Abstract
Our study reviewed nine patients who were treated with the VAC™ Abdominal Dressing System after suffering pelvic fractures and soft tissue loss after high‐energy pelvic trauma. Between March 2008 and August 2009, our clinic treated nine patients with complicated perineal injuries from high‐energy pelvic trauma with multiple irrigation and debridement procedures and broad‐spectrum antibiotics. Protective ostomies were created for all nine patients. Required interventions were made for associated injuries, and VAC™ application was started. All patients were male, with an average age of 24·3 (range 21–32) years, and a mean injury severity score of 36·4 (range 16–59). Wound diameters ranged from 15 to 30 cm, and wound depths ranged from 5 to 25 cm. The injuries included one traumatic bilateral hemipelvectomy, and three unilateral and two bilateral lower extremity amputations. Intensive care unit length of stay averaged 12 (6–19) days, and average hospital length of stay was 44·12 (31–64) days. Beginning at an average of day 17 (±5·9 days) post‐injury, wound cultures detected no bacterial colonisation. One patient died on the sixth day after injury from septic complications. Two patients' wounds were closed by primary closure, and six patients' wounds were closed by split thickness grafts after an average of 31·4 (17–50) days. Optimal treatment of high‐energy perineal injuries requires early and extensive debridement and rich irrigation. The application of the VAC™ system as temporary coverage of large complex wounds in the pelvic region enhances wound healing and facilitates an early grafting process.
Keywords: High‐energy perineal trauma, Vacuum‐assisted closure, Wound care
INTRODUCTION
High‐energy trauma to the perineal region, usually resulting from traffic accidents, gunshot or land mines, can produce pelvic bone fractures, catastrophic bleeding, perineal injuries and extensive soft tissue damage 1, 2, 3, 4. In such scenarios, the main objective is to preserve life. Secondary objectives are to ensure limb function and restore urinary and intestinal integrity as much as possible. When encountering these patients, the first step is to control bleeding. The next step is to eliminate perineal contamination, which may require urinary and intestinal diversion 2, 3. After necrotic tissue is debrided and infection is controlled, reconstruction procedures are begun.
In high‐energy perineal trauma, pelvic bones are frequently separated from one another, tissues are dissected and peeled off the surface and a large wound is usually present. The end result for many patients is lower extremity amputation and loss of the pelvic floor muscles with gas/faecal incontinence and urinary incontinence 2, 3, 4. A high risk of infection accompanies this type of trauma, most commonly trauma from mine injuries. In these patients, exudate and haemorrhagic or inflammatory drainage most likely can be seen (3).
The anatomical structures of the pelvic/perineal area and the natural curves of the site make bandages and dressings difficult to apply. The extensive haemorrhagic and exudative discharge necessitates changing the dressing at least several times a day; this requires much staff time and causes a high level of patient discomfort. Not changing the dressings often enough or failure to close this region well increases the risk of infection.
In recent years, negative pressure wound closure systems began to be used for injuries to the perineal region 5, 6, 7, 8, 9, 10. The aim of our study was to review the use of negative pressure wound therapy with the VAC™ system (Kinetic Concepts Inc., San Antonio, TX) as a closure system for our patients with complicated perineal injuries. There is little information in the literature on management of complex perineal injuries with vacuum‐assisted closure (VAC) 3, 11; we therefore seek to share our experience and describe our technique to show how we applied VAC to achieve excellent results.
METHODS
We used the VAC™ system to treat a series of nine patients who sustained complex perineal injuries from March 2008 to August 2009. We managed these patients as follows:
A Foley catheter was placed. In patients whose urethral injuries precluded insertion of a Foley catheter, cystostomy was performed. Patients with deteriorating condition and unstable haemodynamics underwent damage control surgery with recovery in the intensive care unit until they became stabilised.
All patients in our series experienced large perineal tissue loss, and, due to the high risk of contamination, underwent a second operation for creation of a protective ostomy. Anal examination and rectoscopy were held in the same operative session, and the status of the anal sphincter was evaluated. Distal rectal washout was also performed at this time, and the infective load of the colon and rectum was reduced.
Once the ostomy was established, the definitive surgical reconstruction process could begin. VAC therapy was started immediately after the ostomy was created. VAC and dressing changes were performed under sterile conditions in the operating room once every 2 days. Once per week, the rectum was washed internally with Octenisept® (Schülke & Mayr GmbH, 22840 Norderstedt, Germany) followed by dressing change. Debridement was performed as necessary.
After confirming, there was no infection around the open pelvic fractures, we stabilised the fractures via internal fixation methods in a third operation. For patients with distorted pelvic stability, external fixation was applied (Figure 1). As needed to help wound contraction, the two tips of the anal sphincter were merged by sutures. In cases where the rectum and anal canal were completely dissected and separated from the surrounding tissue, causing a tendency to escape inward, sutures were placed along the anal canal contour with downward traction to assist the rectum and anal canal to adhere to their normal positions. The VAC negative pressure applied to the perineal region also helped restore the rectum and anal canal to their proper positions. Whenever possible, the anal canal contour closest to the perineal skin was sutured; in time, additional sutures were placed and the anal canal was seated in its normal position.
Figure 1.

Open wound.
VAC™ application
In our use of VAC, we placed strips prepared from polyurethane foam included with the VAC™ system between the perineum and pelvic muscles where tissue defects had formed around the rectum and bladder and into the cavities. Next, foam pieces cut into appropriate sizes and shapes were placed on the wound. For covering the wound, the VAC™ package provides a transparent air‐ and waterproof self‐adhesive drape and a seal. Before applying the drape, the surrounding skin was dried and degreased by alcohol wipes as necessary to ensure full adhesion. In difficult areas, Whitehead's varnish was first applied to the firm skin area adjacent to the wound to improve skin adhesion; then the drape was affixed. For application of the vacuum, a 1‐cm diameter hole was cut in the drape, and this hole was pasted on top of the TRAC™ (Kinetic Concepts Inc., San Antonio, TX) pad. An adjustable negative pressure hose on the back of the TRAC™ pad was attached to the vacuum machine, and a 125‐mmHg vacuum was created which applied a continuous topical negative pressure to the area. Prepared foam strips were placed into the defects and cavities at the perineal region to achieve the negative pressure, where exudate drained and led to the rapid filling of granulation into these areas. In due course, the pararectal and paravesical cavities filled with granulation tissue, while the volume of the foam strips placed in those areas decreased. The granulation process progressed until the cavities were filled by the surrounding tissue. The top of the injured tissue became covered by healthy granulation; VAC™ application was continued until the area was, in effect, covered as with a graft. When possible, the wound was then closed with delayed primary suturation; wounds in which primary closure could not be achieved were closed by split thickness grafts.
Concurrent with the VAC™ application, any required surgical procedures and treatments for accompanying thorax, abdomen and limb injuries were performed.
RESULTS
A total of nine patients were included in our study. All the patients were male. Their ages are shown with the patient clinical data in Table 1. All the patients had perineal wounds with large tissue loss. There was urethral full‐cut in three patients, and four patients had bladder injuries. Two patients suffered pubic separation, one of 12 cm and the other 8 cm; because of this separation, the sacroiliac joints were also broken. Three patients had completely torn pelvic floor muscles; the rectum had dissected and separated from the tissue around the roundabout, resulting in the loss of integrity of the internal and external anal sphincter muscles. One patient had a 4‐cm rectal injury beginning at 5 cm above the dentate line. All the patients injured by land mines required amputation of one or both the lower extremities at different levels. The smallest wound size at admission was 15 cm in diameter, and the largest was 30 cm. The wound bases were irregular in structure, and most of the wounds had deep extensions featuring cavities and pockets. In three patients, the anal canal and surrounding rectal tissues were dissected completely on both sides of the pelvic floor; the muscles were completely fragmented, the bladder was dissected and injured, and the penis root and prostate were completely visible. In none of our study patients could the pelvic floor be sutured and repaired.
Table 1.
Clinical characteristics of our series of patients having complex perineal wounds and receiving VAC treatment
| Age (years) | ISS | Injury type | Associated injuries | ICU stay (day) | Hospital stay (day) | Definite closure time (day)/type |
|---|---|---|---|---|---|---|
| 21 | 41 | Land mine explosion | CPI + PS + left leg amputation from knee | 14 | 39 | 29/STG |
| 22 | 50 | Land mine explosion | CPI + PS + bilateral leg amputation from knee + pneumothorax + rib fracture + urethral incision | 16 | 57 | 44/STG |
| 27 | 45 | Land mine explosion | CPI + PF + right leg upper knee amputation + eye injury | 11 | 42 | 30/STG |
| 21 | 29 | Gunshot injury | CPI + PF + left hand injury + urethral incision | 7 | 39 | 27/suture |
| 32 | 24 | Traffic accident | CPI + pneumothorax + fibula fracture | 12 | 33 | 19/suture |
| 21 | 16 | Gunshot injury | CPI + PF | 6 | 31 | 17/suture |
| 32 | 59 | Land mine explosion | CPI + PF + bilateral hemipelvectomy + haemo/pneumothorax + lung contusion | Exitus day 6 | – | −/− |
| 21 | 57 | Land mine explosion | CPI + one site foot and the other site upper knee amputation + flail chest | 19 | 64 | 50/STG |
| 22 | 34 | Land mine explosion | CPI + right lower knee amputation | 11 | 48 | 35/STG |
ISS, injury severity score; ICU, intensive care unit; CPI, complex perineal injury; STG, split thickness graft; PS, pubic seperation; PF, pubic fracture.
The depth of the perineal wounds ranged between 5 and 25 cm. In four of the patients, the perineal wounds were so deep that they connected into the abdominal cavity; some of these wounds extended between the subcutaneous tissue and the lumbar muscles. Weekly wound measurements were taken during the VAC™ dressing changes. Decreases in the sizes of the wounds were detected each week at the rate of approximately 0·5 − 1 cm in wound depth and approximately 5 − 10% in wound diameter. Beginning at an average of day 17 (±5·9 days) post‐injury, wound cultures detected no bacterial colonisation. One of our patients died on the sixth day after injury from sepsis‐related multiorgan failure. This patient's injury severity score (ISS) was calculated as 59; he had bilateral traumatic hemipelvectomy, complex perineal injury, haemo/pneumothorax and lung contusion. Of the remaining eight surviving patients, two were able to receive delayed primary suturation, and the remaining six patients' wounds were closed with split thickness grafts at an average of 31·4 (17–50) days after injury. All the surviving patients were discharged with resolution of their wounds. The average length of stay in the intensive care unit was 12 (range 6–19) days, and hospital length of stay averaged 44·12 (range 31–64) days. 2, 3, 4, 5, 6, 7 illustrate our treatment in two of the cases.
Figure 2.
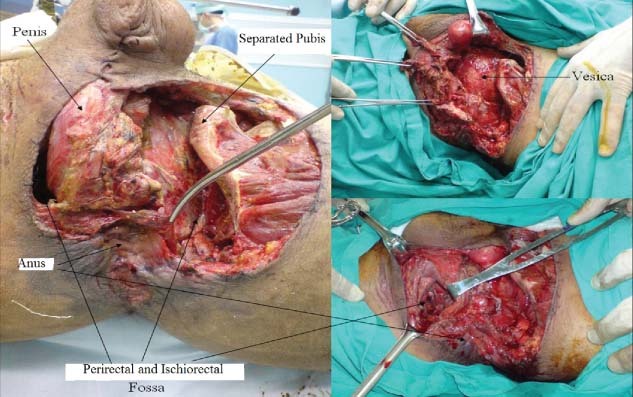
Open wound different view.
Figure 3.

Applicated VAC.
Figure 4.

Approximation sutures (above), graft application (below).
Figure 5.

Injured sphincters (left), VAC Application (right).
Figure 6.

Anal region after VAC application.
Figure 7.

The last view of the same patient.
DISCUSSION
As indicated in the literature, VAC has been successfully used not only for chronic wounds with open fractures but also for temporary wound closure of complicated acute injuries 7, 8, 9. VAC therapy provides temporary protection of soft tissue defects by means of polyurethane foam, which is sealed airtight by a polyvinyl foil. A negative topical pressure gradient is generated by a VAC unit (5). In clinical practice, VAC therapy increases the formation of granulation tissue on the wound surface, both quantitatively and qualitatively 5, 12, 13, 14. It is well documented that 4 days of VAC therapy produces significantly improved microperfusion, an increased partial oxygen pressure in the tissue, and a reduction in bacterial colonisation (bacterial clearance) 5, 12, 13, 14, 15. The increased microvessel density, which develops with time, results in improved granulation formation and speeds wound healing (5). Because of these features, we decided to use the VAC™ system for our patients.
Each patient in our series had a high ISS: not only were the wounds severe, but also each patient's overall condition was critical. Early closure and reconstruction was not possible in these cases. Morel–Lavallee lesions 15, 16 accompanied the contaminated areas, and the injuries were extensive. Tissue damage and necrosis of a large part of the perineal region are known to accompany high‐energy injuries caused by high‐pressure blasts such as from land mines or traffic accidents 16, 17, 18, 19, 20, 21. Foreign bodies and infective particles move deep into the perineum, capillary circulation is severely damaged, and in the days following, the additional problem of necrosis begins. Rectal and anal sphincter damage often accompanies these injuries 4, 5, 16. These high‐impact wounds should receive early and frequent debridement. They should be left open without suturing, and good drainage should be provided. Any new foci of necrosis found in daily follow‐up should be redebrided. Faecal diversion is recommended for this type of patient with open pelvic fracture 17, 18, 20. During treatment with the VAC™ system, we changed the dressing once every 2 days; the transparent drape allowed us to see the development of new skin tissue around the wound. Although the dressing was not changed as often as usual, necrosis nevertheless did not develop; in contrast, the wound revitalised.
Death from these types of injuries is usually caused by exsanguination in the early period and by sepsis in later stages 5, 19. When these types of patients reach the hospital, most of them are hypothermic, acidotic and experiencing coagulopathic disorders. Patients in this condition cannot tolerate a long operation. It is therefore recommended to perform immediate damage control surgery, followed by definitive surgery in 24‐48 hours (19). We performed damage control surgery for the patients in our series. In patients with high‐energy injuries, who trend towards sepsis and necrotising fasciitis, early and aggressive surgery is crucial to successful healing. In the first operation (damage control), we performed aggressive debridement. Following this, we focused on resolving regional oedema and reducing bacterial colonisation. To promote exudation by allowing the wound to drain at the appropriate humidity, restore the microcirculation and increase oxygenation to the tissues, we decided to use the VAC™ system.
Use of the VAC™ system promoted rapid granulation. The polyurethane foam could be cut into shapes exactly fitted to the wounds. In addition, foam strips could be placed into the deepest wounds. Even in the most difficult drainage area, negative pressure could be transmitted to the closed cavities. The cavities readily filled with renewed tissue, and in a short time the wound bases become smooth and raised, creating appropiate grafting. The most important point is that the tips of the sponge filaments (foam pieces) placed in the main wound must be in contact with the sponges that are placed in the satellite (smaller) wound cavities. This allows the subatmospheric pressure to be transmitted to the smaller cavities, enabling complete drainage of these areas.
We found that another benefit of the rapid development of the high‐quality granulation facilitated by the VAC™ was that the tips of the anal sphincter muscle (which had been fragmented) came together tip‐to‐tip, restoring the original anatomical structure. This was shown in three of our cases where the anal sphincter muscle had been fragmented and lost its integrity. After VAC therapy, improvements were showed by magnetic resonance imaging (MRI). Immediately after injury, pelvic MRI scans had showed loss of anal sphincter integrity; after VAC therapy, new MRI scans showed that the missing parts of the anal sphincter had become filled with granulated tissue. The MRI scans showed the granulation tissue to be isointense with muscle on T1 sequences and mildly hyperintense on fat‐suppressed T2 sequences. Where there had been loss of the anal sphincter muscles, there was now granulation tissue connecting the muscle contours, allowing the anal canal to function again. In these three patients, their anal manometry results before VAC therapy showed anal canal resting pressures of 27, 31 and 23 mmHg (normal values: 59–74 mmHg) and maximum squeeze pressure of 51, 47 and 41 mmHg (normal range: 100–140 mmHg), respectively. While the post‐treatment manometric measurements showed that the resting and squeeze pressures did not attain normal values, the values did reach to about half of normal. The patients were instructed in Kegel exercises to improve sphincter function.
In three of the patients, the completeness of the anal sphincter and rectum was deteriorated such that the tips of the anal sphincter and rectum tended to go into the abdominal cavity. To prevent this problem, we attached the anal sphincter to the pelvic muscles with stitches. One of the most important factors which helped pull out the rectum and anal canal into place was the negative pressure created by the VAC™ system at this site. The application of negative pressure via the foam strips that were placed into the deep space surrounding the rectum facilitated drainage of this area, speeding the development of granulation and accelerating the healing period. All of this allowed the rectum to return to its normal position.
Blast injuries cause deterioration of the microcirculation, resulting in a great deal of necrosis (21). In our series, we found that very little debridement needed to be carried out after the initial large‐scale debridement performed in the first operation. We believe this is because damage to the microcirculation blocks or limits oxygenation; the increase in blood flow from the application of negative pressure allows better oxygenation and, therefore, better neovascularisation 5, 12, 13, 14, 15.
We believe the VAC™ system played a prominent role in the rapid successful healing of the large wounds of our patients. Although the patient's wound diameters ranged from 15 cm to as large as 30 cm, with wound depth reaching 25 cm, we nevertheless were able to close the wounds by delayed primary suturation in two of our patients. The other six patients' wounds became eligible for grafting at 21–43 days post‐injury, which is quite soon for such large wounds.
As recommended in the literature, we applied a negative pressure of 125 mmHg (3). In the four cases in which the perineal injuries communicated with the abdominal cavity, we started the vacuum application at 50 mmHg. Our aim was to prevent possible intestinal fistulation caused by a high vacuum. In addition, because some large‐scale vessels such as the iliac artery and vein were in contact with the VAC™ sponges, we preferred an initial low negative pressure to prevent damage to these structures. Our caution in beginning treatment with low pressure served us well because we did not observe any complications related to fistulation or the vascular structures. After four dressing changes, sufficient granulation tissue developed, and we gradually increased the negative pressure to 125 mmHg. By inserting the foam in contact with the intestine in such a way as to prevent intestinal fistulation and to prevent adhesion and contact of the foam with the intestine, the polyethylene sheet with the encapsulated foam included in VAC™ Abdominal Dressing System can be used safely.
In conclusion, to prevent high mortality and morbidity from high‐energy injuries of the pelvic and perineal area resulting from such incidents as mine explosions, gunshot and traffic accidents, multiple sessions of debridement and surgery are required, and the injury site(s) must be protected with appropriate and frequent changing of dressings. The excellent results obtained in our series of nine patients shows that the VAC™ system is a good choice for treating high‐energy perineal injuries by reducing wound infection, increasing wound neovascularisation and oxygenation, enhancing wound healing and enabling early wound closure.
ACKNOWLEDGEMENT
The authors thank Marilyn Carlson for her editorial assistance.
REFERENCES
- 1. Giannoudis PV, Pape HC. Damage control orthopaedics in unstable pelvic ring injuries. Injury 2004;35:671–7. [DOI] [PubMed] [Google Scholar]
- 2. Jeffery LC. Advanced wound therapies in the management of severe military lower limb trauma: a new perspective. Eplasty 2009;9:e28. [PMC free article] [PubMed] [Google Scholar]
- 3. Labler L, Trentz O. The use of vacuum assisted closure (VAC) in soft tissue injuries after high energy pelvic trauma. Langenbecks Arch Surg 2007;392:601–9. [DOI] [PubMed] [Google Scholar]
- 4. Wightman JM, Gladish SL. Explosions and blast injuries. Ann Emerg Med 2001;37:664–78. [DOI] [PubMed] [Google Scholar]
- 5. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–77. [PubMed] [Google Scholar]
- 6. Demir A, Demirtas Y, Ciftci M, Ozturk N, Karacalar A. Our topical negative pressure vacuum assisted closure (VAC) applications. Türk Plast Rekonstr Est Cer Derg 2006;14:171–7. [Google Scholar]
- 7. Fleischmann W, Strecker W, Bombelli M, et al. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg 1993;96:488–92. [PubMed] [Google Scholar]
- 8. Herscovici D Jr, Sanders RW, Scaduto JM, Infante A, DiPasquale T. Vacuum‐assisted wound closure (VAC therapy) for the management of patients with high‐energy soft tissue injuries. J Orthop Trauma 2003;17:683–88. [DOI] [PubMed] [Google Scholar]
- 9. Labler L, Keel M, Trentz O. Vacuum assisted closure (VAC®) for temporary coverage of soft tissue injury in type III open fracture of lower extremities. Eur J Trauma 2004;30:305–12. [Google Scholar]
- 10. Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum assisted closure therapy in the management of Fournier's gangrene. Am J Surg 2009;197:660–5. [DOI] [PubMed] [Google Scholar]
- 11. Bronchard R, de Vaumas C, Lasocki S, Jabbour K, Geffroy A, Kermarrec N, Montravers P. Vacuum‐assisted closure in the treatment of perineal necrotizing skin and soft tissue infections. Intensive Care Med 2008;34:1345–7. [DOI] [PubMed] [Google Scholar]
- 12. Labler L, Oehy K. Vacuum sealing of problem wounds. Swiss Surg 2002;8:266–72. [DOI] [PubMed] [Google Scholar]
- 13. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 14. Müllner T, Mrkonjic L, Kwasny O, Vecsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. Br J Plast Surg 1997;50:194–9. [DOI] [PubMed] [Google Scholar]
- 15. Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel‐Lavallée lesion. J Trauma 1997;42:1046–51. [DOI] [PubMed] [Google Scholar]
- 16. Woods RK, O’Keefe G, Rhee P, Routt ML Jr, Maier RV. Open pelvic fracture and fecal diversion. Arch Surg 1998;133:281–6. [DOI] [PubMed] [Google Scholar]
- 17. Jones AL, Powell JN, Kellam JF, McCormack RG, Dust W, Wimmer P. Open pelvic fractures. A multicenter retrospective analysis. Orthop Clin North Am 1997;28:345–50. [DOI] [PubMed] [Google Scholar]
- 18. Pell M, Flynn WJ Jr, Seibel RW. Is colostomy always necessary in the treatment of open pelvic fractures? J Trauma 1998;45:371–73. [DOI] [PubMed] [Google Scholar]
- 19. Simsek A, Ozer MT, Eryilmaz M, Ozturk E, Ozerhan IH, Gorgulu S, Peker Y, Tufan CT. The results of damage control surgery in abdominal trauma. BMMR 2007;10:136–40. [Google Scholar]
- 20. Smektala R, Hahn MP, Henkel M, Muhr G. Surgical management of complications of open pelvic injuries. Zentralbl Chir 1995;120:893–8. [PubMed] [Google Scholar]
- 21. Stuhmiller J, Phillips YY, Richmond DR. The physics and mechanisms of primary blast injury. Washington, DC: Office of the Surgeon General of the US Army, 1991. [Google Scholar]


