Abstract
The concept that undisturbed wound healing, optimised by dressing choice, improves wound outcomes has become a focal point of consideration when evaluating wound management regimens in recent years. However, little evidence exists related to wound contact layers and the potential detrimental effects of the intimate contact with the wound bed. The aim of this study was to evaluate the effects of atraumatic wound contact dressings on the healing of partial‐thickness wounds in comparison to untreated air‐exposed wounds. Using an in vivo porcine wound model the handling properties of each dressing in terms of adhesion were analysed. Methods of wound characterisation included histological analysis of granulation tissue formation and epithelialisation and this was correlated with various clinical observations. Differences were found between dressings in terms of adherence to the wound bed and surrounding skin, capacity to retain wound exudates and enhancement of healing.
Keywords: Adherence, Dressings, Porcine model, Wound healing
Introduction
When choosing the most suitable dressing for a wound, one needs to consider using a dressing that does not disturb the wound healing process. Proper dressing choice which includes materials that do not cause additional tissue injury would most likely lead to significant improvements in wound outcomes. While the intimate contact between the wound dressing and the wound bed is a common feature of modern dressings, the potential for disturbance to the wound bed and periwound skin upon removal of the dressing exists. A number of factors may lead to disturbed healing as a result of poor dressing choice including inadequate moisture balance, dressing adherence, mechanical stress, dressing residues, temperature and chemical imbalance and chemical stress 1, 2, 3, 4, 5. Evidence from case studies suggests that removal of adherent wound dressings can cause pain, with patients often requiring administration of analgesia at dressing changes 6, and can also cause reinjury to the wound, which hampers the healing process 7. Furthermore, dressing removal is particularly important in elderly patients, whose skin is often fragile and easily wounded 6.
The development of atraumatic wound contact layers, defined as a non‐adherent interface between the wound and secondary dressing 8, has been a major advancement in wound care. These dressings aim to prevent the problems associated with ‘skin stripping’, which damage the newly forming epithelium and surrounding tissue. Incorporating soft silicone into the dressing material allows for gentle adhesion without tissue trauma during removal 7, 9.
Despite the increasing popularity of atraumatic dressings in wound care practice, little clinical evidence exists regarding the effectiveness of these dressings. The purpose of this study was to assess the adherent properties of a range of atraumatic dressings and their effect on the healing of deep partial‐thickness wounds in a well‐established in vivo model.
Materials and methods
Institutional policies and regulations
The experimental animal protocols used for this study have been approved by the University of Miami Institutional Animal Care and Use Committee and all procedures followed the federal guidelines for the care and use of laboratory animals (U.S. Department of Health and Human Services, U.S. Department of Agriculture). The studies were conducted in compliance with the University of Miami's Department of Dermatology and Cutaneous Surgery Standard Operating Procedure. Animals were monitored daily for any observable signs of pain or discomfort. In order to help minimise possible discomfort, two analgesics (buprenorphine and fentanyl transdermal system) were used.
Experimental animals
A porcine model was used because of the morphological similarities between swine skin and human skin 10. A total of three specific‐pathogen‐free (B. G. Looper Farm, Granite Falls, NC) female pigs weighing 35–40 kg were kept in‐house for several days prior to initiating the experiment in order for the animals to acclimatise to the environment. They were fed a basal diet ad libitum and housed individually in the animal facilities [American Association for Accreditation of Laboratory Animal Care (AAALAC) accredited] with controlled temperature (19‐21°C) and lighting (12/12‐hour light : dark cycle).
Animal preparation
The animals were anaesthetised and hair on the backs of the experimental animals were clipped with standard animal clippers on the first day of experimentation. The skin on both sides of each animal was prepared for wounding by washing with a non‐antibiotic soap (Neutrogena Soap Bar; Johnson and Johnson, Los Angeles, CA) and sterile water. Finally, they were blotted dry with sterile gauze.
Wounding technique and experimental design
Sixty‐three rectangular wounds measuring 10 × 7 × 0·5 mm (L × W × D) were made in the paravertebral and thoracic areas with a specialised electrokeratome on each animal. Three animals were used for a total of 189 wounds for evaluation. The wounds were separated from one another by 15 mm of unwounded skin and randomly assigned to seven treatment regimens in different anatomical areas. Each treatment group contained nine wounds (Table 1).
Table 1.
Treatment groups
| Dressing typea | Trade name |
|---|---|
| Soft silicone (SS) primary dressing | Mepitel® (Mölnlycke Health Care) |
| One‐sided soft silicone (OSS) primary dressing | Mepitel‐One® (Mölnlycke Health Care) |
| Cellulose acetate mesh with soft silicone (CASS) primary dressing | Adaptic Touch® (Systagenix) |
| Flexible polyester mesh with lipido‐colloid (FPLC) primary dressing | Urgotul® (Urgo Medical) |
| Polyester mesh with soft silicone (PSS) primary dressing | Silfex® (Advancis Medical) |
| Polyester mesh with neutral triglycerides (PNT) primary dressing | Atrauman® (Hartmann) |
| Untreated air exposed |
All primary dressings were covered with secondary foam dressing Mepilex® (Mölnlycke Health Care).
Treatment regimen
Wounds were first dressed within 20 minutes of creation on day 0 with one of the primary dressings listed in Table 1. Primary dressings (1·5 × 1·5 inches) were kept in place until assessment. The secondary dressings (2·0 × 2·0 inches) that were taped in place were changed on days 1 and 2. They were weighed prior to application and during first and second dressing changes to determine dressing absorption (the amount of wound exudate allowed to pass through the primary dressings). All dressings were covered and secured by wrapping the animal with self‐adherent elastic bandages (Coban; 3M, St. Paul, MN).
Wound recovery
Three wounds from each treatment group were assessed by one of the investigators on days 3, 4 and 6 after wounding. The same investigator performed all evaluations. Two biopsies were taken from each wound. One excisional wedge biopsy, obtained through the centre of the wound with normal adjacent skin on both sides, was recovered for histological analysis.
Histological analysis
All excisional specimens were placed in formalin and then stained with haematoxylin and eosin. One section per block was analysed. The specimens were evaluated using light microscopy by a dermatopathologist who was unaware of treatment received and examined histologically for the parameters outlined in Table 2.
Table 2.
Histological measurements and scale codes
| Wound characteristic | Type of measurement | Scale of measurement |
|---|---|---|
| Epithelialisation | Measurement of the length of the wound surface covered with epithelium in relation to non‐epithelialised surface | % of wound epithelialised |
| Epithelial thickness | Thickness of the epithelium in micron measured on five equal distance points from each other in the biopsy and averaged (epithelial thickness may vary from area to area within the biopsy) | Cell layers (µm) |
| White cell infiltrate | Presence and amount of subepithelial mixed leucocytic infiltrates | 1 = absent, 2 = mild, 3 = moderate, 4 = marked, 5 = exuberant |
| Granulation tissue | Approximate amount of new granulation tissue formation (dermis) | 0 = 0%, 0·5 < 10%, 1 = 10–30%, 2 = 31–50%, 3 = 51–70%, 4 = 71–90%, 5 > 90% |
| Tissue damage | Microscopic damage to epithelium and damage to blood vessels | 1 = absent, 2 = mild, 3 = moderate, 4 = marked, 5 = exuberant |
| Dressing residue | Dressing residue present in the wound and observations of foreign body giant cells | 1 = absent, 2 = mild, 3 = moderate, 4 = marked, 5 = exuberant |
Clinical observations
Representative photos of wounds were taken throughout the study and observations were made during all assessment days 3, 4, 6. Wounds were recorded for any signs such as erythema (redness), reinjury, clinical signs of infection and oedema. In addition, material adherence to both wound and normal skin was assessed as described below:
Material adherence to normal skin and ability to stay in place.
Material adherence to wound bed (degree to which dressing is attached to wound bed causing reinjury).
Scab formation or crust formation.
Erythema (redness)—indicative of the amount of inflammation present.
Infection (clinical observation: oedema/purulent exudate).
Score for (i) and (ii) is as follows: 1 = completely adherent, 2 = mostly adherent, 3 = moderately adherent, 4 = slightly adherent and 5 = none adherent. Score for (iii), (iv) and (v) is as follows: 1 = absent, 2 = mild, 3 = moderate, 4 = marked and 5 = exuberant.
Statistical analysis
The data from three pigs were combined, and a total of nine samples from each treatment group at each time point were analysed together. A one‐way analysis of variance (ANOVA) was used for statistical analysis. A P value of less than 0·05 was considered significant.
Results
Clinical observations
Adherence of the primary dressing to the surrounding skin (ability to remain in situ)
There was no variation between the different primary dressings in terms of adhesion to the normal surrounding skin on days 4 and 6 with all showing a non‐adherent characteristic. However, at day 3 the majority of the one‐sided soft silicone (OSS) squares had some adherence to the surrounding skin.
Adherence of the primary dressing to the wound bed
On day 3, the soft silicone (SS), OSS, flexible polyester mesh with lipido‐colloid (FPLC), polyester mesh with soft silicone (PSS) and polyester mesh with neutral triglycerides (PNT) dressings were all significantly less adhered (P < 0·05) to the wound bed than cellulose acetate mesh with soft silicone (CASS) dressing (see Figure 1). The SS dressing displayed the lowest adhesion overall with all the materials showing no attachment to wound bed at day 3. The PSS dressing was the next best at resisting attachment, followed closely by OSS and PNT dressings. The tendency of the FPLC dressing to bind with the wound bed was noticeably higher.
Figure 1.
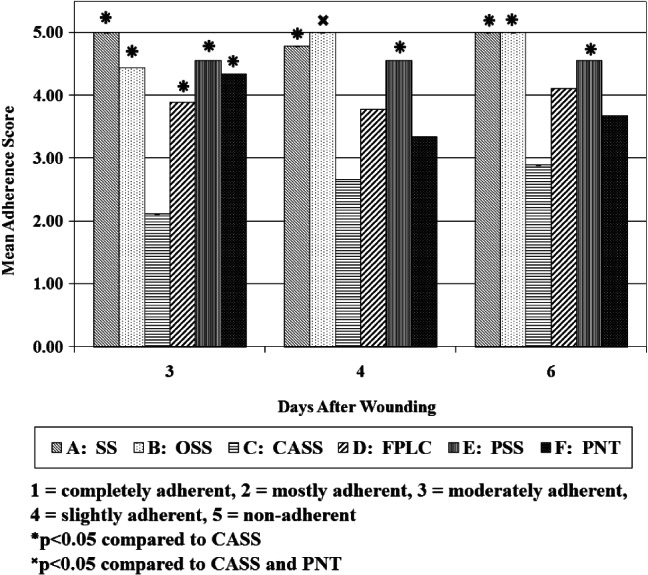
Adherence of primary dressing to the wound bed.
The degree of dressing adhesion to the wound bed decreased significantly (P < 0·05) over the following days for OSS. Adherence for the SS dressing, on the other hand, increased slightly. For the PSS dressing, qualitative observations remained unchanged from day 3. Both the SS and PSS dressings continued to be significantly less adhesive than the CASS dressing (P < 0·05). The number of FPLC and PNT dressings moderately attached was much higher on day 4, and was no longer superior to CASS dressing; adherence decreased slightly in the latter group at day 4, but was still noticeably more than each of the other dressings.
Adherence decreased or remained the same for all treatment groups between days 4 and 6. Both the SS and OSS dressings displayed total non‐adhesion at day 6. Almost all the individual dressings from the CASS group were graded as mostly adherent at day 3 with little decrease in adherence over the remainder of the assessment period with the dressings causing slight reinjury (see Figure 2A,B).
Figure 2.
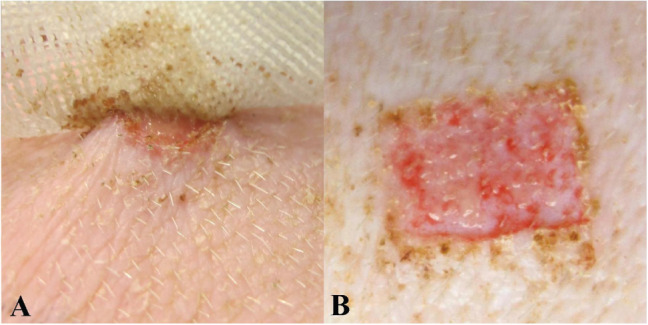
Adherence of cellulose acetate mesh with soft silicone (CASS) dressing. (A) Dressing attachment to wound bed. (B) Punctuate bleeding due to reinjury.
Adherence of the secondary dressing to the surrounding skin
The degree of adherence of the secondary dressings to the surrounding skin varied only slightly between treatment groups at the day 3 assessment. The general ability of these dressings to stick to the skin was poor, which may have been due to the amount of material that had contact to normal surrounding skin (only one quarter of an inch was in contact with skin). On day 3, secondary dressings covering the OSS dressings were the ones most securely attached to normal skin. The SS and CASS dressings were the only other treatment groups in which the majority of the secondary dressings displayed any adhesive characteristics on day 3. No differences were seen in the secondary dressing's adherence on days 4 and 6 among any of the treatment groups.
Adherence of the secondary dressing to the primary dressing
The trends relating to the adhesion of the secondary dressings to the primary dressings were reasonably similar throughout the three assessment days. Those covering the PNT dressing showed no signs of adhesion on each of the assessment days (see Figure 3). This was also the case for the FPLC dressings with the exception of day 3; some remained slightly attached to their coupled primary material on this assessment day, making the degree of adhesion statistically significant (P < 0·05) compared with the other two assessment days. All the secondary dressings covering the SS primary dressings had slight adherence on each assessment day, thus characterising the treatment group as significantly distinct from the two aforementioned dressings (P < 0·05). The majority of the dressing pairs in the CASS group had similar adherent qualities to the SS dressing. Day 3 assessment of the OSS dressing characterised all as moderately adhered to the secondary dressings. However, there was some fluctuation in the degree of cohesion between the OSS and secondary dressings throughout the course of the study. This fluctuation in cohesion was considered significant (P < 0·05) between assessment days 3 and 4. The PSS dressings were more attached to the secondary dressings when compared with all other dressings on days 3 and 4 of assessment with a 95% certainty confirmed by ANOVA. By day 6, all the individual pieces had become only moderately stuck to their secondary counterpart. The treatment group remained significantly distinct from each of the others with the exception of OSS (P < 0·05).
Figure 3.
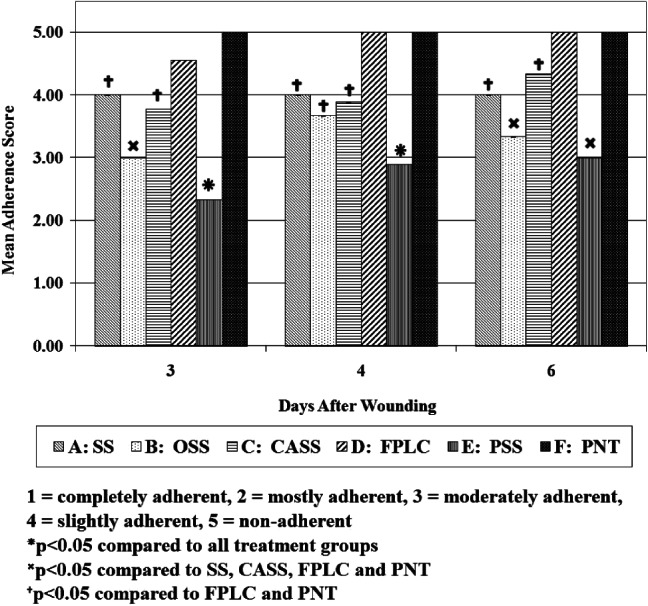
Adherence of the secondary dressing to the primary dressing.
Scab formation
Assessment on day 3 indicated that the wounds covered by the CASS dressings produced the least amount of scab (graded from mild to moderate) (see Figure 4). Wounds treated with each of the other dressings had mild to moderate scabs although much less than untreated air‐exposed wounds. On day 4 of assessment, wounds treated with SS, OSS and FPLC demonstrated increased scab formation, with marked scab formation to wounds treated with SS and OSS by day 6. Scab formation increased marginally in those wounds treated with CASS over the 6‐day period. Wounds treated with the PSS dressing showed a steady increase in scab formation over the treatment period, whereas those treated with PNT remained significantly lower in terms of scab formation than the untreated air‐exposed wounds.
Figure 4.
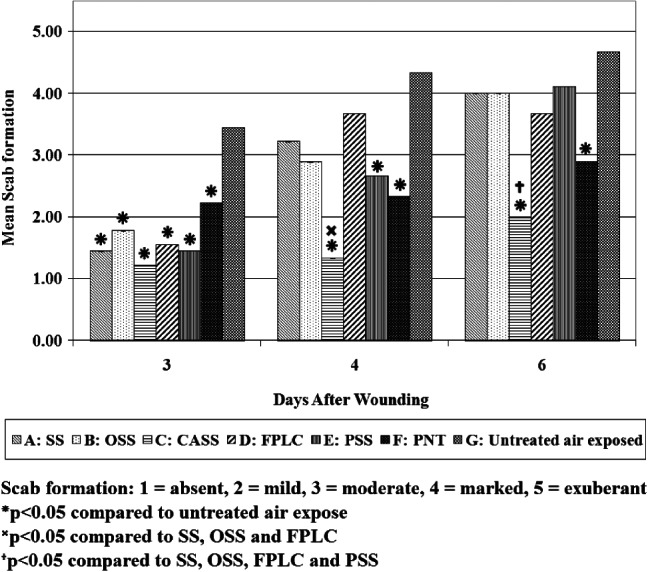
Scab formation of wounds treated with each of the primary test dressings.
Erythema and infection
No signs of erythema, purulent exudate or swelling were observed during the entire study in any of the three animals.
Dressing absorption
The average degree of wound fluid absorption into the secondary dressing was assessed and measurements indicated that the PNT dressings allowed the largest amount of wound exudate to pass into the secondary dressings between days 0 and 1. The average change in mass found in these dressings was approximately 167·78 mg of fluid. The FPLC dressings had the second highest level of fluid loss, with a mean 135·22 mg absorbed by the secondary dressings. The SS, OSS, CASS and PSS dressings were substantially better at fluid retention between these days compared with the PNT dressings (P < 0·05). PSS was the overall best, allowing only 56·51 mg of exudate to pass into the secondary dressings, followed by SS with 61·30 mg. OSS and CASS were the third and fourth least permeable; the secondary dressings covering the former brand contained an average of 70·85 mg of fluid, whereas those covering the latter held 71·07 mg.
The overall amount of wound fluid in most of the secondary dressings was found to be much higher between days 1 and 2. Furthermore, the correlation between dressing type and fluid retention was quite different from what was seen on assessment day 1. The CASS dressing was the least effective of all; its secondary dressings contained a mean 170·7 mg of exudates. PNT, the most porous of all the dressings between days 0 and 1, leaked the second largest amount of 144·52 mg. PSS, FPLC and SS were slightly better, losing 138·04, 129·15 and 128·93 mg, respectively. The secondary dressings covering the OSS absorbed the least wound fluid of all six treatments on day 2. With an average 100·33 mg in each, this group had the best exudate retention at day 2. However, there were no statistically significant differences between any of the materials on this day.
Histological analysis
The histological data from the three pigs were combined and analysed together, providing a total of nine samples from each treatment group at each assessment point. A one‐way ANOVA followed by a Student's t‐test were used for statistical analysis. A P value of less than 0·05 was considered significant.
Percentage of reepithelialisation
The percentage of reepithelialisation represents the degree of the wound area covered by newly formed epithelium or the epidermis with one or more layers of keratinocytes, which is a useful index for the speed of keratinocyte migration and the first step of reepithelialisation.
Generally, the wounds treated with SS, OSS, CASS, FPLC, PSS and PNT exhibited dramatically faster rate of reepithelialisation than those left untreated and exposed to air at all three time points (P < 0·001 for all dressings versus untreated air‐exposed wounds) (see Figure 5). This was especially observed in the earlier stage of healing, with reepithelialisation over 60% at day 3 and completely (or almost) reepithelialised at day 6. SS, OSS and CASS performed slightly better than FPLC, PSS and PNT with significant differences identified at day 4 between OSS and FPLC and PNT (both P ≤ 0·01), and between CASS and FPLC and PNT (both P < 0·05).
Figure 5.
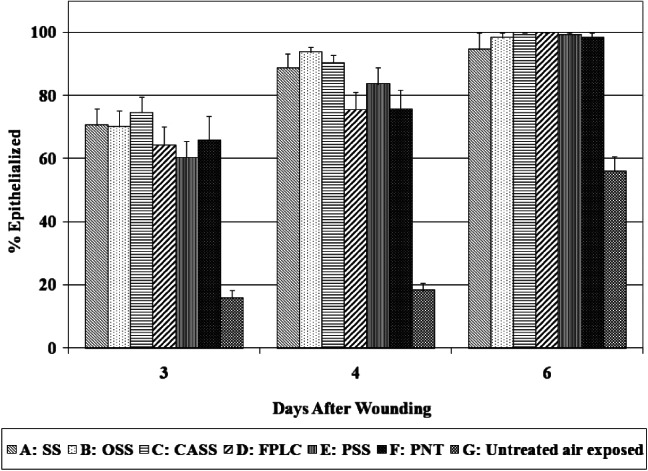
Percentage of reepithelialised wound area.
Epithelial thickness
The epithelial thickness is a measure of the average thickness of five points of newly formed epithelium. It reflects the process of keratinocyte proliferation, differentiation and epidermal maturation. In the early stage of wound healing, the thickness of newly formed epidermis gradually increases as keratinocytes proliferate rapidly. In the late stage of epidermal maturation, keratinocytes become flattened and the epidermal thickness gradually decreases to the original level.
Wounds treated with SS, OSS, CASS, FPLC, PSS and PNT produced thicker epidermis than untreated air‐exposed wounds. However, no difference was observed between any other groups (see Figure 6).
Figure 6.
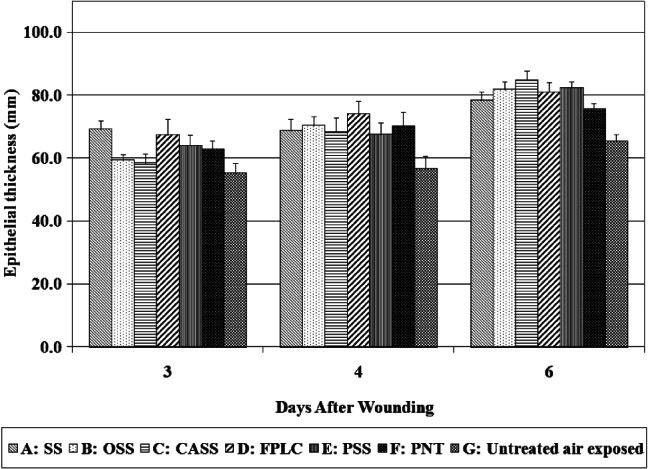
Epithelial thickness measurements.
White cell infiltration (WCI)
WCI is used to assess the degree of inflammation, which could be a normal process of wound repair, a response to microbial infection or the tissue reaction to foreign materials in the wound. No significant differences were seen among all groups in WCI at each assessment day (see Figure 7).
Figure 7.
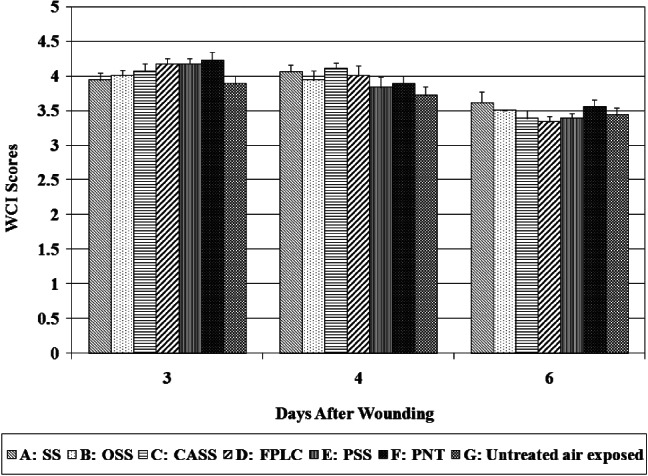
White cell infiltrate scores.
Granulation tissue formation
The dermal reconstitution begins in about 3–4 days after injury with the hallmark of granulation tissue formation, which includes new blood vessel formation (angiogenesis) and the accumulation of fibroblasts and collagen extracellular matrices.
In general, normal patterns of granulation tissue formation were observed in those wounds treated with SS, OSS, CASS, FPLC, PSS and PNT. Granulation tissue formation was observed as early as day 3 with these treatments with complete development in the wound bed at day 6. Strikingly, no obvious granulation tissue was seen in the untreated air‐exposed wounds at days 3 and 4, and with only 10–20% granulation tissue formation at day 6.
Tissue damage
No visible microscopic damage to the epithelium or blood vessels was noted with any of the treatment groups.
Dressing residues
Small pieces of dressing residues surrounded by foreign body giant cells were noticed within two of the wounds treated with PSS (day 3: two animals). Similarly, it was noted that wounds treated with CASS and SS dressings also contained small pieces of the dressing (day 6: one wound, one animal).
Discussion
Identifying a dressing that allows for optimal adhesion to the periwound area without disturbing the wound bed is a fundamental part of wound management. The ideal dressing should maintain stability over the wound area while enabling easy and pain‐free removal during dressing changes, creating an undisturbed healing environment. Adhesive‐backed wound dressings may cause rewounding of otherwise normally healing wounds and cause pain 11, 12. Indeed, repeated application and removal of dressings can cause mechanical reinjury to the wound bed, skin irritation, blistering and epidermal skin stripping 2, 13, 14, which collectively results in further disturbance to the wound healing process.
In this study, the findings demonstrate that overall the SS and OSS dressings performed very favourably in comparison to all the other dressings in terms of adhesion, wound exudate containment and speed of healing. Interestingly, although we showed clinical signs of tissue reinjury during removal of some of the dressings, we could neither detect any microscopic damage from dressing adherence to newly forming tissue nor correlate this with the amount of material residue found within the wounds. The lack of the microscopic damage could be due to the limit of tissue biopsy or section where the clinical reinjury was not present.
Several clinical evaluations and case studies encouraged the use of SS dressings for a variety of wound types. For instance, Thomas 7 highlighted the capacity of SS to remain in place and prevent friction damage. This feature may also help to reduce the required number of dressing changes, thus minimising treatment costs. Furthermore, Burton 15 noted that SS was easy to remove from acute traumatic and surgical wounds. In the clinical setting, SS has also proved to be superior to other dressings in terms of stability, given the allowance of exudate to escape without reducing its adhesive properties on dry surfaces, while dressing changes can be conducted without disrupting regenerating skin owing to its non‐stick nature towards the moist skin 16. In a randomised prospective study by Dahlstrøm 17, SS was found to be the preferred dressing because it adhered less to the wound bed, caused less pain and bleeding and the time required to change the dressing was shorter. Similarly, OSS has been rated well in terms of providing an optimal wound environment. In their case study series, Cooper et al. 18 reported that OSS conformed well to the shape of the wound, helped to keep topical agents in place over the wound bed and could be left in place for several days.
In this study, the OSS dressing was superior in terms of its ability to adhere to the normal skin surrounding the wound sites. In fact, it was the only material to demonstrate any adherent qualities to unwounded skin on day 3. It is important to note that occlusion of the wound to the surrounding environment via an appropriate dressing helps to promote optimal healing by acting as a physical barrier from invasion by pathogenic bacteria (including resistant strains) and environmental toxins 19, 20, 21, 22. Furthermore, over the first 24 hours of wound healing, neutrophil antimicrobial activity within the wound fluid may help to destroy potentially harmful bacteria 21; the ability of the SS dressing to retain wound fluid without overt leakage into the secondary dressing and drying of the wound could help to maintain an optimal moist environment over the wound area.
OSS also produced relatively good results with regard to the assessment of its adhesion to the secondary dressing. Strong adhesion was an especially desirable characteristic in this experiment because of the limited adhesive qualities possessed by the primary dressings themselves. The PSS dressing was the most adherent material with the secondary dressing throughout the study, while the OSS dressing was the second most adherent at each assessment point. With regard to healing, wounds treated with both SS and OSS as well as the CASS dressings showed the highest percentage of epithelialisation.
Overall, the SS and OSS dressings performed extremely well in this wound model. The wound healing response requires each phase of healing to be undisturbed in order to maximise wound closure in a timely manner. The reduction of further trauma to both the wound area and the surrounding skin is likely to aid the healing process. The use of SS wound dressings has been demonstrated to be an effective way to minimise tissue disturbance, with both SS and OSS demonstrating an ability to adhere to the periwound skin without adhering to the wound bed thus preventing reinjury. Both dressings also displayed an inherent ability to retain wound fluid.
Acknowledgement
This study was supported in part by a grant from Molnlycke Healthcare.
References
- 1. Achterberg V, Mayer‐Ingold W. Hydroactive dressings and serum proteins: an in vitro study. J Wound Care 1996;5:79–82. [PubMed] [Google Scholar]
- 2. Cutting KF. Impact of adhesive surgical tape and wound dressings on the skin, with reference to skin stripping. J Wound Care 2008;17:157–62. [DOI] [PubMed] [Google Scholar]
- 3. Gray M, Weir D. Prevention and treatment of moisture‐associated skin damage (maceration) in the periwound skin. J Wound Ostomy Continence Nurs 2007;34:153–7. [DOI] [PubMed] [Google Scholar]
- 4. Rogers AA, Walmsley RS, Rippon MG, Bowler PG. Adsorption of serum‐derived proteins by primary dressings: implications for dressing adhesion to wounds. J Wound Care 1999;8:403–6. [DOI] [PubMed] [Google Scholar]
- 5. Wilkinson LJ, White RJ, Chipman JK. Silver and nanoparticles of silver in wound dressings: a review of efficacy and safety. J Wound Care 2011;20:543–9. [DOI] [PubMed] [Google Scholar]
- 6. Meaume S, Van De Looverbosch D, Heyman H, Romanelli M, Ciangherotti A, Charpin S. A study to compare a new self‐adherent soft silicone dressing with a self‐adherent polymer dressing in stage II pressure ulcers. Ostomy Wound Manage 2003;49:44–51. [PubMed] [Google Scholar]
- 7. Thomas S. Atraumatic dressings. World Wide Wounds 2003. URL http://www.worldwidewounds.com/2003/january/Thomas/Atraumatic‐Dressings.html [accessed on January 2003].
- 8. Seaman S. Dressing selection in chronic wound management. J Am Podiatr Med Assoc 2002;92:24–33. [DOI] [PubMed] [Google Scholar]
- 9. White R. Evidence for atraumatic soft silicone wound dressing use. Wounds UK 2005;1:104–9. [Google Scholar]
- 10. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76. [DOI] [PubMed] [Google Scholar]
- 11. Hollinworth H, Collier M. Nurses' views about pain and trauma at dressing changes: results of a national survey. J Wound Care 2000;9:369–73. [DOI] [PubMed] [Google Scholar]
- 12. Zitelli J. Wound healing for the clinician. Adv Dermatol 1987;2:243–67. [PubMed] [Google Scholar]
- 13. Waring M, Butcher M. An investigation into the conformability of wound dressings. Wounds UK 2011;7:14–24. [Google Scholar]
- 14. Waring M, Bielfeldt S, Mätzold K, Wilhelm KP, Butcher M. An evaluation of the skin stripping of wound dressing adhesives. J Wound Care 2011;20:412–22. [DOI] [PubMed] [Google Scholar]
- 15. Burton F. An evaluation of non‐adherent wound‐contact layers for acute traumatic and surgical wounds. J Wound Care 2004;13:371–3. [DOI] [PubMed] [Google Scholar]
- 16. Newman JP, Fitzgerald P, Koch J. Review of closed dressings after laser resurfacing. Dermatol Surg 2000;26:562–71. [DOI] [PubMed] [Google Scholar]
- 17. Dahlstrøm KK. A new silicone rubber dressing used as a temporary dressing before delayed split skin grafting. A prospective randomised study. Scand J Plast Reconstr Surg Hand Surg 1995;29:325–7. [DOI] [PubMed] [Google Scholar]
- 18. Cooper P, Gray D, Russell F, Stringfellow S. Case reports using Mepitel One wound contact dressing with Safetac technology. Wounds UK 2010;11(Suppl):1–11. [Google Scholar]
- 19. Helfman T, Ovington L, Falanga V. Occlusive dressings and wound healing. Clin Dermatol 1994;12:121–7. [DOI] [PubMed] [Google Scholar]
- 20. Hutchinson JJ, McGuckin M. Occlusive dressings: a microbiological and clinical review. Am J Infect Control 1990;18:257–68. [DOI] [PubMed] [Google Scholar]
- 21. Mertz PM, Marshall DA, Eaglstein WH. Occlusive wound dressings to prevent bacterial invasion and wound infection. J Am Acad Dermatol 1985;12:662–8. [DOI] [PubMed] [Google Scholar]
- 22. Eaglstein WH. Moist wound healing with occlusive dressings: a clinical focus. Dermatol Surg 2001;27:175–82. [DOI] [PubMed] [Google Scholar]


