Staphylococcus lugdunensis is a species of coagulase-negative staphylococcus (CoNS) that causes serious infections in humans akin to those of S. aureus. It was often misidentified as S. aureus, but this has been rectified by recent routine use of matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) in diagnostic laboratories. It encodes a diverse array of virulence factors for adhesion, cytotoxicity, and innate immune evasion, but these are less diverse than those encoded by S. aureus.
KEYWORDS: endocarditis, fibrinogen, heme, invasive infection, macrophage, probiotic, surface proteins, von Willebrand factor
SUMMARY
Staphylococcus lugdunensis is a species of coagulase-negative staphylococcus (CoNS) that causes serious infections in humans akin to those of S. aureus. It was often misidentified as S. aureus, but this has been rectified by recent routine use of matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) in diagnostic laboratories. It encodes a diverse array of virulence factors for adhesion, cytotoxicity, and innate immune evasion, but these are less diverse than those encoded by S. aureus. It expresses an iron-regulated surface determinant (Isd) system combined with a novel energy-coupling factor (ECF) mechanism for extracting heme from hemoproteins. Small cytolytic S. lugdunensis synergistic hemolysins (SLUSH), peptides related to phenol-soluble modulins of S. aureus, act synergistically with β-toxin to lyse erythrocytes. S. lugdunensis expresses a novel peptide antibiotic, lugdunin, that can influence the nasal and skin microbiota. Endovascular infections are initiated by bacterial adherence to fibrinogen promoted by a homologue of Staphylococcus aureus clumping factor A and to von Willebrand factor on damaged endothelium by an uncharacterized mechanism. S. lugdunensis survives within mature phagolysosomes of macrophages without growing and is released only following apoptosis. This differs fundamentally from S. aureus, which actively grows and expresses bicomponent leukotoxins that cause membrane damage and could contribute to survival in the infected host. S. lugdunensis is being investigated as a probiotic to eradicate S. aureus from the nares of carriers. However, this is contraindicated by its innate virulence. Studies to obtain a deeper understanding of S. lugdunensis colonization, virulence, and microbiome interactions are therefore warranted.
INTRODUCTION
Staphylococcus lugdunensis was first identified as a new species of coagulase-negative staphylococcus (CoNS) in the late 1980s (1). It is a component of the human skin microbiome that is mainly associated with the lower parts of the body and the extremities, particularly in moist areas such as the inguinal fold and the perineum and under the large toe nail, in up to 67% of the population (2). Also, S. lugdunensis can be found in the nasal cavity but less frequently than at other body sites (2, 3). Unlike other CoNS, S. lugdunensis can cause severe infections akin to those caused by S. aureus (4–7). It causes a wide range of infections, including skin and soft tissue infections, bone and joint infections, prosthetic joint infections, vascular catheter-related infections, and abscesses (5, 8, 9) (Table 1). It is notorious for causing a destructive form of infective endocarditis (IE) (10–12). Although it is responsible for only about 1% of IE cases, these can have a mortality rate of up to 40% (10, 12, 13). The incidence of infections caused by S. lugdunensis was likely underestimated in earlier publications because of the use of latex agglutination tests for clumping factor to distinguish S. aureus from CoNS which can also agglutinate S. lugdunensis (14). CoNS isolates from blood cultures were often not identified to the species level or were even discarded as contaminants, raising doubts about the actual number of invasive infections caused by S. lugdunensis (15). Nowadays, the routine use of matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI TOF MS) allows accurate identification of staphylococcal clinical isolates to the species level (15). For example, routine use of this technique increased the identification of S. lugdunensis in clinical specimens 18-fold (16). Furthermore, a selective and differential growth medium is now available (17).
TABLE 1.
Infections caused by S. lugdunensis
| Type of infection |
Comment(s) | Reference(s) |
|---|---|---|
| Endocarditis | Native valve. Left-sided infection predominates. Causes vegetations. Surgery often required. High mortality. Occasional cerebrovascular incident. Community acquired. | 5, 10–12 |
| Prosthetic valve. Pacemaker associated. | 5 | |
| Bacteremia sepsis | Frequent complication of IE. | 11, 104, 105 |
| Bone and joint | Prosthetic joint infection (hip and knee arthroplasty, knee more common, underlying comorbidities). | 106, 107 |
| Fracture fixation devices. Vertebral osteomyelitis. Disk space infection. Infective arthritis, osteomyelitis. | 108, 109 | |
| Skin and soft tissue | Predominantly wound infection & abscesses. Primarily in perineal and pelvic girdle region. Predominantly community acquired. | 110, 111 |
| Peritonitis | Continuous ambulatory peritoneal dialysis. | 5 |
In this review, we discuss how recent advances in pathogenomics have led to the identification of a plethora of potential virulence factors that distinguish S. lugdunensis from other CoNS. However, detailed molecular analysis has been patchy and attempts to define virulence factors using experimental infections in rodents have been frustrated by their failure to recapitulate features of human infections. Consideration is given to the possible mechanisms of innate immune evasion and IE based on comparisons with S. aureus.
GENOME AND POPULATION STRUCTURE
Phylogenetic analysis of S. lugdunensis genome sequences indicates that the species is closely related to S. aureus and forms a unique cluster group (18, 19). S. aureus, S. epidermidis, S. capitis, and S. warneri have genomes that are open to acquisition of foreign DNA by horizontal gene transfer (20). This is indicated by the constant increase of the total gene number identified for the species when new genomes are added to the database (21, 22).
In contrast, S. lugdunensis shows a closed pangenome (18, 23) and the clonal populations show low genetic diversity (24). There is a high degree of amino acid and DNA sequence identity, suggesting that only limited horizontal transfer has occurred. CRISPR/Cas loci and toxin/antitoxin systems are key features of S. lugdunensis genomes (18, 25), and lineage-specific sets of restriction-modification systems shape clonality (26). It is likely that these strict barriers to horizontal gene transfer contribute to the slow development of antibiotic resistance.
Multilocus sequence typing has revealed 7 clonal complexes (24, 27). Increased discriminatory power can be achieved by several schemes, namely, multiple-virulence-locus sequence typing, variable number tandem repeat sequence typing, and repeat length variation in the gene encoding the fibrinogen binding protein Fbl (27–29). No link was shown between the clinical setting (e.g., hematogenic origin) and clonal complex or subgroup.
ANTIBIOTIC RESISTANCE
Until recently, clinical strains were susceptible to most antibiotics. Methicillin resistance was first reported in 2003 and has been increasingly encountered, particularly in the Far East. A prospective study revealed that 4.7% of 106 clinical isolates in one Singapore hospital between 2004 and 2006 were resistant (14), and 8.3% of 252 isolates in a Hong Kong hospital were found to be resistant (30). Resistance to methicillin and most β-lactam antibiotics is conferred by the mecA gene, which is acquired by horizontal transfer of mobile genetic elements called staphylococcal cassette chromosome mec elements (SCCmec) (31). At least 11 different SCCmec types have been described in S. aureus and other staphylococci.
There is incomplete information about the SCCmec types present in methicillin-resistant S. lugdunensis. Some strains carried elements that were variants of SCCmec type V (32), while a recently sequenced Japanese strain carried a large, complex, and untypeable element that harbored two loci encoding cell wall-anchored (CWA) surface proteins (33). The former strains were from multilocus sequence type 6 (ST6), while the latter was from ST3. A comprehensive analysis of the SCCmec types present in S. lugdunensis STs from different geographic locations is required to gain a complete understanding of the acquisition and spread of resistance to β-lactams.
GENETIC MANIPULATION
The growing evidence that S. lugdunensis represents an unusually virulent CoNS sparked efforts to investigate the molecular mechanisms of pathogenicity of this organism. Genetic manipulation using transformation with recombinant plasmids and allelic exchange is key for this. Originally, S. lugdunensis was found to be transformable with recombinant plasmids by the use of protoplast transformation (34). A more convenient genetic system is now available that allows efficient genetic manipulation (26). Electroporation protocols for plasmid DNA isolated from Escherichia coli were optimized. S. lugdunensis does not encode a SauUSI-type restriction system such as represents the primary barrier in S. aureus to the uptake of cytosine-methylated DNA from dcm-positive (dcm+) E. coli strains (26). However, type 1 restriction modification systems consisting of HsdS, HsdM, and HsdR subunits are present in S. lugdunensis. These systems govern the frequency of transformation of S. lugdunensis with plasmid DNA. Plasmid artificial modification (PAM) uses recombinant E. coli strains to produce methylated plasmid DNA mimicking the methylation pattern of the target species in order to increase transformation frequencies (35). A S. lugdunensis CC1-specific E. coli PAM strain (SL01) was constructed for use with the frequently studied N920143 and HKU09-01 strains (26). The frequency of plasmid transfer to CC1 strains as well as CC2 strains was improved 10-fold to 90-fold using SL01-derived DNA, showing that CC2 strains have the same HsdRMS system. No increase was observed with strains from CC3, CC4, and CC5, indicating that they have different systems. Plasmid vectors used for allelic exchange in S. aureus have been used to isolate targeted mutations by allelic exchange in chromosomal genes of S. lugdunensis (26, 34, 36).
Also, phage transduction of S. lugdunensis using the S. aureus ST395-derived phage Φ187 was described previously (37). This allows transfer of recombinant plasmids from S. aureus to S. lugdunensis. However, transduction of chromosomal markers has yet to be established.
Another genetic tool for improved phenotypic characterization of S. lugdunensis mutants is a vector containing the integrase gene and attachment site attP of a lysogenic bacteriophage. This allows stable integration at a single site in the chromosome of strains from CC1 to CC5 (38). Integrants were not compromised for fitness in vitro. The vector can be used for labeling strains with inducible antibiotic resistance determinants for growth competition experiments, for controlled gene expression experiments, or for complementation experiments where constitutive expression from a multicopy plasmid might be deleterious. Although not tested in animal experiments, such a stable vector should not be subject to plasmid segregation, which often occurs during growth in vivo.
POTENTIAL VIRULENCE FACTORS
Analysis of the genome sequences of S. lugdunensis strains reveals that the species lacks many of the virulence factors that are characteristic of S. aureus such as coagulase, innate immune evasion proteins, protein A, β-barrel pore forming cytolytic toxins, and enterotoxins (23, 25). The repertoire of potential virulence factors is summarized in Table 2. Few have been subjected to analysis, so very little is known about the role of these factors in pathogenesis. There are several toxins that could damage host cells, extracellular polysaccharides, and poly-d-glutamic acid that could help evade phagocytosis by neutrophils and macrophages, several CWA proteins that could promote adhesion to host cells and tissues, and enzymes that modify the bacterial cell surface to confer resistance to host antimicrobial factors. S. lugdunensis harbors a locus encoding iron-regulated surface determinant (Isd) proteins that enable bacteria to acquire iron from heme in the infected host.
TABLE 2.
Virulence-associated factors of S. lugdunensisa
| Virulence-associated factor |
Comment(s) | Reference(s) |
|---|---|---|
| Capsular polysaccharide Cap | CC3 Cap homologous to type 8 Cap of S. aureus. Others type 5. | 23, 112 |
| Polysaccharide intercellular adhesin Ica | Homology to ica locus of S. epidermidis and S. aureus. | 23 |
| Polyglutamic acid capsule Pga | Homology to the pga locus of S. epidermidis. | 23, 113 |
| β-Hemolysin Hlb | Homologous to β-hemolysin, sphingomyelinase of S. aureus. | 25 |
| Hemolysin III | Homologous to hemolysin III of Bacillus cereus. | 114 |
| S. lugdunensis synergistic hemolysins SLUSH | Three synergistic hemolytic peptides. β-Type phenol-soluble modulins. Some isolates lack SLUSH-C. SLUSH-B activates FPR2. | 23, 54, 55, 115 |
| ESAT-6 like secretion system Ess/type VII | Highly variable CC-dependent genetic organization. | 23 |
| Multiple peptide resistance factor MprF | Synthesis of lysyl-phosphatidylglycerol. Resistance to antimicrobial peptides. | 116 |
| Dlt | d-Alanylation of teichoic acids. Resistance to antimicrobial peptides. | 116 |
| Peptidoglycan O-acetyltransferase Oat | Survival in macrophages. Resistance to lysozyme. | 40 |
| Lugdulysin | Metalloprotease. Expression in vitro associated with infective isolates. Homology to the ShpI protease of S. hyicus but not to S. aureus serine or cysteine proteases. | 9 |
| Superoxide dismutase SodA | 90% identical to SodA in S. aureus. Reduction in reactive oxygen species in phagocytes. | |
| Nuclease | Homologue of S. aureus thermonuclease. | 23 |
| Fibrinogen binding protein of S. lugdunensis Fbl | CWA protein of MSCRAMM family closely related to ClfA of S. aureus. | 76 |
| Expression in vitro associated with infective isolates. | 23 | |
| von Willebrand factor binding protein of S. lugdunenesis vWbl | CWA surface protein binding von Willebrand factor? Frameshift mutation or stop codon in several CCs. No evidence of promoting adhesion to vWF. | 23, 73, 74 |
| S. lugdunensis Sl surface protein | Variable repeat numbers cause length differences in SlsA, SlsG, and SlsE. | 23, 25 |
| SlsA absent from CC3 and CC5. | 23 | |
| Iron-regulated surface determinant Isd proteins | Uptake of heme from hemoglobin. Growth in iron-restricted medium with hemoglobin as sole source of iron. Locus duplicated in HKU09-01. | 25, 36, 39 |
| Iron-regulated ECF heme transporter Lha | Uptake of heme from hemoglobin and myoglobin. | 47 |
Most genes are present in 100% of genome sequences analyzed by (Lebeurre et al., 2019) (23).
TESTING MOLECULAR KOCH’S POSTULATES
Isogenic mutants of S. lugdunensis have been isolated for the Isd, Fbl, and vWbl CWA proteins, Hts and Sir siderophore uptake systems, the peptidoglycan modifying enzyme Oat, the type 1 restriction subunit HsdA, and the housekeeping recombinase RecA (26, 39–41). Comparison of wild-type strains with mutants in in vitro and in vivo models is an established protocol employed to determine the role of potential virulence factors, allowing Koch’s postulates to be tested at the molecular level (42). Expression of factors in surrogate hosts such as S. carnosus or Lactococcus lactis is a complementary approach that has been used to a limited extent in S. lugdunensis (36).
Genome-wide association studies (GWAS) comparing sequences of many strains from different clonal complexes representing both commensal and disease-causing isolates can identify factors or allelic variants associated with a clinical condition. This has been performed with S. epidermidis (43) but has not been performed so far with S. lugdunensis. However, strains expressing in vitro fibrinogen binding activity correlated significantly with bacteremia and strains expressing a metalloprotease were enriched in isolates from bone and joint infections, bacteremia, and deep infections (9). There was no significant difference in the levels of virulence of isolates associated with carriage or invasive disease in humans when investigated in the Galleria mellonella infection model (23).
ACQUISITION OF NUTRITIONAL IRON
Molecular iron is essential as a cofactor or prosthetic group for many processes in central metabolism. During invasive infection, iron availability is actively limited by the host, a process referred to as nutritional immunity (44, 45). Iron acquisition strategies of S. lugdunensis have been studied in some detail. There are similarities to, but important differences from, the well-characterized system in S. aureus (46). However, the inability of IsdB to bind to murine hemoglobin has hindered attempts to fulfil Koch’s postulates at the molecular level for the Isd system (and other potential virulence factors) of S. lugdunensis using mouse infection models.
Acquisition of Heme
Many invasive pathogens access heme from the host to fulfil their requirement for iron. S. lugdunensis carries a locus that encodes iron-regulated surface determinant (Isd) proteins. A similar locus occurs in S. aureus which facilitates the acquisition of nutritional heme (46). Such systems represent a hallmark of bacterial pathogens. It has been suggested that the presence of Isd might explain the increased virulence potential of S. lugdunensis. This sparked molecular and biochemical characterization exceeding that of any other potential virulence trait of S. lugdunensis.
In S. aureus, the near iron transporter (NEAT) motif containing cell wall-anchored IsdB and IsdH proteins binds hemoproteins, captures heme, and transfers it across the cell wall via IsdA and IsdC and the membrane employing IsdEF. In the cytoplasm, the IsdG and IsdI heme oxygenases release iron (46). isd genes are expressed only under iron-limited conditions under the control of the Fur transcriptional regulator. This allows bacteria to grow in human tissue where availability of iron is strictly limited.
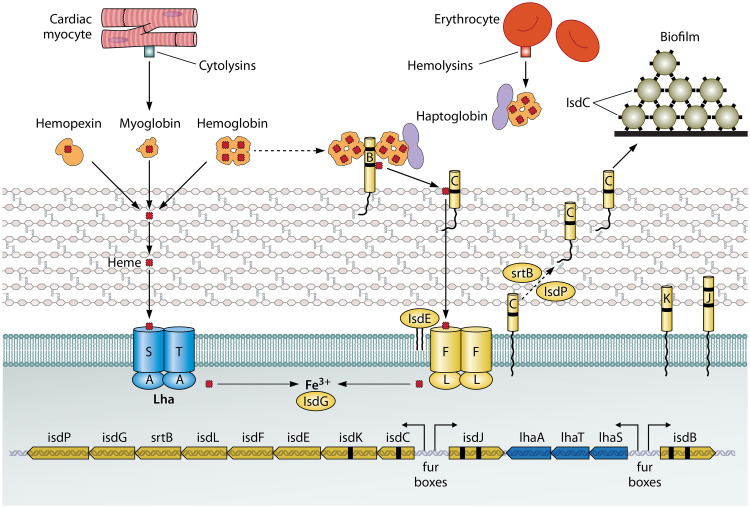
There are several important differences between the isd locus of S. lugdunensis and that of S. aureus (25, 36). S. lugdunensis Isd does not have IsdH or IsdA proteins but instead has an IsdA-like protein with two NEAT motifs called IsdJ (Fig. 1). Genes encoding an energy-coupling factor (ECF)-type transporter (lhaSTA) are located between isdB and isdJ (47). There is a single canonical heme oxygenase, IsdG, in S. lugdunensis, whereas S. aureus has two. The S. lugdunensis isd locus uniquely expresses an autolysin called IsdP and also a single NEAT motif-containing protein, IsdK, that has no orthologue in S. aureus (48).
FIG 1.
Iron acquisition from heme. The lower part of the diagram shows the genetic organization of the isd locus of S. lugdunensis. The locations of fur boxes and the directions of transcription following induction by iron limitation are shown by arrows. The upper part of the diagram shows the cell membrane and cell wall peptidoglycan and the locations of the Isd proteins (right side) and the Lha proteins (left side). Hemoproteins are shown with their bound heme molecules (red rectangles), including four in hemoglobin and one in myoglobin and hemopexin. Myoglobin and hemoglobin are released from myocytes and erythrocytes, respectively, by the action of cytolytic toxins. Black dashes in genes and proteins indicate NEAT domains. IsdB NEAT1 can bind hemoglobin and the haptoglobin-hemoglobin complex. The passage of heme across the cell wall and membrane is shown, along with release of Fe3+ by intracellular hemoxygenase. The predicted second hemoxygenase is not shown. IsdC proteins on different cells also promote biofilm formation.
A model for the capture of heme and its transportation across the cell envelope into the cytoplasm has been formulated (Fig. 1) (36, 48). This emanated from studies involving separation of the bacterial cell envelope into membrane and cell wall fractions following protoplast formation and performing Western immunoblotting with specific antisera. The ability of wild-type bacteria and specific mutants to grow under iron-restricted conditions with hemoglobin as the sole source of iron was also measured.
IsdB is a sortase A-anchored protein located in the cell wall fraction that is exposed on the cell surface (36). IsdB possesses two NEAT motifs. NEAT1 binds hemoglobin and hemoglobin-haptoglobin complexes, whereas NEAT2 binds heme. Heme is transferred from IsdB NEAT2 to the single NEAT motif of IsdC, a reaction that was demonstrated in vitro with recombinant proteins. In order for IsdC to be anchored to peptidoglycan by sortase B, the autolysin IsdP is required to cleave peptidoglycan between the lysine of the stem peptide and the fifth glycine of the cross-bridge (48). This exposes an amino group in cross-linked peptidoglycan for SrtB-promoted linkage of IsdC directly from the membrane-bound intermediate rather than to the peptidoglycan precursor lipid II. The need for IsdP-dependent peptidoglycan cleavage is an interesting difference from S. aureus, which does not need a similar activity for sorting of IsdC. While the reasons for this are speculative, the results might reflect different degrees of peptidoglycan cross-linkage in S. aureus and S. lugdunensis.
The majority of the IsdJ protein fractionates with the membrane, despite the precursor having an SrtA sorting signal, implying inefficient sortase activity (36). IsdK possesses a single NEAT motif and the characteristic hydrophobicity profile of CWA proteins but lacks the SrtA recognition motif. Accordingly, the protein is found in membrane fractions and culture supernatants. The roles of the IsdJ and IsdK proteins in heme uptake are unclear, and their elucidation will require the study of nonpolar mutants to avoid affecting expression of other genes in the locus.
Three genes encoding an energy-coupling factor (ECF) transporter, lugdunesis heme acquisition (lhaSTA), are uniquely present within the S. lugdunensis isd locus (47). This acts as a second heme uptake system allowing bacteria to scavenge heme directly from diverse hemoproteins, including hemoglobin, myoglobin, and hemopexin (Fig. 1). The broad range of the abilities of LhaSTA to accept heme does not seem to be dependent on direct interaction with the hemoproteins and was attributed to the high affinity of ECF transporters for their ligands, allowing diffusion-dependent extraction. However, its relevance during invasive diseases such as endocarditis or bacteremia awaits validation.
A single canonical heme monooxygenase encoded by the S. lugdunensis isd locus has been functionally validated (49). However, a so-far-unknown heme degrading enzyme must additionally be present in S. lugdunensis because an isdG deletion mutant retained the ability to degrade nutritional heme.
The isd locus of the first S. lugdunensis genome to be sequenced (HKU09-01) was duplicated by a rare unequal recombination event that occurred between identical 19-bp sequences located in two genes that are separated by 32 kb (39). This created a hybrid gene that is unique to the duplicated strain. The duplication included not only the isd locus but also genes encoding the SirABC transporter and a MarR family transcriptional regulator and 10 genes encoding proteins that could be involved in metabolism and nutrient transport. Long tandem repeats can amplify and contract at high frequency by homologous RecA-dependent recombination. Indeed, the duplication is intrinsically unstable, and revertants as well as amplifications occur at high frequency. Amplification results in higher levels of expression of Isd proteins and of binding of hemoglobin to the cell and provides a growth advantage for cells that are competing for low levels of hemoglobin as the sole source of iron. This helps to overcome nutritional immunity. The amplification of the Isd locus in strain testifies to its importance, but this is counterbalanced by the failure to observe the duplication in other strains.
As is the case with the S. aureus Isd proteins, several of those of S. lugdunensis have additional functions (50–52). The S. lugdunensis IsdC protein contributes to biofilm formation as described below (Fig. 1). Like IsdA, the IsdJ protein binds cytokeratin 10, loricrin, and fibrinogen in vitro. However, it is not exposed on S. lugdunensis cell surface, most likely due to a failure in sorting, and does not support bacterial adhesion to fibrinogen. In contrast, IsdJ conferred partial resistance against antimicrobial fatty acids (36).
Acquisition of Nonheme Iron
The production of iron-scavenging siderophores is another microbial strategy to acquire iron. Unlike other staphylococci, S. lugdunensis is unable to produce siderophores, suggesting dependence on xenosiderophore acquisition in polymicrobial settings (41). Indeed, S. lugdunensis expresses acquisition systems for the S. aureus siderophores staphyloferrin A and staphyloferrin B. Furthermore, the presence of S. aureus enhances the growth of S. lugdunensis under iron-limited conditions in a staphyloferrin-dependent manner. Additionally, S. lugdunensis expresses the receptors for hydroxamate siderophores (FhuD), and the genes encoding the Sst catecholate siderophore acquisition system are duplicated in many clinical isolates (23, 25). It can therefore be speculated that S. lugdunensis is also able to acquire iron from host hormones such as epinephrine. However, this awaits experimental validation.
POTENTIAL VIRULENCE FACTORS
Cytolysins
The benefit of heme acquisition systems is coupled to the induction of host cell damage, which allows access to the intracellular pool of hemoproteins. Additionally, secreted toxins could target immune effector cells to prevent pathogen elimination and damage endothelial and epithelial cells. The S. lugdunensis genomes harbor several loci with the potential to encode cytolytic toxins (Table 2), and S. lugdunensis strains are hemolytic toward human erythrocytes (47, 53). However, the extent to which the putative hemolysins contribute to lysis of erythrocytes or host immune cells has not been investigated. S. lugdunensis strains have a locus comprising three closely linked genes encoding S. lugdunensis synergistic hemolysins (SLUSH) (5, 53–55). The peptides act synergistically to potentiate hemolysis by S. aureus sphingomyelinase (β-toxin) and most likely by the S. lugdunensis β-toxin orthologue.
The SLUSH peptides belong to the group of well-known phenol-soluble modulins (PSMs) from S. aureus with important functions in host cell damage as well as in activation and modulation of the immune response (56). The ability of staphylococci to cause invasive disease correlates with the presence of loci encoding PSMs (56, 57).
The Innate Immune Evasion Conundrum
S. lugdunensis is capable of causing serious invasive infections similar to those caused by S. aureus. It is thus surprising that so little is known about its pathogenic mechanisms in general or its innate immune evasion mechanisms in particular. Despite lacking the plethora of immune evasion factors elaborated by S. aureus, S. lugdunensis must express a minimal repertoire of factors in order to survive in vivo. Perhaps its reduced armory in part explains the low incidence of infection compared to S. aureus. Nevertheless, it is instructive to speculate about such mechanisms in light of current understanding of S. aureus (see Fig. 3).
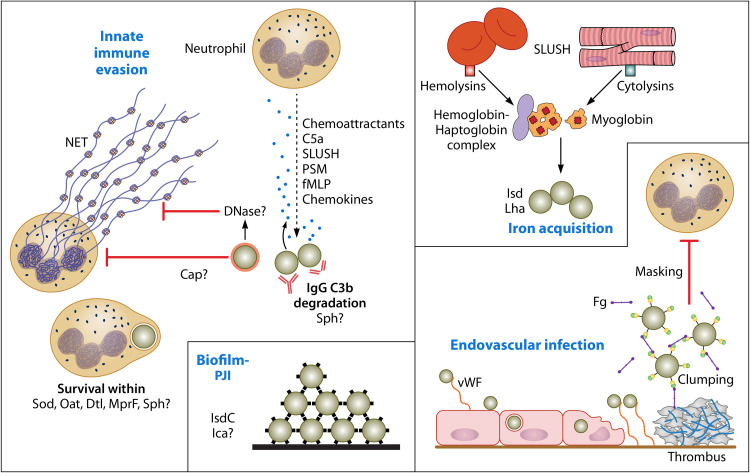
FIG 3.
Roles of potential virulence factors. A summary of factors that might contribute to pathogenesis of infection based on knowledge of S. aureus innate immune evasion and virulence mechanisms is presented. Upon initiation of infection, many chemoattractants generated by the bacteria and nearby host cells stimulate neutrophils to migrate from the bloodstream toward the site of infection. Interference with opsonophagocytosis is probably mediated by expression of a capsule. Secreted proteases might promote opsonin degradation. DNase could degrade neutrophil extracellular traps (NETs) and prevent entrapment and killing by antimicrobial substances. Once phagocytosis has occurred, several mechanisms likely contribute to resistance to neutrophil killing mechanisms. S. lugdunensis strains form biofilm, which likely contributes to prosthetic joint infections (PJI). IsdC-promoted cell accumulation is shown. Endovascular infections could be initiated by an as-yet-unknown surface component (not vWbl) binding to surface-attached vWF on inflamed endothelial cells. Bacteria can invade endothelial cells in vitro. This might trigger endothelial cell damage. Bacteria attach to vWF bound to collagen in the exposed extracellular matrix. The ability to bind fibrinogen/fibrin via Fbl is likely to contribute to immune evasion in the bloodstream by masking opsonins and by facilitating adherence to thrombi on damaged heart valves. Access to iron is crucial. This occurs by cytolysins releasing hemoglobin from erythrocytes and myoglobin from cardiac myocytes.
The ability to evade innate immune responses is crucial for the success of a pathogen that causes invasive infections. S. aureus is well endowed with factors that (i) interfere with neutrophil activation and migration in response to host and bacterial chemotactic factors; (ii) cause lysis of neutrophils and degrade neutrophil extracellular traps; (iii) interfere with opsonization, opsonins, and neutrophil phagocytosis; and (iv) promote bacterial survival within the neutrophil (for reviews, see references 58–61).
Measuring the ability of S. aureus to survive in human blood where neutrophils and complement are active is a simple approach to the study of innate immune evasion mechanisms, for example, by measuring the differences between wild-type and mutant strains (62). Surprisingly, there are no reports of experiments testing blood survival of S. lugdunensis.
Neutrophils are activated and stimulated to migrate toward the infecting organisms by signals generated at the early stages of infection, for example, host chemokines, complement degradation products, and bacterial factors such as formylated peptides and β-type phenol-soluble modulins. IgG and complement deposited on the cell surface act as opsonins. The bacterial metalloprotease lugdulysin (9) probably contributes to opsonin degradation similarly to aureolysin of S. aureus. Capsular polysaccharides and poly-d-glutamic acid, along with host plasma proteins such as fibrinogen coating the cell surface, block opsonin recognition by neutrophils. S. lugdunensis has the ability to secrete an extracellular nuclease that might degrade neutrophil extracellular traps like the thermonuclease of S. aureus.
S. lugdunensis survives within macrophages (and Kupffer cells) due in part to its ability to resist lysozyme via acetylation of muramic acid in peptidoglycan (40, 63, 101). The same mechanism likely contributes to survival in neutrophils. Survival of S. lugdunensis in the macrophage phagosome is also due to its failure to proliferate and the lack of bicomponent leukotoxins that would otherwise damage the phagosome membrane, releasing bacteria into the cytosol.
Addition of d-alanine residues to teichoic acid (TA) molecules by the activity of the Dtl system and synthesis of lysyl phosphatidyl glycerol on the outer surface of the cytoplasmic membrane by MprF result in the creation of positively charged surfaces which repel cationic antimicrobial peptides (AMPs). The metalloprotease lugdulysin could contribute to survival by degrading AMPs. Finally, expression of superoxide dismutase degrades reactive oxygen species elaborated during the oxidative burst.
BIOFILM
The ability to form biofilm is an important mechanism of pathogenesis and is a major factor in staphylococcal infections associated with indwelling-device-related infection. Biofilm formation could be of particular importance for S. lugdunensis prosthetic joint infection (PJI).
The mechanistic basis of biofilm formation by S. lugdunensis is unclear. All 21 strains from 5 clonal complexes whose genomes have been sequenced carry the ica locus, which is a homologue in terms of gene content and organization to the well-characterized loci of S. aureus and S. epidermidis that control expression of the polysaccharide intercellular adhesin PNAG (23). However, studies of biofilm formed in vitro by uncharacterized clinical isolates failed to detect the presence of PNAG with specific antibody or lectin (5, 64). Biofilms could be dispersed by protease treatment but not by periodate treatment, which implies that the matrix is proteinaceous. Indeed, when bacteria are growing under iron-restricted conditions, expression of IsdC (Fig. 1) promotes biofilm accumulation by homophilic protein-protein interactions (65, 66). Similar protein-dependent biofilm formation promoted by fibronectin binding proteins and SdrC of S. aureus allows multiple low-affinity interactions that cumulatively stimulate cells to attach to each other (67, 68). Analyzing the proteome of the biofilm matrix and extending analysis to biofilms growing under in vivo-like conditions is required. Comparing disease-causing and commensal isolates and inclusion of strains from all clonal complexes are important for future studies.
CELL WALL-ANCHORED SURFACE PROTEINS
Interactions of bacteria with the environment are shaped by the composition and structure of the cell surface. Gram-positive bacteria decorate their envelope by sortase-dependent attachment of proteins to the peptidoglycan. Cell wall-anchored (CWA) proteins of S. aureus are known to be crucial for pathogenesis by promoting attachment to host cells and matrix proteins as well as immune evasion, biofilm formation, and nutrient acquisition (69, 70). S. aureus possesses two CWA proteins that facilitate adherence to damaged heart valves and the pathogenesis of endocarditis, i.e., the von Willebrand factor (vWF) binding protein and fibrinogen binding protein clumping factor A (69, 71, 72). This provided a rationale for studying binding to fibrinogen and vWF to investigate the mechanism of endocarditis caused by S. lugdunensis.
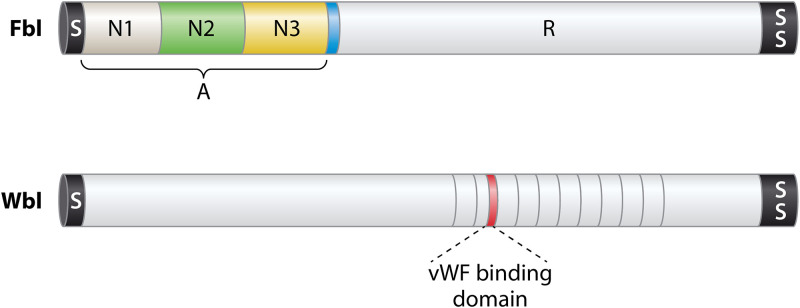
The repertoire of S. lugdunensis CWA surface proteins was identified from genome sequences by screening for open reading frames specifying a secretory signal sequence at the N terminus and a sorting signal for anchorage to cell wall peptidoglycan at the C terminus (25). Apart from the Isd proteins described above, nine additional CWA proteins were identified but only the fibrinogen binding protein Fbl and the vWF binding protein vWbl have so far been investigated (Fig. 2). The function of vWbl is unclear because investigations following its original identification have not supported a role in binding vWF.
FIG 2.
Cell wall-anchored surface proteins. A schematic diagram showing the two CWA proteins apart from the Isd proteins (Fig. 1) that have been analyzed at the molecular level is presented. The preproteins have a signal sequence (S) that promotes secretion across the membrane via the Sec apparatus at the N terminus and a sorting signal (SS) at the C terminus for anchorage to peptidoglycan. The A domain of Fbl is composed of three separately folded subdomains, with N2 and N3 being the minimum required for fibrinogen binding. Region R comprises SDSDSA repeats, which form a flexible stalk. The minimum putative vWF binding domain of Wbl is shown in red.
vWbl
The vWbl protein of CC1 strains carries a distinctive 67-amino-acid repeat sequence (73). The vWF binding domain was identified from phagemid clones expressing peptide fragments derived from S. lugdunensis genomic DNA fused to a capsid protein. The phagemid library was panned against vWF, and phages that bound vWF were captured and eluted and the insertions sequenced. The minimal binding domain was localized to a 24-residue sequence at the C terminus of repeat 2. No further molecular characterization of the protein has been performed. It is unclear why only R2 repeat segments and no other repeats were detected. Biochemical and biophysical analysis of purified proteins is required.
Additionally, isogenic mutants of S. lugdunensis deficient in vWbl or srtA retained their ability to adhere to immobilized vWF (74). Thus, it is unclear if vWbl supports adhesion of whole cells to vWF and so it must be concluded that least one non-sortase-anchored but surface-associated protein or a nonproteinaceous surface component promotes S. lugdunensis binding to vWF.
Fbl
Similarly to vWbl, the fibrinogen binding protein of S. lugdunensis (Fbl) was identified using phage panning experiments (75). Fbl is related in sequence and structural organization to clumping factor A of S. aureus (Fig. 2) (76) and confers clumping factor activity to 32% to 65% of clinical isolates (9, 28). The N2 and N3 subdomains have 60% amino acid sequence identity to these regions of ClfA. The C-terminal repeat domain comprises 6 residue motifs (SDSDSA). These differ slightly from region R of ClfA, which is composed of dipeptide SD repeats. The R domain of Fbl ranges in length from 9 to 52 repeats (28). The repeat length correlates with the clonal complex, implying that it is stable and that length variants emerging by unequal crossover results are rare. This is likely to be due to optimized DNA sequence diversity employing all 6 serine codons. The diversity of repeat numbers and variations in the 18-bp coding sequences has been exploited as a typing scheme (28). In S. aureus, a minimum number of residues (>40) is required for optimal ClfA functional activity on the surface of the bacterium (77). The presence of too few repeats may prevent conformational changes in region A required for ligand binding. In S. lugdunensis, strains with longer repeats bound fibrinogen more strongly than those with shorter repeats (28).
The A domain of Fbl binds to the same residues in the C terminus of the γ chain of fibrinogen as ClfA (76). This occurs by the dock lock latch (DLL) mechanism (69, 70, 102, 103).
PATHOGENESIS OF ENDOVASCULAR INFECTIONS
The ability to bind vWF released by inflamed endothelial cells or exposed by damage to the endothelium under conditions of shear stress is a hallmark of S. aureus endovascular infections (71). S. lugdunensis alone among the CoNS can attach to immobilized vWF in vitro under conditions of shear forces (74). The steps involved in the pathogenesis of IE are summarized in Fig. 3. Use of an in vivo mesenteric vein inflammation model and a mouse endocarditis model involving localized histamine-induced inflammation of the cardiac valve demonstrated that S. lugdunensis can bind to vWF in vivo. Studies performed in vitro using isogenic mutants defective in Fbl and vWbl failed to demonstrate a role for either protein or indeed for any sortase-anchored cell surface protein in promoting bacterial adhesion to vWF. The surface component(s) of S. lugdunensis that promotes attachment to vWF remains to be identified. This contrasts with the behavior of S. aureus seen in studies using the same models where the bifunctional small secreted von Willebrand factor binding protein acts as a bridge between ClfA on the bacterial cell surface and exposed vWF and the endothelial cell αV β3 integrin (69, 71, 72). It is anticipated that Fbl promotes adhesion to thrombi in a manner similar to that seen with ClfA of S. aureus (78, 79).
Adhesion to and invasion of endothelial cells are important in the pathogenesis of endocarditis caused by S. aureus (78). Several clinical isolates of S. lugdunensis were previously shown to invade endothelial cells in vitro (80). Invasiveness may contribute to the development of IE by promoting damage to the endothelium and exposing collagen in the underlying extracellular matrix, which in turn attracts binding of circulating vWF. Neither the bacterial adhesin nor the host cell ligand was identified, so the mechanistic basis of uptake is not known.
In contrast, sortase-anchored proteins are required for S. lugdunensis to adhere to sterile rat cardiac valve vegetations caused by damage induced by a sterile catheter introduced through the carotid artery (26). S. lugdunensis caused smaller vegetations and lower mortalities than S. aureus, while the levels of bacteremia and dissemination were similar. The low virulence in the rat model of IE might be due to an inability to acquire sufficient heme due to low hemolysis/cytolysis and poor recognition of rodent hemoglobin by Isd.
Mutants defective in Fbl or Wbl alone were not significantly less virulent than the wild type, although a trend toward lower virulence was observed. Using more animals and a double mutant defective in both proteins might have revealed a significant difference.
HOST SPECIFICITY
S. lugdunensis appears to be a human-specific organism, but sporadic reports have shown that it can cause infections in companion animals (81, 82). Studies investigating asymptomatic colonization of companion or farm animals by S. lugdunensis are lacking, and it remains unclear if animals can serve as a reservoir for human infection or vice versa.
Investigation of the virulence of S. lugdunensis in mouse models of infection is compromised because the IsdB protein has a low affinity for murine hemoglobin (36, 47). This limitation could be overcome by using a transgenic mouse that expresses human hemoglobin or by murinizing the S. lugdunesis IsdB protein to recognize human hemoglobin more efficiently. Despite this problem, S. lugdunensis was previously shown to be able to colonize the inflamed cardiac endothelium of mice although the ability of the bacteria to multiply and cause further tissue damage was not evaluated (74).
Mice were reported to show hardly any signs of disease when S. lugdunensis was injected intravenously (47). This could have been due to an inability to capture heme from murine hemoglobin and/or the inability of S. lugdunensis membrane-damaging toxins to lyse murine erythrocytes (47). Thus, mice are not well suited for in vivo models of S. lugdunensis pathogenicity and alternative organisms such as rats, guinea pigs, pigs, or rabbits should be considered in the future.
MODULATION OF SKIN AND NASAL MICROBIOTA
Genome sequences show that S. lugdunensis encodes two nonribosomal peptide synthesis systems (25). One of these was shown to be responsible for the synthesis of as well as secretion and self-resistance to a new class of cyclic peptide antibiotic called lugdunin which has important antimicrobial and immunomodulatory properties (83, 84). Lugdunin is bactericidal toward S. aureus, and individuals who harbor S. lugdunensis in their nares are less likely to be S. aureus carriers. Lugdunin acts synergistically with host skin-derived antimicrobial peptides (85). It also acts on primary human keratinocytes to trigger synthesis of the antimicrobial peptide LL37 and the neutrophil proinflammatory cytokine CXCL8 (interleukin-8 [IL-8]). This enhances the innate immune response and helps suppress the growth of pathogens such as S. aureus.
In line with this experimental evidence, S. lugdunensis, along with other CoNS species present in the skin microbiome, was found to contribute to suppression of the growth of S. aureus in healthy skin and the nares (86). This suggests that S. lugdunensis might have potential as a probiotic with the ability to prevent or even eradicate colonization with S. aureus when applied to individuals at risk of infection by their endogenous S. aureus strain.
DISCUSSION
Staphylococcus lugdunensis represents an ambiguous staphylococcal species, and, 4 decades after its first description, there is growing evidence that it should be regarded as a dangerous opportunistic pathogen rather than just as a harmless skin commensal. Without doubt, S. lugdunensis represents the most aggressive species of CoNS.
Strategies to prevent or eradicate S. aureus colonization in the light of increasing antibiotic resistance are needed. S. lugdunensis appears to have interesting characteristics in this regard. Due the production of the antibiotic lugdunin, it can kill S. aureus in vitro and its presence in the nares was shown to be negatively correlated with S. aureus nasal colonization (83). This suggests that S. lugdunensis might be usable as a probiotic to reduce or eliminate S. aureus carriage when applied to nasal cavities. Interestingly, S. lugdunensis also interacts with S. aureus by usage of staphyloferrin B (SfB). Among the members of the nasal microbiome, only S. aureus produces SfB. The presence of the SfB acquisition system Sir and lack of SfB biosynthesis strongly suggest evolutionary adaptation of S. lugdunensis to the presence of S. aureus. While it has been shown that the presence of S. aureus enhances S. lugdunensis proliferation under iron-restricted conditions (41), it can only be speculated that SfB capture by S. lugdunensis reduces proliferation of S. aureus. However, this is likely, as siderophore production is physiologically costly and usurpation of siderophores by nonproducers impairs the fitness of producers (87). Due to the presence of lactoferrin in nasal secretions, it seems plausible that siderophore acquisition is important to sustain nasal colonization and that the observed protective effects of S. lugdunensis colonization against S. aureus might therefore be multifactorial and depend on antibiotic action as well as on nutritional competition.
There is compelling evidence that S. lugdunensis is adapted to the presence of S. aureus. The reasons for this are speculative, and many open questions remain. Maybe S. aureus and S. lugdunensis share the same niche within the nasal cavity and live in close spatial proximity with limited adhesion sites. Under such conditions, it might be an advantage for S. lugdunensis to hold S. aureus at bay by antibiotic action when bacterial numbers are high while profiting from the presence of S. aureus when bacterial numbers are low. We know that S. aureus nasal colonization is multifactorial and relies on CWA proteins such as ClfB or SdrD as well as on wall teichoic acid (WTA) molecules interacting with receptors on nasal epithelial cells (88). We know nothing regarding the factors that facilitate S. lugdunensis nasal colonization. However, it seems unlikely that S. lugdunensis shares the same adhesion strategies. ClfB and SdrD are not encoded by S. lugdunensis (25), and it does not express a homologue of Aap of S. epidermidis (89). Although its WTA possesses amphipathic properties due to the action of Dlt, the backbone structure and glycosylation are not conserved between the species (90). The mechanism of S. lugdunensis adherence to skin and nasal corneocytes should be investigated.
Additionally, it needs to be considered that the predominant niches of S. lugdunensis lie in the moist areas of the skin rather than the nasal cavity (2). Interestingly, the skin is rarely colonized by S. aureus. It might be speculated that S. lugdunensis helps to exclude S. aureus from healthy skin. However, neither skin colonization by S. lugdunensis nor its interactions with the skin microbiota have been investigated experimentally. The skin of pigs is very similar to human skin, and pig models of skin colonization might be well suited to use in studies in this regard (91). We need to address these issues to better understand the ambivalent relation between S. aureus and S. lugdunensis and the probiotic potential of S. lugdunensis.
There is a debate as to whether CoNS species are actually pathogenic because they are primarily skin commensals and usually infect only individuals who are hospitalized and have indwelling medical devices that provide a breach of the epidermal defenses (92). However, S. lugdunensis has innate virulence potential because of its ability to cause more-severe invasive infections such as IE and skin and soft tissue infections. These infections are rare compared to S. aureus infections, but in the case of IE, such infections are destructive and have a high mortality rate.
S. lugdunensis has a number of potential virulence factors that could aid in pathogenesis (Table 2; see also Fig. 3), although it lacks the extensive repertoire of adhesins, toxins, and immune evasion proteins elaborated by S. aureus. A thorough investigation of innate immune evasion mechanisms is warranted. Of particular importance is the Isd system that allows bacteria to extract heme from hemoglobin. In S. lugdunensis, Isd is supplemented by the ECF transporter LhaSTA, which extracts heme from several hemoproteins by a novel diffusion-dependent mechanism. A fruitful area for future research on pathogenesis will be that of defining the role of the ESAT-6-like type VII secretion system (23), which in S. aureus is known to be required for virulence in mouse infection models and for survival within phagocytic cells and in bacterial competition (93–98).
A major obstacle to attempting to fulfil Koch’s postulates at the molecular level is the failure of rodent infection models to recapitulate human diseases. S. lugdunensis is poorly hemolytic for mouse erythrocytes compared to human cells. In addition, the S. lugdunensis IsdB protein has a low affinity for murine hemoglobin. This severely impairs the growth of S. lugdunensis in the rodent host, particularly as the organism does not produce siderophores. It was previously observed that S. lugdunensis can colonize damaged tissue in rodent models but fails to proliferate and cause the same level of tissue damage as S. aureus (26, 74). Better animal models are required for progress to be made by, for example, employing transgenic mice that have been engineered to express the human version of a receptor or plasma protein to create humanized mouse infection models (99, 100).
The ability of staphylococci to adhere to von Willebrand factor released by activated endothelial cells is key to the pathogenesis of endovascular infections. In S. aureus, CWA protein clumping factor A and the small secreted bifunctional vWBFBP play key roles in in vitro and in vivo models (71). The adhesion of S. lugdunensis to vWF occurs by a mechanism different from that seen with S. aureus. It is independent of the ClfA homologue Fbl and the so-called vWbl. Indeed, no sortase-anchored surface protein seems to be involved. Identification and characterization of the factor that allows the bacterium to attach to vWF are crucial to understanding the pathogenesis of S. lugdunensis IE.
S. lugdunensis seems an interesting candidate for a probiotic to eradicate S. aureus nasal colonization in at-risk individuals (83). However, the benefits and risks of S. lugdunensis colonization need to be balanced carefully and more information regarding the molecular mechanisms of S. lugdunensis pathogenicity as well as of S. lugdunensis-S. aureus interactions are needed. Attenuated S. lugdunensis mutants rather than wild-type strains might be considered as probiotic candidates.
The past decade has produced significant insights into the population structure of S. lugdunensis, its mechanisms of pathogenicity, and its ability to shape the microbiome. New directions for future research include more-detailed understanding of features of virulent strains, for example, from genome-wide association studies such as have been applied to S. epidermidis (43); developing animal models of infection that better reflect human diseases; and defining crucial virulence factors for IE and other invasive infections. Transformation of this knowledge into strategies to prevent infection and to combat S. lugdunensis is the challenge of the future.
ACKNOWLEDGMENTS
The work of S.H. was supported by the German Science Foundation (DFG) (Project HE8381/3-1). Additionally, S.H. received infrastructural funding from the DFG (Cluster of Excellence EXC 2124 Controlling Microbes to Fight Infections).
Biographies

Simon Heilbronner is an Independent Research Group Leader in the Department of Infection Biology at the University of Tübingen (Germany). He studied microbiology and immunology in Tübingen and Uppsala (Sweden) and earned his diploma in 2009. During his Ph.D. studies at Trinity College Dublin (Ireland), he established genetic tools for Staphylococcus lugdunensis research and conducted the first molecular investigations of virulence factors and iron acquisition strategies in this neglected pathogen. Using a Marie Skłodowska-Curie fellowship, he moved back to Tübingen in 2014 and has been leading his own group since 2017. His research focuses on the prokaryotic strategies to create population diversity using gene amplification. Additionally, he studies staphylococcal iron acquisition systems and their role in host colonization and competition with other members of the human microbiome.

Timothy J. Foster is Fellow Emeritus in Trinity College Dublin, having retired from being Professor of Molecular Microbiology in 2014. He was awarded a Ph.D. from the University of Bristol in 1972. He spent his academic career in the Microbiology Department at Trinity College Dublin apart from a sabbatical in Harvard University in 1978 to 1979. Initially, he studied antibiotic resistance mechanisms, plasmids, and transposons in Gram-negative bacteria before turning his attention to Staphylococcus aureus. He was among the first researchers to apply molecule genetic techniques to studying secreted and cell wall-associated virulence factors and to generate site-specific mutations by allele replacement and transposon mutagenesis. Identification and characterization of fibrinogen binding protein clumping factor A led to a succession of projects on cell wall-anchored surface proteins. His group was the first to use the genome sequence of Staphylococcus lugdunensis as a springboard for studies on virulence factors of this important coagulase-negative staphylococcus.
REFERENCES
- 1.Fleurette J, Bes M, Brun Y, Freney J, Forey F, Coulet M, Reverdy ME, Etienne J. 1989. Clinical isolates of Staphylococcus lugdunensis and S. schleiferi: bacteriological characteristics and susceptibility to antimicrobial agents. Res Microbiol 140:107–118. doi: 10.1016/0923-2508(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 2.Bieber L, Kahlmeter G. 2010. Staphylococcus lugdunensis in several niches of the normal skin flora. Clin Microbiol Infect 16:385–388. doi: 10.1111/j.1469-0691.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu CM, Price LB, Hungate BA, Abraham AG, Larsen LA, Christensen K, Stegger M, Skov R, Andersen PS. 2015. Staphylococcus aureus and the ecology of the nasal microbiome. Sci Adv 1:e1400216. doi: 10.1126/sciadv.1400216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker K, Heilmann C, Peters G. 2014. Coagulase-negative staphylococci. Clin Microbiol Rev 27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank KL, Del Pozo JL, Patel R. 2008. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev 21:111–133. doi: 10.1128/CMR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parthasarathy S, Shah S, Raja Sager A, Rangan A, Durugu S. 2020. Staphylococcus lugdunensis: review of epidemiology, complications, and treatment. Cureus 12:e8801. doi: 10.7759/cureus.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natsis NE, Cohen PR. 2018. Coagulase-negative staphylococcus skin and soft tissue infections. Am J Clin Dermatol 19:671–677. doi: 10.1007/s40257-018-0362-9. [DOI] [PubMed] [Google Scholar]
- 8.Douiri N, Hansmann Y, Lefebvre N, Riegel P, Martin M, Baldeyrou M, Christmann D, Prevost G, Argemi X. 2016. Staphylococcus lugdunensis: a virulent pathogen causing bone and joint infections. Clin Microbiol Infect 22:747–748. doi: 10.1016/j.cmi.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Argemi X, Prevost G, Riegel P, Keller D, Meyer N, Baldeyrou M, Douiri N, Lefebvre N, Meghit K, Ronde Oustau C, Christmann D, Cianferani S, Strub JM, Hansmann Y. 2017. VISLISI trial, a prospective clinical study allowing identification of a new metalloprotease and putative virulence factor from Staphylococcus lugdunensis. Clin Microbiol Infect 23:334.e1–334.e8. doi: 10.1016/j.cmi.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Kyaw H, Raju F, Shaikh AZ, Lin AN, Lin AT, Abboud J, Reddy S. 2018. Staphylococcus lugdunensis endocarditis and cerebrovascular accident: a systemic review of risk factors and clinical outcome. Cureus 10:e2469. doi: 10.7759/cureus.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Non LR, Santos CA. 2017. The occurrence of infective endocarditis with Staphylococcus lugdunensis bacteremia: a retrospective cohort study and systematic review. J Infect 74:179–186. doi: 10.1016/j.jinf.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Liu PY, Huang YF, Tang CW, Chen YY, Hsieh KS, Ger LP, Chen YS, Liu YC. 2010. Staphylococcus lugdunensis infective endocarditis: a literature review and analysis of risk factors. J Microbiol Immunol Infect 43:478–484. doi: 10.1016/S1684-1182(10)60074-6. [DOI] [PubMed] [Google Scholar]
- 13.Anguera I, Del Rio A, Miro JM, Matinez-Lacasa X, Marco F, Guma JR, Quaglio G, Claramonte X, Moreno A, Mestres CA, Mauri E, Azqueta M, Benito N, Garcia-de la Maria C, Almela M, Jimenez-Exposito MJ, Sued O, De Lazzari E, Gatell JM, Hospital Clinic Endocarditis Study Group . 2005. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart 91:e10. doi: 10.1136/hrt.2004.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan TY, Ng SY, He J. 2008. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol 46:2393–2395. doi: 10.1128/JCM.00740-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argemi X, Riegel P, Lavigne T, Lefebvre N, Grandpre N, Hansmann Y, Jaulhac B, Prevost G, Schramm F. 2015. Implementation of matrix-assisted laser desorption ionization-time of flight mass spectrometry in routine clinical laboratories improves identification of coagulase-negative staphylococci and reveals the pathogenic role of Staphylococcus lugdunensis. J Clin Microbiol 53:2030–2036. doi: 10.1128/JCM.00177-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elamin WF, Ball D, Millar M. 2015. Unbiased species-level identification of clinical isolates of coagulase-negative staphylococci: does it change the perspective on Staphylococcus lugdunensis? J Clin Microbiol 53:292–294. doi: 10.1128/JCM.02932-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho PL, Leung SM, Tse H, Chow KH, Cheng VC, Que TL. 2014. Novel selective medium for isolation of Staphylococcus lugdunensis from wound specimens. J Clin Microbiol 52:2633–2636. doi: 10.1128/JCM.00706-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argemi X, Matelska D, Ginalski K, Riegel P, Hansmann Y, Bloom J, Pestel-Caron M, Dahyot S, Lebeurre J, Prevost G. 2018. Comparative genomic analysis of Staphylococcus lugdunensis shows a closed pan-genome and multiple barriers to horizontal gene transfer. BMC Genomics 19:621. doi: 10.1186/s12864-018-4978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamers RP, Muthukrishnan G, Castoe TA, Tafur S, Cole AM, Parkinson CL. 2012. Phylogenetic relationships among Staphylococcus species and refinement of cluster groups based on multilocus data. BMC Evol Biol 12:171. doi: 10.1186/1471-2148-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argemi X, Hansmann Y, Prola K, Prevost G. 2019. Coagulase-negative staphylococci pathogenomics. Int J Mol Sci 20:1215. doi: 10.3390/ijms20051215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlan S, Mijares LA, NISC Comparative Sequencing Program, Becker J, Blakesley RW, Bouffard GG, Brooks S, Coleman H, Gupta J, Gurson N, Park M, Schmidt B, Thomas PJ, Otto M, Kong HH, Murray PR, Segre JA. 2012. Staphylococcus epidermidis pan-genome sequence analysis reveals diversity of skin commensal and hospital infection-associated isolates. Genome Biol 13:R64. doi: 10.1186/gb-2012-13-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosi E, Monk JM, Aziz RK, Fondi M, Nizet V, Palsson BO. 2016. Comparative genome-scale modelling of Staphylococcus aureus strains identifies strain-specific metabolic capabilities linked to pathogenicity. Proc Natl Acad Sci U S A 113:E3801–E3809. doi: 10.1073/pnas.1523199113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebeurre J, Dahyot S, Diene S, Paulay A, Aubourg M, Argemi X, Giard JC, Tournier I, Francois P, Pestel-Caron M. 2019. Comparative genome analysis of Staphylococcus lugdunensis shows clonal complex-dependent diversity of the putative virulence factor, ess/type VII locus. Front Microbiol 10:2479. doi: 10.3389/fmicb.2019.02479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chassain B, Lemee L, Didi J, Thiberge JM, Brisse S, Pons JL, Pestel-Caron M. 2012. Multilocus sequence typing analysis of Staphylococcus lugdunensis implies a clonal population structure. J Clin Microbiol 50:3003–3009. doi: 10.1128/JCM.00988-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heilbronner S, Holden MT, van Tonder A, Geoghegan JA, Foster TJ, Parkhill J, Bentley SD. 2011. Genome sequence of Staphylococcus lugdunensis N920143 allows identification of putative colonization and virulence factors. FEMS Microbiol Lett 322:60–67. doi: 10.1111/j.1574-6968.2011.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heilbronner S, Hanses F, Monk IR, Speziale P, Foster TJ. 2013. Sortase A promotes virulence in experimental Staphylococcus lugdunensis endocarditis. Microbiology (Reading) 159:2141–2152. doi: 10.1099/mic.0.070292-0. [DOI] [PubMed] [Google Scholar]
- 27.Dahyot S, Lebeurre J, Argemi X, Francois P, Lemee L, Prevost G, Pestel-Caron M. 2018. Multiple-locus variable number tandem repeat analysis (MLVA) and tandem repeat sequence typing (TRST), helpful tools for subtyping Staphylococcus lugdunensis. Sci Rep 8:11669. doi: 10.1038/s41598-018-30144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahyot S, Lebeurre J, Laumay F, Argemi X, Dubos C, Lemee L, Prevost G, Francois P, Pestel-Caron M. 2019. fbl-typing of Staphylococcus lugdunensis: a frontline tool for epidemiological studies, but not predictive of fibrinogen binding ability. Front Microbiol 10:1109. doi: 10.3389/fmicb.2019.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Didi J, Lemee L, Gibert L, Pons JL, Pestel-Caron M. 2014. Multi-virulence-locus sequence typing of Staphylococcus lugdunensis generates results consistent with a clonal population structure and is reliable for epidemiological typing. J Clin Microbiol 52:3624–3632. doi: 10.1128/JCM.01370-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho PL, Leung SM, Chow KH, Tse CW, Cheng VC, Tse H, Mak SK, Lo WK. 2015. Carriage niches and molecular epidemiology of Staphylococcus lugdunensis and methicillin-resistant S. lugdunensis among patients undergoing long-term renal replacement therapy. Diagn Microbiol Infect Dis 81:141–144. doi: 10.1016/j.diagmicrobio.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Chen D, Peters BM, Li L, Li B, Xu Z, Shirliff ME. 2016. Staphylococcal chromosomal cassettes mec (SCCmec): a mobile genetic element in methicillin-resistant Staphylococcus aureus. Microb Pathog 101:56–67. doi: 10.1016/j.micpath.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 32.Liu MC, Cao H, Lau A, Chow KH, Lai EL, Tse CW, Wu AK, Ho PL. 2019. Structures of SCCmec elements in methicillin-resistant Staphylococcus lugdunensis are closely related to those harboured by community-associated methicillin-resistant Staphylococcus aureus. J Med Microbiol 68:1367–1372. doi: 10.1099/jmm.0.001013. [DOI] [PubMed] [Google Scholar]
- 33.Shibuya R, Uehara Y, Baba T, Teruya K, Satou K, Hirano T, Kirikae T, Hiramatsu K. 2020. Complete genome sequence of a methicillin-resistant Staphylococcus lugdunensis strain and characteristics of its staphylococcal cassette chromosome mec. Sci Rep 10:8682. doi: 10.1038/s41598-020-65632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marlinghaus L, Becker K, Korte M, Neumann S, Gatermann SG, Szabados F. 2012. Construction and characterization of three knockout mutants of the fbl gene in Staphylococcus lugdunensis. APMIS 120:108–116. doi: 10.1111/j.1600-0463.2011.02819.x. [DOI] [PubMed] [Google Scholar]
- 35.Monk IR, Tree JJ, Howden BP, Stinear TP, Foster TJ. 2015. Complete bypass of restriction systems for major Staphylococcus aureus lineages. mBio 6:e00308-15. doi: 10.1128/mBio.00308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zapotoczna M, Heilbronner S, Speziale P, Foster TJ. 2012. Iron-regulated surface determinant (Isd) proteins of Staphylococcus lugdunensis. J Bacteriol 194:6453–6467. doi: 10.1128/JB.01195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winstel V, Kuhner P, Krismer B, Peschel A, Rohde H. 2015. Transfer of plasmid DNA to clinical coagulase-negative staphylococcal pathogens by using a unique bacteriophage. Appl Environ Microbiol 81:2481–2488. doi: 10.1128/AEM.04190-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heilbronner S, Monk IR, Foster TJ. 2013. The phage integrase vector pIPI03 allows RecA-independent, site-specific labelling of Staphylococcus lugdunensis strains. Plasmid 70:377–384. doi: 10.1016/j.plasmid.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Heilbronner S, Monk IR, Brozyna JR, Heinrichs DE, Skaar EP, Peschel A, Foster TJ. 2016. Competing for iron: duplication and amplification of the isd locus in Staphylococcus lugdunensis HKU09-01 provides a competitive advantage to overcome nutritional limitation. PLoS Genet 12:e1006246. doi: 10.1371/journal.pgen.1006246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flannagan RS, Watson DW, Surewaard BGJ, Kubes P, Heinrichs DE. 2018. The surreptitious survival of the emerging pathogen Staphylococcus lugdunensis within macrophages as an immune evasion strategy. Cell Microbiol 20:e12869. doi: 10.1111/cmi.12869. [DOI] [PubMed] [Google Scholar]
- 41.Brozyna JR, Sheldon JR, Heinrichs DE. 2014. Growth promotion of the opportunistic human pathogen, Staphylococcus lugdunensis, by heme, hemoglobin, and coculture with Staphylococcus aureus. Microbiologyopen 3:182–195. doi: 10.1002/mbo3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falkow S. 2004. Molecular Koch's postulates applied to bacterial pathogenicity–a personal recollection 15 years later. Nat Rev Microbiol 2:67–72. doi: 10.1038/nrmicro799. [DOI] [PubMed] [Google Scholar]
- 43.Meric G, Mageiros L, Pensar J, Laabei M, Yahara K, Pascoe B, Kittiwan N, Tadee P, Post V, Lamble S, Bowden R, Bray JE, Morgenstern M, Jolley KA, Maiden MCJ, Feil EJ, Didelot X, Miragaia M, de Lencastre H, Moriarty TF, Rohde H, Massey R, Mack D, Corander J, Sheppard SK. 2018. Disease-associated genotypes of the commensal skin bacterium Staphylococcus epidermidis. Nat Commun 9:5034. doi: 10.1038/s41467-018-07368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer LD, Skaar EP. 2016. Transition metals and virulence in bacteria. Annu Rev Genet 50:67–91. doi: 10.1146/annurev-genet-120215-035146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammer ND, Skaar EP. 2011. Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu Rev Microbiol 65:129–147. doi: 10.1146/annurev-micro-090110-102851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jochim A, Adolf L, Belikova D, Schilling NA, Setyawati I, Chin D, Meyers S, Verhamme P, Heinrichs DE, Slotboom DJ, Heilbronner S. 2020. An ECF-type transporter scavenges heme to overcome iron-limitation in Staphylococcus lugdunensis. Elife 9:e57322. doi: 10.7554/eLife.57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrand AJ, Haley KP, Lareau NM, Heilbronner S, McLean JA, Foster T, Skaar EP. 2015. An iron-regulated autolysin remodels the cell wall to facilitate heme acquisition in Staphylococcus lugdunensis. Infect Immun 83:3578–3589. doi: 10.1128/IAI.00397-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haley KP, Janson EM, Heilbronner S, Foster TJ, Skaar EP. 2011. Staphylococcus lugdunensis IsdG liberates iron from host heme. J Bacteriol 193:4749–4757. doi: 10.1128/JB.00436-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke SR, Andre G, Walsh EJ, Dufrene YF, Foster TJ, Foster SJ. 2009. Iron-regulated surface determinant protein A mediates adhesion of Staphylococcus aureus to human corneocyte envelope proteins. Infect Immun 77:2408–2416. doi: 10.1128/IAI.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, Mond JJ, Tarkowski A, Foster SJ. 2007. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe 1:199–212. doi: 10.1016/j.chom.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Visai L, Yanagisawa N, Josefsson E, Tarkowski A, Pezzali I, Rooijakkers SHM, Foster TJ, Speziale P. 2009. Immune evasion by Staphylococcus aureus conferred by iron-regulated surface determinant protein IsdH. Microbiology (Reading) 155:667–679. doi: 10.1099/mic.0.025684-0. [DOI] [PubMed] [Google Scholar]
- 53.Hebert GA. 1990. Hemolysins and other characteristics that help differentiate and biotype Staphylococcus lugdunensis and Staphylococcus schleiferi. J Clin Microbiol 28:2425–2431. doi: 10.1128/JCM.28.11.2425-2431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donvito B, Etienne J, Denoroy L, Greenland T, Benito Y, Vandenesch F. 1997. Synergistic hemolytic activity of Staphylococcus lugdunensis is mediated by three peptides encoded by a non-agr genetic locus. Infect Immun 65:95–100. doi: 10.1128/IAI.65.1.95-100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donvito B, Etienne J, Greenland T, Mouren C, Delorme V, Vandenesch F. 1997. Distribution of the synergistic haemolysin genes hld and slush with respect to agr in human staphylococci. FEMS Microbiol Lett 151:139–144. doi: 10.1111/j.1574-6968.1997.tb12562.x. [DOI] [PubMed] [Google Scholar]
- 56.Cheung GY, Joo HS, Chatterjee SS, Otto M. 2014. Phenol-soluble modulins–critical determinants of staphylococcal virulence. FEMS Microbiol Rev 38:698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rautenberg M, Joo HS, Otto M, Peschel A. 2011. Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence. FASEB J 25:1254–1263. doi: 10.1096/fj.10-175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spaan AN, van Strijp JAG, Torres VJ. 2017. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol 15:435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buchan KD, Foster SJ, Renshaw SA. 2019. Staphylococcus aureus: setting its sights on the human innate immune system. Microbiology (Reading) 165:367–385. doi: 10.1099/mic.0.000759. [DOI] [PubMed] [Google Scholar]
- 60.de Vor L, Rooijakkers SHM, van Strijp JAG. 2020. Staphylococci evade the innate immune response by disarming neutrophils and forming biofilms. FEBS Lett 594:2556–2569. doi: 10.1002/1873-3468.13767. [DOI] [PubMed] [Google Scholar]
- 61.Rooijakkers SH, van Strijp JA. 2007. Bacterial complement evasion. Mol Immunol 44:23–32. doi: 10.1016/j.molimm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Smith EJ, Visai L, Kerrigan SW, Speziale P, Foster TJ. 2011. The Sbi protein is a multifunctional immune evasion factor of Staphylococcus aureus. Infect Immun 79:3801–3809. doi: 10.1128/IAI.05075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flannagan RS, Heit B, Heinrichs DE. 2016. Intracellular replication of Staphylococcus aureus in mature phagolysosomes in macrophages precedes host cell death, and bacterial escape and dissemination. Cell Microbiol 18:514–535. doi: 10.1111/cmi.12527. [DOI] [PubMed] [Google Scholar]
- 64.Frank KL, Patel R. 2007. Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect Immun 75:4728–4742. doi: 10.1128/IAI.00640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Missineo A, Di Poto A, Geoghegan JA, Rindi S, Heilbronner S, Gianotti V, Arciola CR, Foster TJ, Speziale P, Pietrocola G. 2014. IsdC from Staphylococcus lugdunensis induces biofilm formation under low-iron growth conditions. Infect Immun 82:2448–2459. doi: 10.1128/IAI.01542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aubourg M, Dhalluin A, Gravey F, Pottier M, Thomy N, Bernay B, Goux D, Martineau M, Giard JC. 2020. Phenotypic and proteomic approaches of the response to iron-limited condition in Staphylococcus lugdunensis. BMC Microbiol 20:328. doi: 10.1186/s12866-020-02016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herman-Bausier P, El-Kirat-Chatel S, Foster TJ, Geoghegan JA, Dufrene YF. 2015. Staphylococcus aureus fibronectin-binding protein A mediates cell-cell adhesion through low-affinity homophilic bonds. mBio 6:e00413-15. doi: 10.1128/mBio.00413-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feuillie C, Formosa-Dague C, Hays LM, Vervaeck O, Derclaye S, Brennan MP, Foster TJ, Geoghegan JA, Dufrene YF. 2017. Molecular interactions and inhibition of the staphylococcal biofilm-forming protein SdrC. Proc Natl Acad Sci U S A 114:3738–3743. doi: 10.1073/pnas.1616805114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foster TJ. 2019. The MSCRAMM family of cell-wall-anchored surface proteins of gram-positive cocci. Trends Microbiol 27:927–941. doi: 10.1016/j.tim.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Foster TJ, Geoghegan JA, Ganesh VK, Hook M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Claes J, Vanassche T, Peetermans M, Liesenborghs L, Vandenbriele C, Vanhoorelbeke K, Missiakas D, Schneewind O, Hoylaerts MF, Heying R, Verhamme P. 2014. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor-binding protein. Blood 124:1669–1676. doi: 10.1182/blood-2014-02-558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liesenborghs L, Meyers S, Lox M, Criel M, Claes J, Peetermans M, Trenson S, Vande Velde G, Vanden Berghe P, Baatsen P, Missiakas D, Schneewind O, Peetermans WE, Hoylaerts MF, Vanassche T, Verhamme P. 2019. Staphylococcus aureus endocarditis: distinct mechanisms of bacterial adhesion to damaged and inflamed heart valves. Eur Heart J 40:3248–3259. doi: 10.1093/eurheartj/ehz175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nilsson M, Bjerketorp J, Wiebensjo A, Ljungh A, Frykberg L, Guss B. 2004. A von Willebrand factor-binding protein from Staphylococcus lugdunensis. FEMS Microbiol Lett 234:155–161. doi: 10.1016/j.femsle.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 74.Liesenborghs L, Peetermans M, Claes J, Veloso TR, Vandenbriele C, Criel M, Lox M, Peetermans WE, Heilbronner S, de Groot PG, Vanassche T, Hoylaerts MF, Verhamme P. 2016. Shear-resistant binding to von Willebrand factor allows Staphylococcus lugdunensis to adhere to the cardiac valves and initiate endocarditis. J Infect Dis 213:1148–1156. doi: 10.1093/infdis/jiv773. [DOI] [PubMed] [Google Scholar]
- 75.Nilsson M, Bjerketorp J, Guss B, Frykberg L. 2004. A fibrinogen-binding protein of Staphylococcus lugdunensis. FEMS Microbiol Lett 241:87–93. doi: 10.1016/j.femsle.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Geoghegan JA, Ganesh VK, Smeds E, Liang X, Hook M, Foster TJ. 2010. Molecular characterization of the interaction of staphylococcal microbial surface components recognizing adhesive matrix molecules (MSCRAMM) ClfA and Fbl with fibrinogen. J Biol Chem 285:6208–6216. doi: 10.1074/jbc.M109.062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hartford O, Francois P, Vaudaux P, Foster TJ. 1997. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol Microbiol 25:1065–1076. doi: 10.1046/j.1365-2958.1997.5291896.x. [DOI] [PubMed] [Google Scholar]
- 78.Que YA, Haefliger JA, Piroth L, Francois P, Widmer E, Entenza JM, Sinha B, Herrmann M, Francioli P, Vaudaux P, Moreillon P. 2005. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med 201:1627–1635. doi: 10.1084/jem.20050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moreillon P, Entenza JM, Francioli P, McDevitt D, Foster TJ, Francois P, Vaudaux P. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun 63:4738–4743. doi: 10.1128/IAI.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szabados F, Marlinghaus L, Korte M, Neumann S, Kaase M, Gatermann SG. 2011. Fbl is not involved in the invasion of eukaryotic epithelial and endothelial cells by Staphylococcus lugdunensis. FEMS Microbiol Lett 324:48–55. doi: 10.1111/j.1574-6968.2011.02382.x. [DOI] [PubMed] [Google Scholar]
- 81.Rook KA, Brown DC, Rankin SC, Morris DO. 2012. Case-control study of Staphylococcus lugdunensis infection isolates from small companion animals. Vet Dermatol 23:476–e90. doi: 10.1111/j.1365-3164.2012.01087.x. [DOI] [PubMed] [Google Scholar]
- 82.Beck KM, Waisglass SE, Dick HL, Weese JS. 2012. Prevalence of meticillin-resistant Staphylococcus pseudintermedius (MRSP) from skin and carriage sites of dogs after treatment of their meticillin-resistant or meticillin-sensitive staphylococcal pyoderma. Vet Dermatol 23:369–375, e66–e67. doi: 10.1111/j.1365-3164.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 83.Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, Burian M, Schilling NA, Slavetinsky C, Marschal M, Willmann M, Kalbacher H, Schittek B, Brotz-Oesterhelt H, Grond S, Peschel A, Krismer B. 2016. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535:511–516. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
- 84.Krauss S, Zipperer A, Wirtz S, Saur J, Konnerth MC, Heilbronner S, Torres Salazar BO, Grond S, Krismer B, Peschel A. 2020. Secretion of and self-resistance to the novel fibupeptide antimicrobial lugdunin by distinct ABC transporters in Staphylococcus lugdunensis. Antimicrob Agents Chemother 65:e01734-20. doi: 10.1128/AAC.01734-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bitschar K, Sauer B, Focken J, Dehmer H, Moos S, Konnerth M, Schilling NA, Grond S, Kalbacher H, Kurschus FC, Gotz F, Krismer B, Peschel A, Schittek B. 2019. Lugdunin amplifies innate immune responses in the skin in synergy with host- and microbiota-derived factors. Nat Commun 10:2730. doi: 10.1038/s41467-019-10646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, Latif H, Kim JN, Lockhart A, Artis K, David G, Taylor P, Streib J, Dorrestein PC, Grier A, Gill SR, Zengler K, Hata TR, Leung DY, Gallo RL. 2017. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9:eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kramer J, Ozkaya O, Kummerli R. 2020. Bacterial siderophores in community and host interactions. Nat Rev Microbiol 18:152–163. doi: 10.1038/s41579-019-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krismer B, Weidenmaier C, Zipperer A, Peschel A. 2017. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat Rev Microbiol 15:675–687. doi: 10.1038/nrmicro.2017.104. [DOI] [PubMed] [Google Scholar]
- 89.Macintosh RL, Brittan JL, Bhattacharya R, Jenkinson HF, Derrick J, Upton M, Handley PS. 2009. The terminal A domain of the fibrillar accumulation-associated protein (Aap) of Staphylococcus epidermidis mediates adhesion to human corneocytes. J Bacteriol 191:7007–7016. doi: 10.1128/JB.00764-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Winstel V, Sanchez-Carballo P, Holst O, Xia G, Peschel A. 2014. Biosynthesis of the unique wall teichoic acid of Staphylococcus aureus lineage ST395. mBio 5:e00869. doi: 10.1128/mBio.00869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vodicka P, Smetana K, Jr, Dvorankova B, Emerick T, Xu YZ, Ourednik J, Ourednik V, Motlik J. 2005. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci 1049:161–171. doi: 10.1196/annals.1334.015. [DOI] [PubMed] [Google Scholar]
- 92.Heilmann C, Ziebuhr W, Becker K. 2019. Are coagulase-negative staphylococci virulent? Clin Microbiol Infect 25:1071–1080. doi: 10.1016/j.cmi.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 93.Cao Z, Casabona MG, Kneuper H, Chalmers JD, Palmer T. 2016. The type VII secretion system of Staphylococcus aureus secretes a nuclease toxin that targets competitor bacteria. Nat Microbiol 2:16183. doi: 10.1038/nmicrobiol.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burts ML, Williams WA, DeBord K, Missiakas DM. 2005. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci U S A 102:1169–1174. doi: 10.1073/pnas.0405620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burts ML, DeDent AC, Missiakas DM. 2008. EsaC substrate for the ESAT-6 secretion pathway and its role in persistent infections of Staphylococcus aureus. Mol Microbiol 69:736–746. doi: 10.1111/j.1365-2958.2008.06324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Korea CG, Balsamo G, Pezzicoli A, Merakou C, Tavarini S, Bagnoli F, Serruto D, Unnikrishnan M. 2014. Staphylococcal Esx proteins modulate apoptosis and release of intracellular Staphylococcus aureus during infection in epithelial cells. Infect Immun 82:4144–4153. doi: 10.1128/IAI.01576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lopez MS, Tan IS, Yan D, Kang J, McCreary M, Modrusan Z, Austin CD, Xu M, Brown EJ. 2017. Host-derived fatty acids activate type VII secretion in Staphylococcus aureus. Proc Natl Acad Sci U S A 114:11223–11228. doi: 10.1073/pnas.1700627114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson M, Ohr RJ, Aly KA, Nocadello S, Kim HK, Schneewind CE, Schneewind O, Missiakas D. 2017. EssE promotes Staphylococcus aureus ESS-dependent protein secretion to modify host immune responses during infection. J Bacteriol 199:e00527-16. doi: 10.1128/JB.00527-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boguslawski KM, McKeown AN, Day CJ, Lacey KA, Tam K, Vozhilla N, Kim SY, Jennings MP, Koralov SB, Elde NC, Torres VJ. 2020. Exploiting species specificity to understand the tropism of a human-specific toxin. Sci Adv 6:eaax7515. doi: 10.1126/sciadv.aax7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peetermans M, Vanassche T, Liesenborghs L, Claes J, Vande Velde G, Kwiecinksi J, Jin T, De Geest B, Hoylaerts MF, Lijnen RH, Verhamme P. 2014. Plasminogen activation by staphylokinase enhances local spreading of S. aureus in skin infections. BMC Microbiol 14:310. doi: 10.1186/s12866-014-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flannagan RS, Heit B, Heinrichs DE. 2015. Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens 4:826–868. doi: 10.3390/pathogens4040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ganesh VK, Liang X, Geoghegan JA, Cohen ALV, Venugopalan N, Foster TJ, Hook M. 2016. Lessons from the crystal Structure of the S. aureus surface protein clumping factor A in complex with tefibazumab, an inhibiting monoclonal antibody. EBioMedicine 13:328–338. doi: 10.1016/j.ebiom.2016.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herman-Bausier P, Labate C, Towell AM, Derclaye S, Geoghegan JA, Dufrene YF. 2018. Staphylococcus aureus clumping factor A is a force-sensitive molecular switch that activates bacterial adhesion. Proc Natl Acad Sci U S A 115:5564–5569. doi: 10.1073/pnas.1718104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi SH, Chung JW, Lee EJ, Kim TH, Lee MS, Kang JM, Song EH, Jun JB, Kim MN, Kim YS, Woo JH, Choi SH. 2010. Incidence, characteristics, and outcomes of Staphylococcus lugdunensis bacteremia. J Clin Microbiol 48:3346–3349. doi: 10.1128/JCM.00609-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zinkernagel AS, Zinkernagel MS, Elzi MV, Genoni M, Gubler J, Zbinden R, Mueller NJ. 2008. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 36:314–321. doi: 10.1007/s15010-008-7287-9. [DOI] [PubMed] [Google Scholar]
- 106.Shah NB, Osmon DR, Fadel H, Patel R, Kohner PC, Steckelberg JM, Mabry T, Berbari EF. 2010. Laboratory and clinical characteristics of Staphylococcus lugdunensis prosthetic joint infections. J Clin Microbiol 48:1600–1603. doi: 10.1128/JCM.01769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li A, Gow N, Atkins BL, Taylor A, Peto T, McNally MA, Matthews PC. 2017. Metalware-associated orthopaedic infections caused by Staphylococcus lugdunensis: an emerging pathogen. J Infect 75:368–370. doi: 10.1016/j.jinf.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seng P, Traore M, Lavigne JP, Maulin L, Lagier JC, Thiery JF, Levy PY, Roger PM, Bonnet E, Sotto A, Stein A. 2017. Staphylococcus lugdunensis: a neglected pathogen of infections involving fracture-fixation devices. Int Orthop 41:1085–1091. doi: 10.1007/s00264-017-3476-4. [DOI] [PubMed] [Google Scholar]
- 109.Seng P, Traore M, Lagier JC, Lavigne JP, Sotto A, Drancourt M, Stein A. 2017. Staphylococcus lugdunensis: an underreported pathogen in osteomyelitis. J Foot Ankle Surg 56:412–413. doi: 10.1053/j.jfas.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 110.Bocher S, Tonning B, Skov RL, Prag J. 2009. Staphylococcus lugdunensis, a common cause of skin and soft tissue infections in the community. J Clin Microbiol 47:946–950. doi: 10.1128/JCM.01024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heldt Manica LA, Cohen PR. 2017. Staphylococcus lugdunensis infections of the skin and soft tissue: a case series and review. Dermatol Ther (Heidelb) 7:555–562. doi: 10.1007/s13555-017-0202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O'Riordan K, Lee JC. 2004. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev 17:218–234. doi: 10.1128/cmr.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kocianova S, Vuong C, Yao Y, Voyich JM, Fischer ER, DeLeo FR, Otto M. 2005. Key role of poly-gamma-DL-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J Clin Invest 115:688–694. doi: 10.1172/JCI23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramarao N, Sanchis V. 2013. The pore-forming haemolysins of Bacillus cereus: a review. Toxins (Basel) 5:1119–1139. doi: 10.3390/toxins5061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kretschmer D, Rautenberg M, Linke D, Peschel A. 2015. Peptide length and folding state govern the capacity of staphylococcal beta-type phenol-soluble modulins to activate human formyl-peptide receptors 1 or 2. J Leukoc Biol 97:689–697. doi: 10.1189/jlb.2A0514-275R. [DOI] [PubMed] [Google Scholar]
- 116.Weidenmaier C, Peschel A, Kempf VA, Lucindo N, Yeaman MR, Bayer AS. 2005. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect Immun 73:8033–8038. doi: 10.1128/IAI.73.12.8033-8038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]