Abstract
The literature has reported that surgical site infections account for 17–22% of health care‐associated infections, while surgical wound dehiscence rates range from 0·25% to 3·0% (post laparatomies), 1·6% to 42·3% (post‐caesarean incisions) and 0·5% to 2·5% (sternal incisions). These types of incisional complications can become a significant cost burden to the health care system because of lengthy hospital stays and readmissions, additional nursing care and added surgical procedures. Therefore, the type of therapy used for surgical incisions plays a critical role in the healing process. The success of negative pressure wound therapy (NPWT; V.A.C.® Therapy; KCI USA, Inc., San Antonio, TX) for open wounds has been well documented and has led to its use over clean, closed surgical incisions. This review will focus on clinician experience and literature review of incisional NPWT and will include clinical cases describing NPWT's successful use over surgical incisions.
Keywords: Negative pressure wound therapy, Surgical incisions, Wound dehiscence
MANAGEMENT OF SURGICAL INCISIONS
Conventional closure methods for surgical incisions have included the use of sutures, staples (1), adhesives (2), paper tape (3) or a combination of these methods. Incisional closure complications can include postoperative wound infection 4, 5, 6, dehiscence 7, 8, 9, and formation of haematomas or seromas, and can lead to delayed healing of the incision. Patient comorbidities (e.g. obesity, diabetes and poor vascularisation) may also adversely affect incisional healing 10, 11.
Treatments used over clean, closed surgical incisions range from traditional gauze dressings (12) to more advanced therapies, such as hydrocolloids (12), growth factors (13), cultured skin (14) and negative pressure wound therapy (NPWT) 15, 16, 17. NPWT was initially developed as an adjunctive therapy to help treat difficult open wounds, and the majority of clinical studies regarding NPWT have documented its successful use on open wounds 18, 19, 20. However, more recent studies evaluating the clinical and scientific effects of NPWT suggest it might be useful over closed surgical incisions as well 16, 17, 21.
USE OF NPWT FOR SURGICAL INCISIONS
A small but growing number of clinical studies have been published (based on a proprietary database with daily searches of PubMed and Google Scholar) to date regarding incisional NPWT. Stannard et al. (16) recently published a Level 1 prospective randomised multicentre study on the use of incisional NPWT compared with standard postoperative dressings on 249 patients treated with open reduction and internal fixation (ORIF) of tibial plateau, pilon and calcaneus fractures. A total of 263 fractures were randomised to the control (n = 122) and incisional NPWT (n = 141) groups; fracture types were evenly distributed between the two groups. There were 23 infections (19%) in control patients and 14 (10%) in incisional NPWT patients (P = 0·049). Twenty (16·5%) control patients developed some wound dehiscence compared with 12 (8·6%) of the patients who had incisional NPWT (P = 0·044). The application of incisional NPWT decreased infection and wound dehiscence in these patients following high‐risk, lower‐extremity fractures treated with ORIF (16).
Other retrospective studies have provided evidence for successful use of NPWT over incisions. Gomoll et al. (22) examined records of 35 orthopaedic patients treated with NPWT over clean, closed incisions following revision hip arthroplasty, proximal femoral and tibial fracture fixation, and foot and ankle trauma. Results showed that no infections occurred during the 3‐month follow‐up (22). In another retrospective review, Atkins et al. (17) reported on 57 adult cardiac surgery patients whose sternotomy incisions were treated with NPWT for 4 days. On the basis of a pooled data risk assessment model (23), these patients were considered high risk for sternal wound infections (SWIs), because the majority were obese, diabetic or both. According to the risk assessment model (average estimated risk of 6·1 ± 4% for postoperative SWI), a minimum of three cases of SWI were anticipated. However, no complications were observed in the NPWT group (17).
Additional published clinical series have reported favourable outcomes on surgical incision healing with NPWT. Reddix et al. (21) initially published on incisional NPWT use in 19 morbidly obese patients (BMI ≥ 40) who underwent ORIF of acetabular fractures. None of the patients developed a postoperative infection or wound dehiscence. This was followed by a large series of 235 patients who received NPWT over surgical incisions following ORIF of acetabular fractures. The authors reported three (1·3%) deep wound infections and one (0·4%) wound dehiscence. Their reported rate of infection compared very favourably with the published infection rate of 4% and was significantly less than the authors' prior infection rate of 6·2% following surgical fixation of acetabular fractures (24). Also, Stannard et al. (25) published a small series of high‐risk patients who had incisional NPWT applied. Results showed that all wounds healed well.
A more recent evolution of NPWT technology used for closed incision management [Prevena™ Incision Management System (Prevena Therapy), KCI USA, Inc., San Antonio, TX] has been developed and consists of a single‐use negative pressure therapy unit, canister and easy peel‐and‐place dressing. This new system incorporates all the functional elements of standard incisional NPWT but in a simplified manner. Recent in vivo studies have provided evidence of improved fluid flow with this new incisional NPWT over clean, closed incisions, showing that its application significantly decreased haematoma/seroma levels in a porcine model (26). Wilkes et al. (27) used a finite element analysis to evaluate the stress impact of this new incisional NPWT on closed surgical incisions. They found that it decreased the lateral stresses in the incision by approximately 50% and changed the direction of the stresses to a distribution typical of intact tissue. The authors calculated that the incisional NPWT applied over a surgically closed incision increased the force required to disrupt the incision by 50%, suggesting that incisional NPWT may help prevent wound dehiscence (27).
The first prospective, randomised, controlled study using this new incisional NPWT was published in 2011 and included 19 consecutive patients treated with either incisional NPWT (n = 9) or standard postoperative dressings (Control; n = 10) over closed incisions following total hip arthroplasty (28). Results showed significantly decreased development and volume of postoperative seromas in the incisional NPWT group versus Control on day 10 (1·97 versus 5·08 ml; P = 0·021). A seroma was present in 44% of the incisional NPWT patients and 90% of Control patients. The incisional NPWT group required significantly fewer days of antibiotics (8·44 ± 2·24 versus 11·8 ± 2·82 days, P = .005), and a secretion in the wound after day 5 was reported in fewer patients in the incisional NPWT group versus the Control (1 versus 5 patients, respectively) (28). Other recent case series and studies have also showed successful use of this new incisional NPWT over clean, closed surgical incisions with no complications 29, 30.
WOUND PREPARATION
A number of basic science and animal studies have determined that NPWT is associated with increased microvascular blood flow 31, 32, 33, 34. Timmers et al. (35) published a study evaluating the flow of blood in closed intact skin of the healthy human forearm. They found that the application of NPWT significantly increased microvascular blood flow using a wide range of negative pressures. This mechanism of action may prove beneficial for clean, closed surgical incisions.
NPWT should only be applied immediately post surgery to clean surgically closed incisions. If a conventional NPWT/reticulated open‐cell foam (ROCF) dressing is used, a nonadherent layer should be placed between the foam dressing and the skin because placing the foam dressing directly against the skin can lead to maceration. Alternatively, the dressings with the new incisional NPWT (i.e. Prevena Therapy) are uniquely designed to be skin‐friendly over clean, closed surgical incisions. These dressings are precut to peel and stick over the incision and are connected to a portable, disposable NPWT device.
INITIATION CRITERIA
Although the precise initiation criteria for incisional NPWT are still being defined, the therapy is primarily suited for patients with a clean, closed postoperative incision that is at high risk for infection and/or wound dehiscence. High‐risk classification can be associated with any of three factors such as (i) injury or fracture type, (ii) soft tissue injury or contusion or (iii) patient factors. Of these factors, incisional NPWT use has been investigated most prevalently with respect to injury or fracture. Examples include NPWT use with high‐risk, lower‐extremity fractures (16), acetabulum fractures (24) and following total hip arthroplasty (28). Post‐incision use of NPWT with respect to high‐risk patient factors, including obesity and diabetes, has been investigated by several studies 17, 21, 29. Additional important patient factors may include immunosuppression and other conditions that might impair wound healing.
TREATMENT GOALS
Incisional NPWT is unique compared to other uses of NPWT. The main treatment goal is prophylaxis against wound complications, rather than treatment of wound complications that have already occurred. The desired outcome is a wound that heals with no infection or wound dehiscence.
CONTRAINDICATIONS
Incisional NPWT is applied over clean, closed surgical incisions. There are no specific contraindications with respect to NPWT use over closed incisions beyond those presented in product labelling and for standard NPWT. The only contraindication for the new incisional NPWT (Prevena Therapy) is sensitivity to silver, which is present in the interface layer for the sole purpose of helping control microbial growth in the layer. When using traditional NPWT, a nonadherent layer should always be placed between the foam dressing and the skin to protect the incision and surrounding skin (36).
DISCONTINUATION CRITERIA
Discontinuation criteria for incisional NPWT have not been clearly defined and may vary according to incision and patient factors. Reported duration of incisional therapy varies between 1 and 5 days in the literature 16, 17, 21, 22, 28, 29. Reddix et al. (21) reported discontinuation of incisional NPWT at the point when no oedema fluid was evident in the canister for 12 hours, usually a time period of 24–72 hours after surgery. The Level 1 study by Stannard et al. (16) had specific discontinuation criteria that involved a surgical incision with minimal drainage. However, that study was initiated prior to the availability of home NPWT and small portable units. Study patients only used the NPWT for an average of 2·5 days because they were ready for discharge. Later studies have reported slightly longer duration of incisional NPWT, likely due to increasing use of home NPWT devices. Additional studies are necessary to identify optimal discontinuation criteria for incisional NPWT. Clearly, NPWT should be discontinued if the wound becomes infected and the patient should undergo surgical treatment of their infection.
TECHNICAL PEARLS
Obtaining a good seal can be difficult when working around an external fixator. If there is a leak, additional drape should be used for patching. When there is no leak, the NPWT device should then be used.
CLINICAL CASES
Case study 1
The patients involved in this case were both enrolled in the prospective randomised study on high‐risk fractures treated with ORIF. Both patients were 38‐year‐old smokers and had (i) sustained four‐part calcaneus fractures that were closed, (ii) Tscherne soft tissue grade of 2 and (iii) surgery on the same day by the same surgeon. Figure 1A shows the surgical incision of the control (standard postoperative dressings) patient on postoperative day 4. Figure 1B shows the surgical incision of the study patient who was treated with NPWT. While both incisions healed without a complication, the control patient had an erythematous and tensely swollen foot and incision. The NPWT patient had soft tissue wrinkles and a surgical incision with no signs of healing difficulty.
Figure 1.
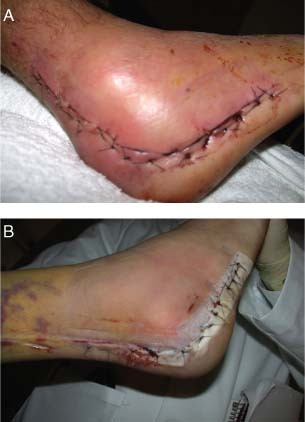
Case study 1: (A) Control incision on postoperative day 4. (B) Negative pressure wound therapy (NPWT) incision on postoperative day 4.
Case study 2
This patient was a 24‐year‐old morbidly obese patient who had sustained a transverse posterior wall acetabulum fracture that was initially treated by another surgeon through a posterior approach. The patient had late displacement of the anterior column requiring an ilioinguinal approach. The patient had a BMI of 50 and a 9·5‐cm adipose layer (Figure 2A). Closure took nearly 2 hours, and there were significant concerns regarding wound dehiscence and infection. NPWT as delivered by V.A.C.Via™ Therapy System (KCI USA, Inc., San Antonio, TX) was applied for 1 week (Figure 2B). The wound looked much improved when NPWT was discontinued a week later (Figure 2C). After 6 weeks, the patient had a well‐healed incision (Figure 2D).
Figure 2.
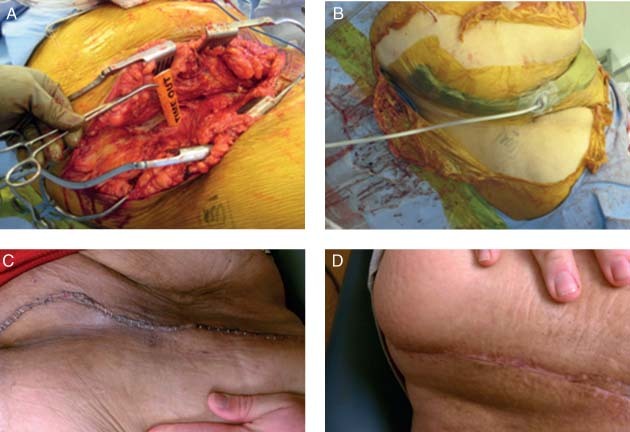
Case study 2: (A) Initial wound presentation. (B) Application of negative pressure wound therapy (NPWT). (C) One‐week post application of NPWT. (D) Healed incision at 6‐week follow‐up.
Case study 3
This patient was a 32‐year‐old male that presented with a failed ventral hernia with mesh. Component separation was initially performed (Figure 3A) followed by application of surgical mesh to repair the abdominal wall defect (Figure 3B and C). Primary closure was then achieved with staples (Figure 3D and E). Next, NPWT as delivered by Prevena Therapy was used over the surgical incision for 7 days with continuous negative pressure preset at −125 mmHg (Figure 3F). At approximately 5 months, follow‐up showed a healed incision (Figure 3G).
Figure 3.
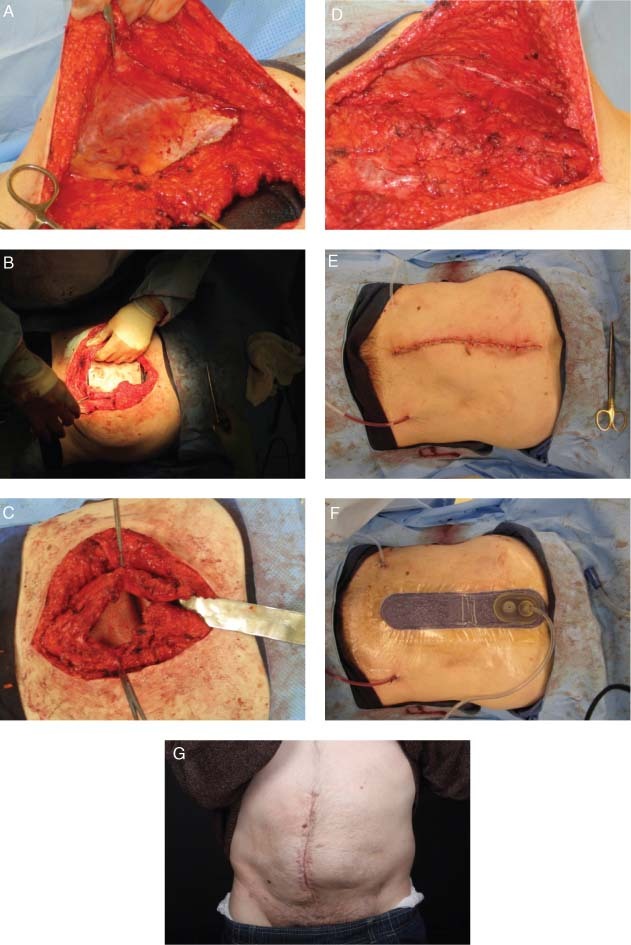
Case study 3: (A) Component separation was performed. (B) Application of surgical mesh underlay. (C) Surgical mesh underlay secured with horizontal mattress sutures. (D) Primary closure of fascia. (E) Primary closure was achieved with staples. (Note: Surgical mesh overlay was applied over fascia before primary closure.) (F) Use of negative pressure wound therapy (NPWT) for 7 days. (G) Healed incision at approximately 5 months follow‐up.
Case study 4
This patient is a 70‐year‐old diabetic male smoker who suffered a high‐grade soft tissue sarcoma of the right upper thigh. The tumour showed a volume of 630 cm3 (Figure 4A). Following wide resection that included a dissection of the main vessels and the periosteum, 2 Redon drains were placed to the deepest area of the wound (Figure 4B). This incision was at high risk for complications; therefore, the wound was closed with staples, and NPWT as delivered by Prevena Therapy was applied over the incision for 5 days followed by dry dressings (Figure 4C). There was <10 ml of wound drainage through the incision during this time (Figure 4D). Incision showed primary healing before removal of the staples 14 days after surgery (Figure 4E) and at 4 months postoperatively after receiving adjuvant local radiotherapy (Figure 4F).
Figure 4.
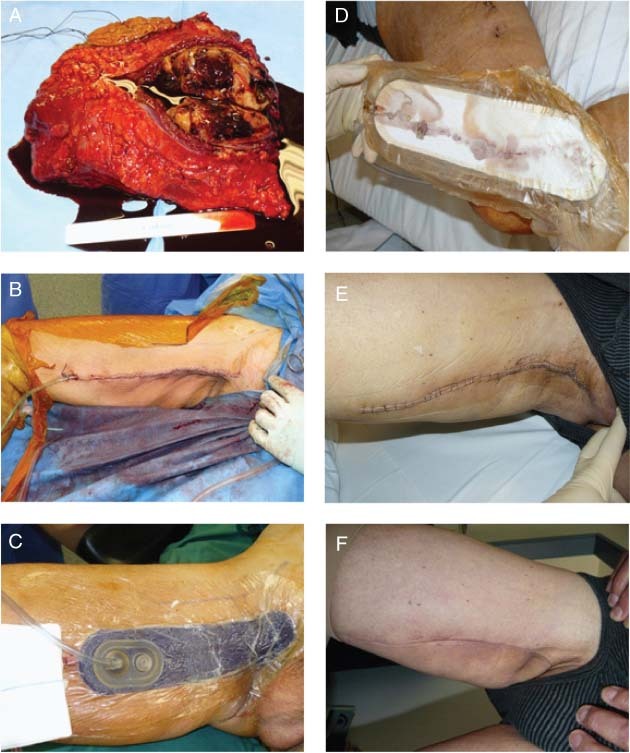
Case study 4: Removal of soft tissue sarcoma. (B) Two Redon drains were placed to the deepest area of the wound. (C) Negative pressure wound therapy (NPWT) was applied for 5 days. (D) There was <10 ml of wound drainage after 5 days of NPWT. (E) Primary healing of incision 14 days after surgery. (F) Healed incision at 4 months postoperatively.
ECONOMIC VALUE AND FUTURE DIRECTIONS
Wound dehiscence and infection are extraordinarily expensive complications that frequently require additional surgery and long‐term intravenous antibiotic treatment 37, 38, 39. Preliminary study data show a decrease in wound dehiscence and infection with use of incisional NPWT, which suggests potential cost savings. However, to date, there have been no comprehensive economic analyses of incisional NPWT use.
Additional well‐designed studies are essential to clearly identify the types of surgical incisions and the appropriate patients that would benefit most from incisional NPWT. Level 1 studies have shown that patients with high‐risk total hip arthroplasty or with lower‐extremity fractures treated with ORIF benefit from incisional NPWT in terms of lower rates of wound dehiscence, infection and seroma development 16, 28. Lower level data suggest that patients with acetabular fractures and sternal incisions, as well as obese patients, may also benefit. Other patients who may benefit are those undergoing total knee replacement who are either obese or have other risk factors for wound healing.
ACKNOWLEDGEMENT
We would like to thank Julissa Ramos, PhD (KCI, Inc.) for assisting with preparation of the manuscript.
CONFLICTS OF INTEREST
Dr JPS, Dr AG and Dr BL have Consulting agreements with Kinetic Concepts, Inc. This article is part of an educational supplement funded by Kinetic Concepts, Inc. to provide an overview of the V.A.C.® Therapy family of products for new users in developing markets. Targeted for distribution at the 2012 World Union of Wound Healing Societies (WUWHS) conference, this supplement article presents a brief literature review and clinical experience treating clean, closed surgical incisions with V.A.C.® Therapy or Prevena™ Incision Management System.
REFERENCES
- 1. Steichen FM, Ravitch MM. Mechanical sutures in surgery. Br J Surg 1973;60:191–7. [DOI] [PubMed] [Google Scholar]
- 2. Coulthard P, Esposito M, Worthington HV, van der Elst M, van Waes OJ, Darcey J. Tissue adhesives for closure of surgical incisions. Cochrane Database Syst Rev 2010;5:CD004287. [DOI] [PubMed] [Google Scholar]
- 3. Atkinson JA, McKenna KT, Barnett AG, McGrath DJ, Rudd M. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer's skin tension lines. Plast Reconstr Surg 2005;116:1648–56. [DOI] [PubMed] [Google Scholar]
- 4. Klevens RM, Edwards JR, Richards CL Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007;122:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. NNIS. National Nosocomial Infections Surveillance report, data summary from October 1986–April 1996 issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) System. Am J Infect Control 1996;24:380–8. [PubMed]
- 6. Scott RD II. The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Atlanta: Centers for Disease Control and Prevention, 2009. [Google Scholar]
- 7. Spiliotis J, Tsiveriotis K, Datsis AD, Vaxevanidou A, Zacharis G, Giafis K, Kekelos S, Rogdakis A. Wound dehiscence: is still a problem in the 21st century: a retrospective study. World J Emerg Surg 2009;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magann EF, Chauhan SP, Rodts‐Palenik S, Bufkin L, Martin JN Jr, Morrison JC. Subcutaneous stitch closure versus subcutaneous drain to prevent wound disruption after cesarean delivery: a randomized clinical trial. Am J Obstet Gynecol 2002;186:1119–23. [DOI] [PubMed] [Google Scholar]
- 9. John LC. Modified closure technique for reducing sternal dehiscence; a clinical and in vitro assessment. Eur J Cardiothorac Surg 2008;33 769–73. [DOI] [PubMed] [Google Scholar]
- 10. Riou JP, Cohen JR, Johnson H Jr. Factors influencing wound dehiscence. Am J Surg 1992;163: 324–30. [DOI] [PubMed] [Google Scholar]
- 11. Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care 2004;17:426–35. [DOI] [PubMed] [Google Scholar]
- 12. Holm C, Petersen JS, Gronboek F, Gottrup F. Effects of occlusive and conventional gauze dressings on incisional healing after abdominal operations. Eur J Surg 1998;164:179–83. [DOI] [PubMed] [Google Scholar]
- 13. Wu L, Mustoe TA. Effect of ischemia on growth factor enhancement of incisional wound healing. Surgery 1995;117:570–6. [DOI] [PubMed] [Google Scholar]
- 14. Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 15. Stannard JP, Robinson JT, Anderson ER, McGwin G Jr, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. J Trauma 2006;60:1301–6. [DOI] [PubMed] [Google Scholar]
- 16. Stannard JP, Volgas DA, McGwin G III. Stewart RL, Obremskey W, Moore T, Anglen JO. Incisional negative pressure wound therapy after high‐risk lower extremity fractures. J Orthop Trauma 2012;26 37–42. [DOI] [PubMed] [Google Scholar]
- 17. Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov 2009;16:140–6. [DOI] [PubMed] [Google Scholar]
- 18. Bovill E, Banwell PE, Teot L, Eriksson E, Song C, Mahoney J, Gustafsson R, Horch R, Deva A, Whitworth I; International Advisory Panel on Topical Negative Pressure. Topical negative pressure wound therapy: a review of its role and guidelines for its use in the management of acute wounds. Int Wound J 2008;5:511–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moues CM, Heule F, Hovius SE. A review of topical negative pressure therapy in wound healing: sufficient evidence? Am J Surg 2011;201:544–56. [DOI] [PubMed] [Google Scholar]
- 20. Xie X, McGregor M, Dendukuri N. The clinical effectiveness of negative pressure wound therapy: a systematic review. J Wound Care 2010;19:490–5. [DOI] [PubMed] [Google Scholar]
- 21. Reddix RN Jr, Tyler HK, Kulp B, Webb LX. Incisional vacuum‐assisted wound closure in morbidly obese patients undergoing acetabular fracture surgery. Am J Orthop 2009;38:32–5. [PubMed] [Google Scholar]
- 22. Gomoll AH, Lin A, Harris MB. Incisional vacuum‐assisted closure therapy. J Orthop Trauma 2006;20:705–9. [DOI] [PubMed] [Google Scholar]
- 23. Fowler VG Jr, O’Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation 2005;112:I358–I365. [DOI] [PubMed] [Google Scholar]
- 24. Reddix RN Jr, Leng XI, Woodall J, Jackson B, Dedmond B, Webb LX. The effect of incisional negative pressure therapy on wound complications after acetabular fracture surgery. J Surg Orthop Adv 2010;19:91–7. [PubMed] [Google Scholar]
- 25. Stannard JP, Atkins BZ, O’Malley D, Singh H, Bernstein B, Fahey M, Masden D, Attinger CE. Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage 2009;55:58–66. [PubMed] [Google Scholar]
- 26. Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen 2011;19:588–96. [DOI] [PubMed] [Google Scholar]
- 27. Wilkes RP, Kilpadi DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov 2012;19:67–75. [DOI] [PubMed] [Google Scholar]
- 28. Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz‐Drost S, Schlechtweg P, Pauser J, Gelse K, Brem MH. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop 2012;36:719–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Colli A. First experience with a new negative pressure incision management system on surgical incisions after cardiac surgery in high risk patients. J Cardiothorac Surg 2011;6:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haghshenasskashani A, Varcoe RL. A new negative pressure dressing (Prevena) to prevent wound complications following lower limb distal arterial bypass. Br J Diabetes Vasc Dis 2011;11:21–4. [Google Scholar]
- 31. Lindstedt S, Malmsjo M, Ingemansson R. Blood flow changes in normal and ischemic myocardium during topically applied negative pressure. Ann Thorac Surg 2007;84:568–73. [DOI] [PubMed] [Google Scholar]
- 32. Petzina R, Gustafsson L, Mokhtari A, Ingemansson R, Malmsjo M. Effect of vacuum‐assisted closure on blood flow in the peristernal thoracic wall after internal mammary artery harvesting. Eur J Cardiothorac Surg 2006;30:85–9. [DOI] [PubMed] [Google Scholar]
- 33. Wackenfors A, Sjogren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum‐assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 2004;12:600–6. [DOI] [PubMed] [Google Scholar]
- 34. Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum‐assisted closure therapy. Ann Thorac Surg 2005;79:1724–31. [DOI] [PubMed] [Google Scholar]
- 35. Timmers MS, Le CS, Banwell P, Jukema GN. The effects of varying degrees of pressure delivered by negative‐pressure wound therapy on skin perfusion. Ann Plast Surg 2005;55:665–71. [DOI] [PubMed] [Google Scholar]
- 36. Vaez‐zadeh S. In response to blister formation with negative pressure dressings. Curr Orthop Pract 2011;22:591. [Google Scholar]
- 37. Wong ES. The price of a surgical‐site infection: more than just excess length of stay. Infect Control Hosp Epidemiol 1999;20:722–4. [DOI] [PubMed] [Google Scholar]
- 38. Holtz TH, Wenzel RP. Postdischarge surveillance for nosocomial wound infection: a brief review and commentary. Am J Infect Control 1992;20:206–13. [DOI] [PubMed] [Google Scholar]
- 39. Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 2003;290:1868–74. [DOI] [PubMed] [Google Scholar]


