Abstract
The prevention of hospital acquired pressure ulcers in critically ill patients remains a significant clinical challenge. The aim of this trial was to investigate the effectiveness of multi‐layered soft silicone foam dressings in preventing intensive care unit (ICU) pressure ulcers when applied in the emergency department to 440 trauma and critically ill patients. Intervention group patients (n = 219) had Mepilex® Border Sacrum and Mepilex® Heel dressings applied in the emergency department and maintained throughout their ICU stay. Results revealed that there were significantly fewer patients with pressure ulcers in the intervention group compared to the control group (5 versus 20, P = 0·001). This represented a 10% difference in incidence between the groups (3·1% versus 13·1%) and a number needed to treat of ten patients to prevent one pressure ulcer. Overall there were fewer sacral (2 versus 8, P = 0·05) and heel pressure ulcers (5 versus 19, P = 0·002) and pressure injuries overall (7 versus 27, P = 0·002) in interventions than in controls. The time to injury survival analysis indicated that intervention group patients had a hazard ratio of 0·19 (P = 0·002) compared to control group patients. We conclude that multi‐layered soft silicone foam dressings are effective in preventing pressure ulcers in critically ill patients when applied in the emergency department prior to ICU transfer.
Keywords: Pressure ulcers, Prevention, Silicone foam dressings
Introduction
The prevention of hospital acquired pressure ulcers in critically ill patients while in the intensive care unit (ICU) remains a persistent and significant clinical challenge. ICU pressure ulcer incidence rates have been reported in the range of 3·3–53·4% depending on type of ICU and show large variation internationally because of study methodology 1, 2, 3, 4. In the case of patients admitted through the emergency department (ED) and subsequently transferred to the ICU, additional factors which may contribute to pressure injuries are often involved. These factors include time spent in the ED and medical imaging on surfaces with limited pressure redistribution capacity 5, 6, and for trauma patients it includes potentially long periods in the operating room (OR) prior to ICU transfer 7, 8, 9, 10, 11. The consequence of these factors and their interaction with the patient's physiological status, such as advanced age, nutritional status and comorbidities 5, degree and duration of hypotension and the use of vasopressor drugs may result in a situation where the pressure ulcers that are subsequently identified in ICU may have had their origin in the pre‐ICU admission period.
The mechanisms of pressure injury involve the mechanical effects of pressure, shear, friction and moisture at the skin/surface interface 12. These forces are transmitted throughout the tissues and contribute to tissue ischaemia 13, 14, cell deformation and consequent cell destruction 15. In the clinical ED/ICU setting the forces implicated in the development of pressure ulcers are often occurring concurrently. For example, an unconscious, mechanically ventilated, febrile and diaphoretic ICU patient positioned with the head of the bed elevated. There is direct pressure exerted on the patient's heels as well as pressure and shear forces to the sacrum as the patient's body weight causes increased loading to the pelvic region including the sacrum as well as sliding forces tangential to the support surface. The transmission of those forces is also modified by the degree of friction at the skin/surface interface as well as by the level of moisture present at that interface. To date studies of these forces have occurred in isolation owing to the complexity of the interactions and the difficulty of accurate and valid measurement in vivo.
The use of wound dressings as prophylaxis for pressure ulceration has been under investigation for some time. Dressings of various constructions have been thought to potentially be able to reduce pressure, friction and shear and to effectively manage moisture levels. Laboratory based studies exploring the possible protective mechanisms of dressings have been focussed on mechanical loading, interface pressures and pressure redistribution 16, 17, 18, 19, 20. These studies have used a range of methodologies including the use of volunteer subjects wearing dressings in bed or while walking as well as exploring animal models with subcutaneous pressure sensors. While encouraging, these studies are difficult to compare because of the differences in dressing materials that were investigated and the study methodologies used.
The question of how a dressing may reduce or modify friction and shear forces has been explored by a number of investigators 18, 20, 21. These studies have demonstrated shear force reduction but used dressings of differing construction in both human and animal models. Generally one can say that some dressings do appear to reduce both interface pressure and shear forces. Dressings have been shown to have the capacity to modify the microclimate at the skin/dressing interface and thereby influence pressure ulcer risk 18, 22. More recently Geffen 23 reported mathematical modelling of the relationship of skin moisture levels under a dressing and pressure ulceration.
In clinical studies, Brindle 24 demonstrated the benefits of using prophylactic multi‐layered soft silicone bordered foam sacral dressings in a multi‐intervention surgical ICU cohort study to significantly reduce pressure ulceration. More recently, Brindle and Wegelin 25 used similar silicone dressings with cardiac surgical patients to once again reduce the incidence of pressure ulcers. Similarly, others have also demonstrated clinically important reductions in incidence rates in ICU patients when using silicone dressings to reduce pressure friction and shear 26, 27. In the ED setting, Cubit 28 reported the positive benefit of multi‐layered soft silicone bordered foam dressings used as sacral pressure ulcer prophylaxis in a small study with historical controls.
A review of published clinical studies reveal a lack of research investigating the effectiveness of dressings in reducing sacral and heel pressure ulcer formation in critically ill patients by intervening as soon as they arrive in the ED and then following them through their ICU stay. The aim of this study was to explore the question of the prophylactic effectiveness of multi‐layered soft silicone foam dressings in pressure ulcer prevention by conducting a prospective randomised controlled trial of critically ill/trauma patients admitted to an Australian university teaching hospital.
Methods
The study was designed as a prospective open‐label randomised controlled trial of 440 trauma and critically ill patients who were admitted to the ED and subsequently transferred to the ICU. Patients who met the study inclusion criteria were randomly allocated to either the control group (n = 221) that received usual pressure ulcer prevention strategies or the trial group (n = 219) that received usual care plus they had a Mepilex® Border Sacrum (Mölnlycke Healthcare AB, Göteborg, Sweden) dressing applied to their sacrum and Mepilex® Heel (Mölnlycke Healthcare AB) dressing applied to each heel in the ED. These dressings are constructed of multiple layers (Mepilex® Border Sacrum has five layers and Mepilex® Heel has three layers). However, in all cases only one dressing was used at each anatomical site at any one time. All patients were examined every 24 hours by a member of the study team while in ICU to identify the development of any hospital acquired pressure ulcers to their sacrum or heels. Patients in the intervention group had their sacral and heel dressings changed every three days or more frequently if they became soiled or dislodged.
Aim
To determine the effectiveness of multi‐layered soft silicone foam dressings in preventing sacral and heel pressure ulcer development in trauma/critically ill ICU patients by applying the dressings on admission to the ED.
Hypothesis
Patients treated with multi‐layered soft silicone foam dressings will have a lower incidence rate of hospital acquired sacral and heel pressure ulcer development than patients receiving standard care.
Primary endpoint
Incidence rates of hospital acquired pressure ulcers in ICU expressed as the total number of pressure ulcers developed in both groups.
Sample and setting
The study was conducted at the Royal Melbourne Hospital (RMH), Australia which is a large university teaching hospital and is part of a multi‐site health care group, Melbourne Health. RMH is one of two adult Trauma Centres in Melbourne. RMH ED has over 60 000 presentations per annum of which approximately 40% are admitted. The ICU is a mixed medical and surgical 24 bed, level three facility with 2000 admissions per annum.
Potential study subjects comprised all major trauma and critically ill patients admitted to ED and that were to be transferred to ICU. Data collection commenced in April 2011 and was completed in December 2012. The study was approved by the Melbourne Health Human Research Ethics Committee in 2010 and registered as a clinical trial with the Australian Therapeutic Goods Administration CTN Scheme and with Clinical Trials.gov (NCT01356459).
Inclusion criteria
ED and ICU admission for critical illness and/or major trauma
Over 18 years of age
Exclusion criteria
Suspected or actual spinal injury precluding the patient being turned
Pre‐existing sacral or heel pressure ulcer
Trauma to sacrum and/or heels
Randomisation
Patients were randomised in the ED to either the intervention group or to the control group by retrieving the next envelope in a pre‐prepared series of envelopes that had been randomised using a computer generated set of random numbers to determine group allocation. The randomisation of participants was undertaken by an ED research nurse when the patients were admitted to ED and following screening to determine if they met the inclusion criteria.
The following procedure was used by the ED research nurse to enrol each participant into the trial:
Potential participant admitted to ED trauma/resuscitation
Determines if patient meets study inclusion criteria
Determines group allocation by retrieving randomisation envelope
If randomised to trial group:
Applies Mepilex® Border Sacrum dressings to sacrum and Mepilex® Heel dressing to both heels
Records time of dressing application
All patients
All patients in the study were cared for in ICU on a Hill‐Rom Versa Care low air loss bed (Hill‐Rom, Batesville, IN) for the duration of their ICU treatment and all study patients received standard RMH ICU pressure ulcer prevention strategies which included ongoing Braden pressure ulcer risk assessment and regular repositioning and skin care.
Intervention group
Intervention group patients who met the inclusion criteria had one Mepilex® Border Sacrum dressing applied to their sacrum and one Mepilex® Heel dressing to each heel and retained with Tubifast® (Mölnlycke Healthcare AB) elastic tubular bandages on admission to ED. In the case of trauma patients requiring emergency surgery, the dressings that were applied in ED were left in place during the duration of the OR procedure. Similarly for patients that required medical imaging studies, dressings were left in place until transfer to ICU. Dressings were maintained on the sacrum and heels throughout the patient's ICU stay and changed every three days unless they became soiled or dislodged.
Measurement and data collection
Initial data were collected for each patient on arrival to the ED. These data included reason for admission, comorbidity, physiological variables, Australasian Triage Scale score 29 and time commenced on mechanical ventilation. The hospital electronic patient management system was used to retrieve data on length of stay in ED, OR and ICU, expressed in hours. In the ICU, all patients had a Braden score 30 calculated and updated daily, APACHE II 31 score calculated and all drugs recorded and all patients were reviewed every 24‐hours for the duration of their ICU stay by a member of the research team to determine if a hospital acquired pressure ulcer had developed. In the intervention group this involved partially peeling back the dressings so that the skin could be visualised and assessed for pressure related damage and then reapplying the dressing.
Pressure ulcers
Pressure related injuries were defined according to the Australian Wound Management Association (AWMA): Clinical practice guidelines for the prediction, prevention and management of pressure ulcers 32. Any pressure ulcer that developed during the course of the study was staged as per the AWMA four point staging system. All members of the research team underwent inter‐rater reliability testing in September 2010 prior to data collection to ensure consistency in pressure ulcer identification and staging.
Sample size
We calculated that to detect a decrease in the ICU pressure ulcer incidence rate of 3·5% (from 4% to 0·5%) in the intervention group with power set at 80% and alpha of 0·05 would require a total of 220 patients per group.
Analysis
The analysis was based on intention to treat (ITT) 33 where all patients randomised to the intervention were analysed regardless of protocol violations. The development of pressure ulcers per group and pressure ulcers by anatomical site per group were compared using Fishers Exact test. A survival analysis was used to determine the difference in pressure ulcer incidence development rates per group and time to provide a hazard ratio (HR) between the groups.
Results
Patient enrolment, allocation, follow‐up and analysis flow through the trial are presented in Figure 1 according to the CONSORT protocol.
Figure 1.
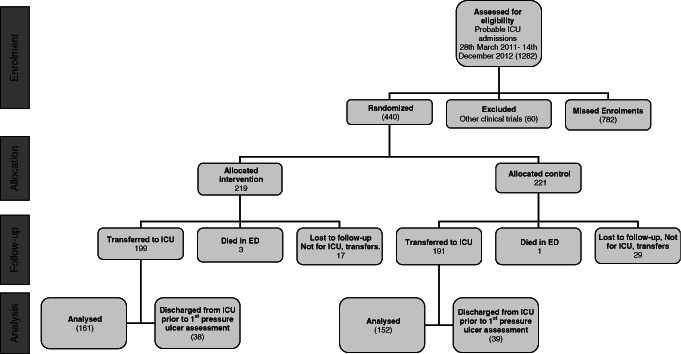
CONSORT diagram of patient flow through the study.
The characteristics of the 440 patients presented in Table 1 reveal that the groups were comparable on major physiological and demographic characteristics on admission to ED.
Table 1.
Patient demographics
| Intervention (n = 219) | Control (n = 221) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Median | (IQR) | Mean | (SD) | Median | (IQR) | |
| Age years | 54 | (20·8) | 53 | (37–72) | 56 | (20·5) | 58 | (38–74) |
| Gender M/F | 126/89 | 132/82 | ||||||
| Physiological variables | ||||||||
| MAP mmHg | 94 | (23·6) | 90 | (78–107) | 93 | (22·7) | 91 | (79–107) |
| Temperature (°C) | 36·1 | (1·6) | 36·2 | (1·6) | ||||
| Heart rate | 99 | (26·4) | 95 | (26·6) | ||||
| FiO2 | 0·97 | 0·98 | ||||||
| Braden score | 12 | (4·2) | 10 | (9–14) | 12 | (3·9) | 11 | (9–15) |
| ATS | 2 | (0·7) | 1 | (1–2) | 2 | (0·8) | 1 | (1–2) |
| Apache II | 19 | 18 | (13–25) | 19·5 | 19 | (13–25) | ||
| ED admission classification | ||||||||
| Critical illness | 141 | 147 | ||||||
| Major trauma | 69 | 65 | ||||||
| Length of stay (hours) | ||||||||
| ED | 6 | (4) | 4 | (3–7) | 6 | (4) | 5 | (3–8) |
| OR | 4 | (2) | 3 | (2–4) | 5 | (4) | 4 | (2–7) |
| ICU | 91 | (112) | 49 | (29–98) | 86 | (101) | 47 | (25–95) |
| Mechanical ventilation | ||||||||
| Yes | No | Yes | No | |||||
| ED | 156 | 54 | 140 | 67 | ||||
| ICU | 155 | 41 | 153 | 39 | ||||
| Cases to OR from ED | ||||||||
| Total cases (No.) | 27 | 20 | ||||||
APACHE II, Acute Physiology & Chronic Health Evaluation II; ATS, Australian Triage Score, 5 tier system of categorising patients urgency (1 = treat immediately, 2 = treat within 10 minutes, 3 = treat within 30 minutes, 4 = treat within 1 hour, 5 = treat within 2 hours); ED, emergency department; FiO2, fraction of inhaled oxygen expressed as a decimal value; ICU, intensive care unit; IQR, interquartile range; MAP, mean arterial pressure; OR, operating room.
Table 2 presents the data on pressure ulcer development from three perspectives: Number of patients who developed a pressure ulcer, the incidence rate per group and the number of pressure ulcers developed by anatomical site. The experimental event rate (EER) was 3·1% whereas the control event rate (CER) was 13·1%; therefore the absolute risk reduction (ARR) was 10% which provides the number needed to treat (NNT) value of 10. There were no adverse events related to the dressings used throughout the study.
Table 2.
Pressure ulcer development by group
| Intervention (n = 161) | Control (n = 152) | P | |
|---|---|---|---|
| Cases | |||
| Developed PU | 5 | 20 | 0·001 |
| Incidence (%) | 3·1 | 13·1 | |
| Anatomical site | |||
| Developed PU | 7 | 27 | 0·002 |
| Sacral PU | 2 | 8 | 0·05 |
| Heel PU | 5 | 19 | 0·002 |
PU, pressure ulcer.
Figure 2 reveals the rate at which each group developed pressure ulcers expressed in days. The Cox regression analysis resulted in a HR for developing a pressure ulcer in the intervention group of 0·198 (95% CI 0·065–0·555) (P = 0·002) compared to the control group.
Figure 2.
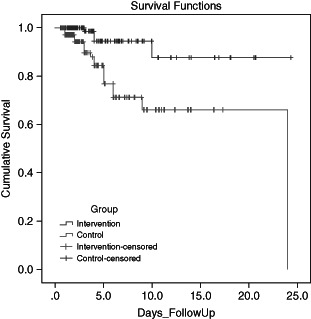
Kaplan–Meier survival analysis.
Discussion
The prevention of hospital acquired pressure ulcers in critically ill ICU patients is a complex on‐going task for clinicians involving risk assessment, repositioning, skin care and the use of pressure redistribution surfaces. These and other interventions are carried out in the context of a critically ill patient. Numerous interventions have been examined and implemented to prevent these wounds from developing yet there remains a consistent incidence of pressure ulceration in these patients that has been resistant to complete elimination. The use of dressings as a prophylactic to pressure ulceration has been sporadically investigated over many years in a number of laboratory studies and a small number of clinical trials 24, 25, 26, 27. These efforts have revealed some positive indications regarding the potential protective effectiveness of dressings.
Our results reveal that the 440 patients enrolled in the study were comparable in terms of their demography, physiology and illness profiles (Table 1). Our study population was younger than that reported by Cubit et al. 28 in their ED pressure ulcer study and we believe that this was because of our ED being a Trauma Centre and therefore having a State‐wide patient referral area, the consequence is that we take a potentially broader and younger population group. Our study was originally powered to detect a 3·5% effect size between the groups and therefore required a total of 440 patients. Owing to the number of patients that were discharged from ICU prior to their first pressure ulcer assessment (Figure 1), we recalculated the power of the study based on the number of patients that were available for final analysis and the detected difference in pressure ulcer incidence between the groups and this recalculation confirmed that the study remained adequately powered.
Our finding shows that the intervention group had significantly less patients who developed a pressure ulcer in ICU (5 versus 20, P = 0·001). This represents a 10% lower incidence rate for the intervention group indicating that NNT is ten patients to prevent one pressure ulcer. This reduction is consistent with observational ICU studies 24, 26, 27 investigating the effectiveness of multi‐layered silicone dressings. In the current study we have also demonstrated that there were significant reductions in both sacral (2 versus 8, P = 0·05) and heel pressure ulceration (5 versus 19, P = 0·002) when compared to controls. Overall the number of pressure ulcers was significantly less in the dressing group than in the control group (7 versus 27, P = 0·002). The survival analysis confirmed that intervention group patients developed pressure ulcer at a significantly slower rate than controls (HR 0·19, P = 0·002).
We note that there were seven study protocol violations in the dressing group where sacral dressings were not always in place due to patient factors. One of these patients developed a stage II sacral pressure injury; during the time he did not have the dressing in place, however, because of the ITT analysis this patient was analysed with all other intervention group patients. The finding of the very large difference in the rate of heel ulceration between intervention group and control group patients is interesting when viewed in the light of the dressing products used on the heel and the sacrum. We used the Mepilex® Heel dressing which is a three layered soft silicone foam dressing retained on the heel by a Tubifast® tubular elastic bandage whereas the Mepilex® Border Sacrum dressing is a five layered soft silicone bordered foam dressing. One would expect that the greater number of layers in the sacral dressing may confer greater protection than a three layer dressing due to enhanced pressure redistribution and protection from shear and friction as well as microclimate control 21, 22, 23. However, this explanation may be inadequate due to the considerable anatomical differences between the sacrum and the heel from the perspectives of blood supply, tissue layer composition and densities as well as the differing topography of the underlying sacral and calcaneal bones; additionally the forces exerted on these sites would also be quantitatively and qualitatively different. This finding warrants further exploration in future studies. We also believe that the finding highlights the issue of dressing construction generally because our study is based on the use of wound dressings rather than a dressing specifically designed for pressure ulcer prevention.
Limitations
Our study is limited by the single site nature of the design. It was also not possible to blind data collectors to the nature of the treatment intervention. Our results can only be viewed in the context of the critically ill patient in the ED and ICU setting and cannot be generalised to other patient populations at this point. Furthermore, it is not possible for us to determine if the success of our intervention in reducing pressure ulceration incidence was solely because of the fact that we commenced the dressing use in ED. Future multi‐site studies could be designed that include this element within the analysis.
Conclusion
Our findings have demonstrated a statistical and clinically significant benefit for the application of multi‐layered soft silicone foam dressings for the prevention of sacral and heel pressure ulcers. When used in combination with thorough risk assessment and evidence‐based pressure ulcer prevention strategies, the intervention resulted in a 10% reduction in pressure ulcer incidence. This reduction represents preventing one patient developing a pressure ulcer for every ten patients treated with the dressings. The clinical applications of our results are that we can now delay or potentially eliminate hospital acquired pressure ulcers in critically ill patients by adding the use of multi‐layered soft silicone foam dressings to our preventative strategies as soon as the patient is admitted to the ED. As a consequence of the findings of this study, our hospital has now mandated the use of these dressings for all patients who are at high risk of pressure ulceration.
References
- 1. Cuddigan J. In: Pieper B, editor. The National Pressure Ulcer Advisory Panel (NPUAP) 2012. Pressure ulcers: prevalence, incidence and implications for the future. Washington, DC: NPUAP, 2012:47–56. [Google Scholar]
- 2. Shanin ES, Dassan T, Halfens RJD. Pressure ulcer incidence in intensive care patients: a literature review. Nurs Crit Care 2008;13:71–9. [DOI] [PubMed] [Google Scholar]
- 3. Shanin ES, Dassan T, Halfens RJD. Pressure ulcer prevention in intensive care patients: guidelines and practice. J Eval Clin Prac 2009;15:370–4. [DOI] [PubMed] [Google Scholar]
- 4. Jiricka MK, Ryan P, Cavahlo MV, Bukvika J. Pressure ulcer risk factors in an ICU population. Am J Crit Care 1995;4:361–7. [PubMed] [Google Scholar]
- 5. Dugaret E, Videau M‐N, Faure I, Gabinski C, Bourdel‐Marchasson I, Salles N. Prevalence and incidence rates of pressure ulcers in an emergency department. Int Wound J 2012. DOI: 10.1111/j.1742-481X.2012.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denbey A, Rowlands A. Stop them at the door: should a pressure ulcer prevention protocol be implemented in the emergency department? J Wound Ostomy Continence Nurs 2010;37:35–8. [DOI] [PubMed] [Google Scholar]
- 7. Schoonhoven L, Defloor T, Grypdonck MH. Incidence of pressure ulcers due to surgery. J Clin Nurs 2002;1:479–87. [DOI] [PubMed] [Google Scholar]
- 8. Lewicki LJ, Mion L, Splane KG, et al. Patient risk factors for pressure ulcers during cardiac surgery. AORN 1997;65:933–42. [DOI] [PubMed] [Google Scholar]
- 9. Bliss M, Simini B. When are the seeds of postoperative pressure sores sown? Often during surgery. Br J Med 1999;319:863–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ganos D, Siddiqui A. In: Pieper B, editor. The National Pressure Ulcer advisory Panel (NPUAP) 2012. Pressure ulcers: prevalence, incidence and implications for the future. Washington, DC: NPUAP, 2012:57–63. [Google Scholar]
- 11. Nixon J, Brown J, McElvenny D, Mason S, Bond S. Prognostic factors associated with pressure sore development in the immediate post‐operative period. Int J Nurs Stud 2000;37:655–63. [DOI] [PubMed] [Google Scholar]
- 12. Wounds International . Pressure ulcer prevention. Pressure, shear, friction and microclimate in context. London: Wounds International, 2010. [Google Scholar]
- 13. CollierM MZ. Etiology and risk factors. In: Romanelli M, Clark M, Cherry G, Colin D, Defloor T, editors. Science and practice of pressure ulcer management. London: Springer Verlag, 2006:27–36. [Google Scholar]
- 14. Mimura M, Ohura T, Takahashi M, Eng D, Kajiwara R, Eng B, Ohura N. Mechanisms leading to the development of pressure ulcers based on shear force and pressure during bed operation: Influence of body types, body positions, and knee positions. Wound Repair Regen 2009;17:789–96. [DOI] [PubMed] [Google Scholar]
- 15. Oomens CWJ, Loerakker S, Bader DL. The importance of internal strain as opposed to interface pressure in the prevention of pressure related deep tissue injury. J Tissue Viability 2009;19:35–42. [DOI] [PubMed] [Google Scholar]
- 16. Clark M. The effect of a pressure‐relieving wound dressing on the interface pressures applied to the trochanter. Decubitus 1990;3:43–6. [PubMed] [Google Scholar]
- 17. Torra i Bou JE, Rueda Lopez J, Camanes G, Herrero Narvaez E, Blanco Blanco J, Martinez‐Esparza EH, Aneas Alcantara J, Verdu Soriano J. Heel pressure ulcers. Comparative study between heel protective bandage and hydrocellular dressing with special form for the heel. Rev Enferm 2002;25:50–6. [PubMed] [Google Scholar]
- 18. Nakagami G, Sanada H, Konya C, Kitagawa A, Tadaka E, Matsuyama Y. Evaluation of a new pressure ulcer preventive dressing containing ceramide 2 with low frictional outer layer [corrected] [published erratum appears in J Adv Nurs 2007 Nov;60(3):357]. J Adv Nurs 2007;59:520–9. [DOI] [PubMed] [Google Scholar]
- 19. Imanishi K, Morita K, Matsuoka M, Hayashi H, Furukawa S, Terashita F, Moriya E, Kanesaki U, Kinukawa N, Nose Y, Moroi Y, Urabe K, Furue M. Prevention of postoperative pressure ulcers by a polyurethane film patch. J Dermatol 2006;33:236–7. [DOI] [PubMed] [Google Scholar]
- 20. Ohura T, Takahashi M, Ohura N. Influences of external forces (pressure and shear force) on superficial layer and subcutis of porcine skin and effects of dressing materials: Are dressing materials beneficial for reducing pressure and shear force in tissues? Wound Repair Regen 2008;16:102–7. [DOI] [PubMed] [Google Scholar]
- 21. Call E. Characterization of wound dressings physical properties and their potential impact on prevention of ulceration. 13th European Pressure Ulcer Advisory Panel; 2010; Birmingham, England, 5.
- 22. Call E, Pedersen J, Bill B, Oberg C. Wound dressings, measuring the microclimate they create. 14th Annual European Pressure Ulcer Advisory Panel; 2011; Oporto, Portugal, 10.
- 23. Gefen A. How do microclimate factors affect the risk for superficial pressure ulcers: a mathematical modelling study. J Tissue Viability 2011;20:81–8. [DOI] [PubMed] [Google Scholar]
- 24. Brindle CT. Outliers to the Braden Scale: Identifying high‐risk ICU patients and the results of prophylactic dressing use. WCET J 2010;30:11–8. [Google Scholar]
- 25. Brindle CT, Wegelin JA. Prophylactic dressing application to reduce pressure ulcer formation in cardiac surgery patients. J Wound Ostomy Continence Nurs 2012;39:133–42. [DOI] [PubMed] [Google Scholar]
- 26. Walsh NS, Blanck AW, Smith L, Cross M, Andersson L, Polito C. Use of a sacral silicone border foam dressing as one component of a pressure ulcer prevention program in an intensive care unit setting. J Wound Ostomy Continence 2012;39:146–9. [DOI] [PubMed] [Google Scholar]
- 27. Chaiken N. Reduction of sacral pressure ulcers in the intensive care unit using a silicone border foam dressing. J Wound Ostomy Continence Nurs 2012;39:143–5. [DOI] [PubMed] [Google Scholar]
- 28. Cubit K, McNally B, Lopez V. Taking the pressure off in the emergency department: evaluation of the prophylactic application of a low shear, soft silicone sacral dressing on high risk medical patients. Int Wound J 2012. DOI: 10.1111/j.1742-481X.2012.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Australasian College for Emergency Medicine . Guidelines on the implementation of the Australasian Triage Scale in emergency departments. ACEM; 2005; Victoria, Australia.
- 30. Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden scale for predicting pressure sore risk. Nurs Res 1987;36:205–10. [PubMed] [Google Scholar]
- 31. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- 32. Australian Wound Management Association . Clinical practice guidelines for the prevention and management of pressure ulcers. West Leederville, WA: Cambridge Publishing, 2001. [Google Scholar]
- 33. Schulz KF, Altman DG, Moher D, for the CONSORT Group . CONSORT2010. Statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med 2010;152:726–32.20335313 [Google Scholar]


