrogenase; MGMT = O6-methylguanine-DNA methyltransferase; rCBV = relative cerebral blood volume; ADC = apparent diffusion coefficient
means statistically significant
Discussion
In the current study, 18F-FDOPA PET and MRI characteristics were evaluated with regard to their association with molecular status and OS in LGG patients. Our investigation revealed that combining PET and MRI information with patient age could predict IDH and 1p19q status more accurately than using PET or MRI information alone. We also confirmed that nSUVmax and 18F-FDOPA hypermetabolic volume were higher in IDHm-codel than IDHm-non-codel LGGs. The Cox regression analysis revealed significant associations of OS with the IDH status, nSUVmax, 18F-FDOPA hypermetabolic volume, and FLAIRROI volume. Because not all recurrent glioma patients in all institutions have a known molecular status at the time point of the previous surgery or biopsy, the results of predicting molecular status and prognosis are useful not only for newly diagnosed LGGs, but also recurrent LGGs.
The molecular status of LGGs is crucial for patient diagnosis and predicting prognosis. As shown previously and in this study, the IDH mutation status was significantly associated with OS with shorter survival for IDHwt LGGs due to the biological similarities to glioblastomas.8, 18 In the evaluation of imaging metrics of LGGs, although some studies have evaluated the association of amino acid tracer uptake and molecular status, the imaging features in different molecular status were not confirmed. One study stratified gliomas into IDHwt and IDHm groups, which included 1p19q non-codeleted and codeleted gliomas, and showed higher tracer uptake in IDHm than IDHwt.3 Other studies stratified gliomas into 1p19q codeleted and non-codeleted groups, which included both IDHm-non-codel and IDHwt gliomas, and showed no significant differences between 1p19q codeleted and non-codeleted gliomas.19, 20 The diverse patient cohort in each study may confuse the issue and mask the associations of the amino acid tracer uptake with molecular status. In contrast, the current study stratified gliomas into three groups (IDHwt, IDHm-non-codel, and IDHm-codel) and confirmed the features of amino acid tracer uptake, including higher nSUVmax and 18F-FDOPA hypermetabolic volume in IDHm-codel than IDHm-non-codel LGGs. This was consistent with previous literatures indicating a higher tracer uptake in oligodendrogliomas compared with astrocytomas, reflecting higher cell density, endothelial hyperplasia, microvascular proliferation, and higher vascular bed in oligodendroglial compared to astrocytic components.8, 10, 21, 22 When comparing between IDHwt and IDHm, the ratio of IDHm-codel LGGs in the IDHm group may affect the differences of 18F-FDOPA uptake because the current study revealed IDHm-codel LGGs to show high tracer uptake.
In the current study, the combination of PET and MRI information with patient age successfully differentiated the IDH mutation and 1p19q codeletion status with AUCs higher than 0.90, although the performance, particularly for the IDH mutation status, was largely dependent on the patient age. A previous study revealed that the IDHm-non-codel group tended to be found in the youngest patients, followed by IDHm-codel and IDHwt;23 hence, the combination of PET and MRI, along with patient age may be helpful for predicting the molecular status.
High 18F-FDOPA uptakes are known to reflect high metabolic activity and predict worse survival outcomes in both newly diagnosed and recurrent gliomas.6, 24 For glioblastomas, hypermetabolic volume was an important factor.25 For newly diagnosed LGGs, the uptake of amino acid tracers was reported to be associated with progression-free survival5, 8 and predicted disease progression after 1-year follow-up.26 No studies have revealed the association of 18F-FDOPA uptake and OS in grade II gliomas alone, partly due to a small cohort population. The current large cohort study revealed that increased nSUVmax of 18F-FDOPA was associated with a worse prognosis in grade II gliomas; furthermore, the 18F-FDOPA hypermetabolic volume was also associated with a worse prognosis. These results were consistent with the strong correlation between the nSUVmax and 18F-FDOPA hypermetabolic volume, detected in this study. Previous studies have indicated that volume of the contrast-enhancing regions was predictive of the OS;27 however, due to the lack of contrast enhancement, such regions cannot be evaluated in most grade II gliomas. Meanwhile, because hypermetabolic volume can be calculated regardless of contrast enhancement, it could be a useful biomarker for gliomas, especially for the non-contrast-enhancing LGGs.
When comparing the newly diagnosed and recurrent LGGs, both nSUVmax and 18F-FDOPA hypermetabolic volume were significantly higher in recurrent LGGs. The treatment-related changes may have affected amino acid tracer uptake, because 18F-FDOPA uptake in normal-appearing brain structures might be altered by temozolomide treatment.28 The blood-brain barrier breakdown, due to cancer progression, may contribute to the extent of amino acid transport in recurrent gliomas, suggesting that the SUV may not directly reflect recurrent tumor activity.29 Malignant transformation at recurrence may also induce an increase in the SUV in some LGGs.24 Unfortunately, the histopathology of recurrent tumors was not available for all patients, and thus it was not analyzed in this study. Several studies evaluated the longitudinal change of amino acids tracer uptake, reporting that a higher rate of temporal change in the 18F-FDOPA uptake was associated with a higher risk of malignant transformation and poor survival in patients with LGGs.9 A study reported that 65% of primary gliomas with a negative 18F-FET uptake, which could not be delineated from the background brain tissue, turned PET-positive during follow-up scans, indicating that gliomas can change their 18F-FET uptake behavior throughout the course of the disease.30 Although 18F-FET PET-negative gliomas in general have a better prognosis than 18F-FET positive gliomas, 18F-FET PET-negative gliomas with photopenic defects were reported to have a higher risk of harboring a higher-grade glioma and an unfavorable outcome than gliomas with indifferent 18F-FET uptake to the background.31, 32 Meanwhile, this study exhibited a better prognosis in patients with lower 18F-FDOPA uptake, and no studies have reported such unfavorable outcomes for 18F-FDOPA hypo-uptake gliomas. The different disease courses may be caused by different amino acid tracers, in particular different metabolic processes of 18F-FDOPA and 18F-FET. Hence, comparison of the glioma pathology and amino acid uptakes with different tracers is desirable, in a longitudinal manner.
The retrospective nature of this study presents one of its limitations; specifically, the clinical information (Karnofsky Performance Status), molecular status, and rCBV/ADC maps were not obtained for all subjects, and the imaging protocols were not exactly matched. Although examination and treatment planning were discussed in weekly tumor boards at our institution, the patient cohort was potentially influenced by selection bias because FDOPA PET examination may have been performed more often for glioma patients who were suspected to have primary or recurrent gliomas but were difficult to be diagnosed based on conventional MRI alone. For three patients with newly diagnosed LGGs, the interval between PET and surgery/biopsy was longer than half a year; however, they did not receive additional treatments between the interval, and the WHO grades remained at grade II when the pathology was confirmed after surgery. Nonetheless, the possibility of temporal change in molecular status during the interval cannot be excluded. Including patients with various previous treatment statuses may have influenced the MRI and/or PET imaging features. As we could not obtain pathologies of all recurrent gliomas after the PET examination, the WHO grade may have been underestimated. Although this study used leave-one-out cross validation for evaluating the predictive performance of molecular status, another independent cohort is required to generalize our classification performance.
Conclusion
This was the largest population study to date evaluating LGGs using both 18F-FDOPA PET and MRI. A combination of PET, MRI, and patient age may be helpful for predicting the molecular status in patients with LGGs, and 18F-FDOPA PET metrics proved useful for estimating the OS.
Supplementary Material
Acknowledgments
Funding:
SNMMI (Tatekawa); ACS Research Scholar Grant (RSG-15-003-01-CCE: Ellingson); ABTA Research Collaborators Grant (ARC1700002: Ellingson); NBTS Research Grant (Ellingson, Cloughesy); NIH/NCI UCLA Brain Tumor SPORE(1P50CA211015-01A1: Ellingson, Lai, Cloughesy, Nghiemphu); NIH/NCI (1R21CA223757-01:Ellingson).
Conflict of interest:
Ellingson is an advisor for Hoffman La-Roche; Siemens; Nativis; Medicenna; MedQIA; Bristol Meyers Squibb; Imaging Endpoints; and Agios Pharmaceuticals. Ellingson is a Paid Consultant for Nativis; MedQIA; Siemens; Hoffman La-Roche; Imaging Endpoints; Medicenna; and Agios. Ellingson received grant funding from Siemens, Agios, and Janssen. Cloughesy is on the advisory board for Roche/Genentech, Amgen, Tocagen, NewGen, LPath, Proximagen, Celgene, Vascular Biogenics Ltd, Insys, Agios, Cortice Bioscience, Pfizer, Human Longevity, BMS, Merck, Notable Lab, and MedQIA.
References
- 1.Galldiks N, Lohmann P, Cicone F, et al. FET and FDOPA PET Imaging in Glioma. In: Pope W, ed. Glioma Imaging. 1st edn. Switzerland: Springer; 2019:211–222. [Google Scholar]
- 2.Huang C, McConathy J. Radiolabeled amino acids for oncologic imaging. J Nucl Med. 2013;54:1007–1010. [DOI] [PubMed] [Google Scholar]
- 3.Verger A, Metellus P, Sala Q, et al. IDH mutation is paradoxically associated with higher (18)F-FDOPA PET uptake in diffuse grade II and grade III gliomas. Eur J Nucl Med Mol Imaging. 2017;44:1306–1311. [DOI] [PubMed] [Google Scholar]
- 4.Kunz M, Thon N, Eigenbrod S, et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011;13:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villani V, Carapella CM, Chiaravalloti A, et al. The Role of PET [18F]FDOPA in Evaluating Low-grade Glioma. Anticancer research. 2015;35:5117–5122. [PubMed] [Google Scholar]
- 6.Patel CB, Fazzari E, Chakhoyan A, et al. (18)F-FDOPA PET and MRI characteristics correlate with degree of malignancy and predict survival in treatment-naive gliomas: a cross-sectional study. J Neurooncol. 2018;139:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. [DOI] [PubMed] [Google Scholar]
- 8.Kertels O, Kessler AF, Mihovilovic MI, et al. Prognostic Value of O-(2-[(18)F]Fluoroethyl)-L-Tyrosine PET/CT in Newly Diagnosed WHO 2016 Grade II and III Glioma. Mol Imaging Biol. 2019;21:1174–1181. [DOI] [PubMed] [Google Scholar]
- 9.Oughourlian TC, Yao J, Schlossman J, et al. Rate of change in maximum (18)F-FDOPA PET uptake and non-enhancing tumor volume predict malignant transformation and overall survival in low-grade gliomas. J Neurooncol. 2020;147:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatekawa H, Hagiwara A, Yao J, et al. Voxel-wise and patient-wise correlation of 18F-FDOPA PET, rCBV, and ADC in treatment-naïve diffuse gliomas with different molecular subtypes. J Nucl Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop A, Satyamurthy N, Bida G, et al. Proton irradiation of [18O]O2: production of [18F]F2 and [18F]F2 + [18F] OF2. Nucl Med Biol. 1996;23:189–199. [DOI] [PubMed] [Google Scholar]
- 12.Nuyts J, Michel C, Dupont P. Maximum-likelihood expectation-maximization reconstruction of sinograms with arbitrary noise distribution using NEC-transformations. IEEE Trans Med Imaging. 2001;20:365–375. [DOI] [PubMed] [Google Scholar]
- 13.Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45:1431–1434. [PubMed] [Google Scholar]
- 14.Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47:904–911. [PubMed] [Google Scholar]
- 15.Leu K, Boxerman JL, Lai A, et al. Bidirectional Contrast agent leakage correction of dynamic susceptibility contrast (DSC)-MRI improves cerebral blood volume estimation and survival prediction in recurrent glioblastoma treated with bevacizumab. J Magn Reson Imaging. 2016;44:1229–1237. [DOI] [PubMed] [Google Scholar]
- 16.Ellingson BM, Kim HJ, Woodworth DC, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252:182–189. [DOI] [PubMed] [Google Scholar]
- 18.Olar A, Wani KM, Alfaro-Munoz KD, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol. 2015;129:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicone F, Carideo L, Scaringi C, et al. (18)F-DOPA uptake does not correlate with IDH mutation status and 1p/19q co-deletion in glioma. Ann Nucl Med. 2019;33:295–302. [DOI] [PubMed] [Google Scholar]
- 20.Yao J, Hagiwara A, Raymond C, et al. Human IDH mutant 1p/19q co-deleted gliomas have low tumor acidity as evidenced by molecular MRI and PET: a retrospective study. Sci Rep. 2020;10:11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi K, Hirata K, Yamaguchi S, et al. Prognostic value of volume-based measurements on (11)C-methionine PET in glioma patients. Eur J Nucl Med Mol Imaging. 2015;42:1071–1080. [DOI] [PubMed] [Google Scholar]
- 22.Naslund O, Smits A, Forander P, et al. Amino acid tracers in PET imaging of diffuse low-grade gliomas: a systematic review of preoperative applications. Acta Neurochir (Wien). 2018;160:1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med. 2015;372:2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karunanithi S, Sharma P, Kumar A, et al. Can (18)F-FDOPA PET/CT predict survival in patients with suspected recurrent glioma? A prospective study. Eur J Radiol. 2014;83:219–225. [DOI] [PubMed] [Google Scholar]
- 25.Suchorska B, Jansen NL, Linn J, et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84:710–719. [DOI] [PubMed] [Google Scholar]
- 26.Rossi Espagnet MC, Romano A, Mancuso V, et al. Multiparametric evaluation of low grade gliomas at follow-up: comparison between diffusion and perfusion MR with (18)F-FDOPA PET. Br J Radiol. 2016;89:20160476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellingson BM, Wen PY, Cloughesy TF. Evidence and context of use for contrast enhancement as a surrogate of disease burden and treatment response in malignant glioma. Neuro Oncol. 2018;20:457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carideo L, Minniti G, Mamede M, et al. (18)F-DOPA uptake parameters in glioma: effects of patients’ characteristics and prior treatment history. Br J Radiol. 2018;91:20170847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fueger BJ, Czernin J, Cloughesy T, et al. Correlation of 6–18F-fluoro-L-dopa PET uptake with proliferation and tumor grade in newly diagnosed and recurrent gliomas. J Nucl Med. 2010;51:1532–1538. [DOI] [PubMed] [Google Scholar]
- 30.Unterrainer M, Schweisthal F, Suchorska B, et al. Serial 18F-FET PET Imaging of Primarily 18F-FET-Negative Glioma: Does It Make Sense? J Nucl Med. 2016;57:1177–1182. [DOI] [PubMed] [Google Scholar]
- 31.Galldiks N, Unterrainer M, Judov N, et al. Photopenic defects on O-(2-[18F]-fluoroethyl)-L-tyrosine PET: clinical relevance in glioma patients. Neuro Oncol. 2019;21:1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen NL, Suchorska B, Wenter V, et al. Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J Nucl Med. 2014;55:198–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.