Abstract
Infection is one of the most important obstacles in the wound‐healing process. Conventional methods used for the treatment of wound infections have their own limitations and hence, are difficult to control. If infection is not addressed well in time, it will further increase morbidity and cost of treatment. An attempt was made to develop a simple and effective treatment modality by using citric acid as the sole antimicrobial agent to control bacterial infections of traumatic wounds. A total of 259 cases of traumatic wounds infected with a variety of bacteria were investigated for culture and susceptibility, and susceptibility to citric acid. Citric acid ointment (3%) was applied to traumatic wounds to determine its efficacy in their treatment of traumatic wounds. In a culture and susceptibility study, a total of 369 aerobic bacteria and 7 fungi were isolated, with Staphylococcus aureus (30·31%) being the most common isolate and ciprofloxacin (61·43%) being the most effective agent. All the isolates were found to be inhibited by citric acid in in vitro studies (minimum inhibitory concentration – 500–2500 µg/ml). Citric acid ointment was found effective in controlling infections. Out of 259 cases, 244 (around 95%) were healed completely in 5–25 applications of 3% citric acid. As citric acid has antibacterial activity and wound‐healing property; hence it is the best alternative for the treatment of traumatic wounds. Besides these properties, citric acid has no adverse effects and it is a good dressing agent.
Keywords: Antibacterial agent; Citric acid treatment; Traumatic wounds; Wound infection
Introduction
Traumatic wounds that do not heal in a timely fashion are treated as chronic wounds. Bacteria and bacterial products such as endotoxins and metalloproteinases are the most significant obstacles in healing processes as slow down the process of healing. It has been experimentally proven that bacteria present at high levels in a wound can inhibit the normal wound‐healing process (1). Thus, infection is one of the major causes for non‐healing of wounds. Treatment of wound infections has always been a problem for clinicians. To the clinicians, it is obvious that reducing the number of bacteria in wounds is ultimately aimed at accelerating wound healing. Although several studies support the value of topical antimicrobials in wound infections, many commonly used antiseptic agents are not approved for use in wounds. The safety and effectiveness of many antiseptics used as topical agents for local wound dressing is a debatable issue. A number of experimental studies, both in vitro and in animal experiments, have suggested that many antiseptics such as betadine, iodine, hydrogen peroxide, alcohol and so on may be toxic to the cells involved in wound healing. They may permit even more virulent organisms to dominate, and hence should be avoided (2, 3, 4, 5, 6). The systemic antibiotics have also been demonstrated as being of little use in the treatment of wounds (7). A variety of chemical agents are available, which are non‐toxic, inexpensive and highly effective against various bacteria commonly associated with wound infections. It has been reported that in some cases of local applications, chemical agents have advantages over antibiotics, especially in controlling multiple antibiotic‐resistant hospital strains (8). These agents can be used locally in the treatment of wound infections. The topical use of various acids, notably acetic acid, boric acid and ascorbic acid has been reported to eliminate Pseudomonas aeruginosa from burn infections and skin and soft tissue infections (9, 10, 11, 12, 13, 14, 15). The use of citric acid has also been reported in the effective treatment of infections of burns, skin and soft tissue 16, 17, 18, 19. In this, an attempt was made to develop an effective and reliable therapeutic approach for the treatment of chronic traumatic wounds in non‐diabetic patients simply by using citric acid as a sole topical agent.
Patients and methods
A prospective open study on traumatic wound infections in non‐diabetic patients was carried out during the period January 2003 to December 2010. The study protocol was approved by the institutional ethical committee and informed consent was taken from the patients who participated in the study. A total of 259 consecutive non‐diabetic cases with traumatic wounds infected with a variety of bacteria and not responding to conventional antibiotic therapy and local wound care with betadine for 2 weeks to 6 months were included in this study. The cause of the infected wound was trauma. The traumatic wounds were mostly present on the leg. The site and size of the wound differed from patient to patient. The wounds were deep and infected because of external contamination and lack of proper care by the patients. The cases were recruited from the hospital where the protocol was to clean and dress the wound with povidone/iodine (betadine) once daily and administer a course of antibiotics to control infection.
The severity of the local infection for each patient was documented before starting the treatment and the diagnosis was made on the basis of local signs of infection. These included the classical signs related to the inflammatory process (20) such as localised erythema and oedema, pain, warmth and so on and presence of slough, discharge from wound and isolation of infecting bacterium from pus in significant numbers, that is a confluent growth on primary and secondary streaking or a minimum 100 colonies.
After thorough clinical examination, routine haematological investigations and investigation for diabetes, a pus swab was collected for culture and susceptibility from each case and processed for aerobic culture by using standard techniques (21). Susceptibility to antimicrobial agents was studied by the Kirby‐Bauer disc diffusion method, using ampicillin (10 µg), amikacin (30 µg), gentamicin (10 µg), ciprofloxacin (5 µg), ceftazidime (30 µg), ceftriaxone (30 µg) and pefloxacin (5 µg) (22). Susceptibility of clinical isolates to citric acid was studied by determining minimum inhibitory concentration (MIC) by the broth dilution method, using different concentrations in the range of 500–3000 µg/ml (23).
After thorough debridement, topical application of 3% citric acid ointment [prepared by mechanical mixing (trituration) of 3 g of citric acid (monohydrate pure obtained from HiMedia laboratories limited) in a mortar with 100 g white soft paraffin (100% pure petroleum jelly – a hydrocarbon base not absorbed by the skin), taking all sterile precautions] to the wound once daily was started and continued until the wound healed completely or showed formation of healthy granulation tissue in case of larger wounds. Before the application of citric acid ointment to wounds, the wound was first irrigated and cleaned with normal saline. Following this, citric acid ointment was applied to the wounds, which were then dressed with a sterile pad. The course of progress was managed by the treating clinician. Citric acid alone was used in most of the cases that were infected, but not showing systemic symptoms such as fever and toxicity. No antibiotics were given during this period of application of citric acid ointment except in nine cases showing systemic symptoms of fever, toxicity and so on. In 15 cases with large raw areas, the wounds were closed by skin grafting after formation of healthy granulation tissue after 5–10 applications of citric acid ointment. In seven cases, showing presence of Candida albicans in addition to bacterial pathogens, oral antifungal agent (tablet fluconazole, 150 mg, daily for 7 days) was given along with local application of 3% citric acid ointment. The wounds in all the patients were observed for adverse reactions after the application of 3% citric acid gel.
Results
A total of 369 aerobic bacteria and 7 fungi (total 376 isolates) were isolated from 259 patients. S. aureus (30·31%) and P. aeruginosa (28·19%) were found to be the most common isolates. Most of the isolates were found to have resistance to more than three to four antibiotics. Ciprofloxacin (61·43%) was the most effective agent, followed by amikacin (57·71%), ceftazidime (52·39%) and ceftriaxone (40·69%). Ampicillin (5·05%) was found to be the least effective agent (Table 1).
Table 1.
Clinical isolates from chronic traumatic wounds in non‐diabetic patients and their antibiogram *
| Serial number | Name of the organism | No. of isolates | Antibiotic susceptibility | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | G | Ak | Cf | Pf | Ca | Ce | |||
| 1. | Staphylococcus aureus | 114 (30·31) | 11 (9·64) | 34 (29·82) | 69 (60·52) | 79 (69·29) | 47 (41·22) | 43 (37·71) | 51 (44·73) |
| 2. | Pseudomonas aeruginosa | 106 (28·19) | 07 (6·60) | 38 (35·84) | 56 (52·83) | 61 (57·54) | 38 (35·84) | 69 (65·09) | 56 (52·83) |
| 3. | Escherichia coli | 61 (16·22) | 02 (3·27) | 22 (36·06) | 34 (55·73) | 34 (55·73) | 19 (31·14) | 33 (54·09) | 11 (18·03) |
| 4. | Klebsiella spp. | 25 (6·64) | 02 (8·00) | 11 (44·00) | 16 (64·00) | 15 (60·00) | 11 (44·00) | 15 (60·00) | 05 (20·00) |
| 5. | Staphylococcus albus | 24 (6·38) | 06 (25·00) | 12 (50·00) | 15 (62·5) | 19 (79·16) | 15 (62·5) | 11 (45·83) | 12 (50·00) |
| 6. | Citrobactor spp. | 20 (5·31) | 01 (5·00) | 07 (35·00) | 12 (60·00) | 12 (60·00) | 10 (50·00) | 12 (60·00) | 09 (45·00) |
| 7. | Proteus spp. | 19 (5·05) | 01 (5·26) | 09 (47·36) | 15 (78·94) | 11 (57·89) | 09 (47·36) | 14 (73·68) | 09 (47·36) |
| 8. | Candida albicans | 07 (1·86) | – | – | – | – | – | – | – |
| Total | 376 | 19 (5·05) | 133 (35·37) | 217 (57·71) | 231 (61·43) | 149 (39·62) | 197 (52·39) | 153 (40·69) | |
A, Ampicillin; G, Gentamicin; AK, Amikacin; Cf, Ciprofloxacin; Pf, Pefloxacin; Ca, Ceftazidime; Ce, Ceftriaxone.
*Figures in parenthesis indicate percentage.
All the isolates were found to be inhibited by citric acid in in vitro studies. The MIC of citric acid in vitro was found in the range of 500–2500 µg/ml against different clinical isolates. P. aeruginosa was found to be most susceptible (MIC 500–1000 µg/ml) and Klebsiella spp. was found to be least susceptible (MIC 2000–2500 µg/ml). S. aureus, Staphylococcus albus, Escherichia coli, Proteus spp. and Citrobacter spp. showed intermediate susceptibility (Table 2).
Table 2.
Minimum inhibitory concentration of citric acid against clinical isolates
| Serial number | Name of microbe | MIC value (µg/ml) |
|---|---|---|
| 1. | Pseudomonas aeruginosa | 500–1000 |
| 2. | Klebsiella spp. | 2000–2500 |
| 3. | Staphylococcus aureus | 900–1000 |
| 4. | E. coli | 1500–2000 |
| 5. | Staphylococcus albus | 1200–1500 |
| 6. | Proteus spp. | 1500–1600 |
| 7. | Citrobacter spp. | 1000–1500 |
Out of 259 cases of chronic traumatic wounds, 244 (around 95%) were healed completely in 5–25 applications of 3% citric acid in 5–25 days. A significant reduction in exudates and pain was observed after 2–3 applications of citric acid. In 15 cases (6%) with large raw areas, skin grafting was required for complete wound closure. No adverse effects were seen in any of the patients except for mild irritation (1, 2, 3).
Figure 1.
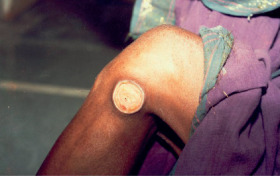
Traumatic wound before application of citric acid.
Figure 2.
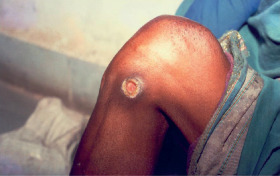
Traumatic wound after 11 applications of citric acid.
Figure 3.
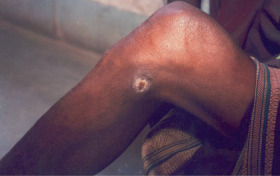
Traumatic wound after 20 applications of citric acid.
Discussion
Wound infections are difficult to manage because of various reasons. Multiple antibiotic resistance in bacteria involved in wound infections makes removal of the causative agent from the infection site more difficult. The use of antiseptic agents in the treatment of wound infections is an often criticised practice. A number of studies have shown that use of these agents should be avoided 2, 3, 4, 5, 6. The systemic antibiotic therapy also has little value in the treatment of infected wounds (7). Thus, it is very difficult to eliminate the infecting organism from the infected site and control infection, a step that is paramount to the success of healing.
In view of this, there is a need of alternative therapy or approach for the treatment of infections in traumatic wounds. In recent years, the use of citric acid has been reported a simple and effective approach towards the treatment of a variety of wounds including burn infections, leprosy ulcers, diabetic foot infections and so on caused by various bacteria including multiple antibiotic‐resistant strains 16, 17, 18, 19.
Citric acid has been reported to have antibacterial activity as indicated by microbiological studies and evidenced by the rapid clearing up of infected surfaces (19). This antibacterial activity may be attributed to the lowering of pH that makes an environment unsuitable for growth and multiplication of bacteria causing wound infections. Citric acid has been shown to enhance epithelisation, which is a major factor in wound healing. Citric acid keeps the wound surface moist and prevents wound desiccation, which is known to retard the healing process, and thus, reduces dehydration necrosis. Histological studies have shown that citric acid has been found to accelerate the wound‐healing process by boosting fibroblastic growth and neovascularisation, which in turn increase microcirculation in the wound, enabling the formation of healthy granulation tissue and thereby leading to faster healing of the wound (24). All these actions of citric acid in coordination increase migration of epithelial cells from the surrounding skin and epithelisation in turn acts as stimulus for depositing of ground substance and formation of granulation tissue. Citric acid also has synergistic antioxidant property. This property may prevent free radical damage and may stabilise lysosomal enzymes needed for collagen synthesis (25).
In this study, the effect of citric acid was studied against pathogens involved in traumatic wounds not responding to conventional antibiotic therapy and local wound care. Citric acid was found effective against these pathogens in in vitro studies and was also found effective in the elimination of bacteria from the infection site and the acceleration of the wound‐healing process. Application of citric acid to the wounds in these cases resulted in rapid cleaning up of infected surfaces and renewal of epithelia. Citric acid was found highly effective in the treatment of these cases with success rate of around 95% in 5–25 applications of 3% citric acid. The rate of healing in these cases is more or less comparable with that of diabetic foot ulcers in which a healing rate of 93% has been reported but it is more when compared with burn infections in which a healing rate of 87% has been reported 16, 17. However, the number of applications needed in these cases is smaller (5–25 applications) and is comparable with that for burn infections in which 7–25 applications are needed, but not comparable with that for diabetic foot ulcers in which a larger number of applications are needed in most of the cases 16, 17 indicating that infections can easily be brought under control in these cases and thereby healing can be enhanced rapidly.
It is evident from the results of this study that citric acid has antibacterial activity, and simultaneously at the same time, it is not toxic to the cells involved in healing process like other routinely used antiseptic agents. It promotes wound healing and is the best alternative for the effective management of traumatic wound infections. Antibacterial activity, wound‐healing property and absence of adverse effects make citric acid a good dressing agent. Finally, we conclude that citric acid is safe and useful in the treatment of traumatic wound infections. These results suggest that there is an opportunity to design a study involving relevant control groups to confirm these preliminary findings and reach more useful and concrete conclusions.
Acknowledgements
The authors declare that they have no conflict of interest.
References
- 1. Robson MC. Wound infection: a failure of wound healing caused by an imbalance of bacteria. Surg Clin N Am 1997;77:637–50. [DOI] [PubMed] [Google Scholar]
- 2. Branemark P, Albrektsson B, Lindstrom J, Lundberg G. Local tissue effects of disinfectants. Acta Chir Scand 1966;357(Suppl):166–76. [PubMed] [Google Scholar]
- 3. Kramer SA. Effect of povidone – iodine on wound healing: a review. J Vasc Nurs 1999;17:17–23. [DOI] [PubMed] [Google Scholar]
- 4. Cooper ML, Laer JA, Hansbrough J. The cytotoxic effects of commonly used antimicrobial agents on human fibroblasts and keratinocytes. Trauma 1991;31:775–84. [DOI] [PubMed] [Google Scholar]
- 5. Russell RCG, Williams NS, Bulstrode CJL. Baily and Love's short practice of surgery, 23rd edn. London: Arnold, 2000:147–162. [Google Scholar]
- 6. Lineaweaver W, McMorris S, Soucy D, Howard R. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg 1985;75:394–6. [DOI] [PubMed] [Google Scholar]
- 7. Robson MC, Edstrom LE, Krizek TJ, Groskin MG. The efficacy of systemic antibiotics in the treatment of granulating wound. J Surg Res 1974;16:299–306. [DOI] [PubMed] [Google Scholar]
- 8. Krasilnikov AP, Adarchenko AA, Bulai PI, and Sobeshchuk OP. A comparative analysis of the antibacterial activity of antiseptics and antibiotics on samples of Pseudomonas aeruginosa . Zhurnal Mikrobiologii, Epidemiologii I Immunobiologii 1991;8:30–33. [PubMed] [Google Scholar]
- 9. Philips I, Lobo AZ, Fernandes R, Gundara NS. Acetic acid in the treatment of superficial wounds infected by Pseudomonas aeruginosa . Lancet 1968;1:11–14. [DOI] [PubMed] [Google Scholar]
- 10. Sloss JM, Cumberland N, Milner SM. Acetic acid used for the elimination of Pseudomonas aeruginosa from burn and soft tissue wounds. J Army Med Corps 1993;139:49–51. [DOI] [PubMed] [Google Scholar]
- 11. Nagoba BS, Deshmukh SR, Wadher BJ, Patil SB. Acetic acid treatment of pseudomonal postoperative wound infection. J Hosp Infect 1997;36:243–4. [DOI] [PubMed] [Google Scholar]
- 12. Adarchenko AA, Krasilnikov AP, Sobeshchuk, OP. Antiseptic sensitivity of clinical strains of Pseudomonas aeruginosa . Antibiotiki I Khimioterapiia 1989;34:902–7. [PubMed] [Google Scholar]
- 13. Husain MT, Karim QN, Tajuri S. Analysis of infection in a burn ward. Burns 1989;15:299–302. [DOI] [PubMed] [Google Scholar]
- 14. Kujath P, Hugelschaffer C. Pseudomonas aeruginosa: pathogenicity, prevention and therapeutic approaches. Zentralbl Chir 1987;112: 558–63. [PubMed] [Google Scholar]
- 15. Mujumdar RK. Treatment of resistant pseudomonas infection in burn patients in tropical climate using acetic medium, oxidizing agent and metronidazole. Ind J Surg 1993;55:501–7. [Google Scholar]
- 16. Nagoba BS, Gandhi RC, Wadher BJ, Rao Ak, Hartalkar AR, Selkar SP. A simple and effective approach for the treatment of diabetic foot ulcers with different Wagner grades. Int Wound J 2010;7:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagoba BS, Gandhi RC, Hartalkar AR, Wadher BJ, Selkar SP. Simple, effective and affordable approach for the treatment of burns infections. Burns 2010;36:1242–7. [DOI] [PubMed] [Google Scholar]
- 18. Nagoba BS, Wadher BJ, Rao AK, Kore GD, Gomashe AV, Ingle AB. Simple and effective approach for the treatment of chronic wound infections caused by multiple antibiotic resistant Escherichia coli . J Hosp Infect 2008;69:177–80. [DOI] [PubMed] [Google Scholar]
- 19. Nagoba BS, Wadher BJ, Chandorkar AG. Citric acid treatment of non‐healing ulcers in leprosy patients. Br J Dermatol 2002;146:1101. [DOI] [PubMed] [Google Scholar]
- 20. Cutting K, Harding K. Criteria for identifying wound infection. J Wound Care 1996;3:198–201. [DOI] [PubMed] [Google Scholar]
- 21. Collee JG, Duguid JP, Fraser AG, Marmion BP. Mackie & McCartney practical medical microbiology, 13th edn. London: Churchill‐Livingston, 1989. [Google Scholar]
- 22. Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966;45:493–6. [PubMed] [Google Scholar]
- 23. Baron EJ, Peterson LR, Finegold SM. Methods for testing antimicrobial effectiveness. In: Diagnostic microbiology, 9th edn. London: Mosby, 1999:168–188. [Google Scholar]
- 24. Nagoba BS, Gandhi RC, Wadher BJ, Potekar RM, Kolhe SM. Microbiological, histopathological and clinical changes in chronic wounds after citric acid treatment. J Med Microbiol 2008;57:681–2. [DOI] [PubMed] [Google Scholar]
- 25. Budhavari S. The Merck index, 11th edn. Rahway: Merck & Co, 1989:2330. [Google Scholar]


