Abstract
It is well shown that chronic wounds are populated by cells unable to respond to re‐epithelising stimulus. Large ulcers that remain unhealed for several months are more difficult to treat probably because of the depletion of active factors. Yet in 1869 Reverdin realised that the partial coverage of an ulcer with small fragments of healthy skin was able to lead to wound healing; unfortunately, its employment was limited to granulating wounds. Recently, the importance of factors such as cytokines, chemokines and adhesion molecules in wound healing, and the involvement of all cellular types resident or transiting in the skin has been partially elucidated. In this study, we proposed to simultaneously provide a new cellular and molecular reservoir with the efficient stimulus to trigger it. We created receiving site inside the ulcer, able to contain a full‐thickness graft taken from a donor site. Our aim was not to cover the entire defect, but to use the minigraft as ‘fount’ of functional cells and to give an acute stress through the chambers created inside the ulcer. A complete wound healing was obtained in all patients treated in a short period of time. This technique does not require special equipment and assistance in maintaining costs at very low levels.
Keywords: Chronic ulcer, Graft, Nested, Wound healing
INTRODUCTION
Wound healing is a complex and well‐regulated process that aims at the reconstruction of damaged tissue (1). It consists of three main phases: inflammation, tissue formation and tissue remodelling. The first phase starts immediately after wounding and is dominated by the release of cytokines and growth factors, influx of neutrophils and macrophages in the wound area and creation of preliminary matrix (Figure 1). Then re‐epithelialisation and granulation tissue formation start within hours after injury and last for several days. Finally, extracellular matrix is reorganised and retracted in the phase of tissue remodelling which lasts for several weeks. Large ulcers which remain unhealed for several months are more difficult to treat successfully probably because of the depletion of active factors and to the deregulation in cellular communication. Recently, the importance of cytokines, transcriptional factors, chemokines, adhesion molecules in wound healing and the involvement of all cellular types resident or transiting in the skin has been partially elucidated 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13. A large number of publications underline both the importance of each single component of this network and its capacity of reciprocal interaction which can regulate other components but need regulation itself 10, 11. On the basis of these findings, the use of different molecules or cells has been proposed for the wound treatment such as haematic cells, growth factors and cytokines. Although these approaches show interesting results they often require complex and expensive laboratory procedures that make them not easily applicable 14, 15, 16, 17, 18, 19.
Figure 1.
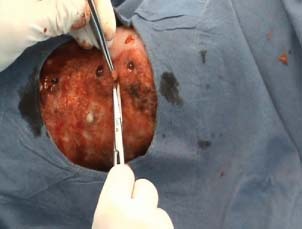
Surgical technique for chambers creation in the ulcer bed.
Furthermore, application of a single biological device shows a partial potential healing depending on the receiving substrate phase. Starting from this knowledge we considered that only healthy skin contains and is able to produce the cellular types and the molecular network involved in wound healing. Reverdin in 1869 realised the regenerative potential of the skin. He first introduced the graft, consisting in removing tiny pieces of skin from a healthy area of the body and seeding them in a location that needs to be covered (Figure 2). All the surgical techniques derived (pinch graft, punch graft, full‐thickness graft, split‐thickness graft, etc.) are based on this principle. Grafts offer a valid approach for chronic leg ulcers management and they are particularly well suited for venous and diabetic ulcers; unfortunately, all these surgical techniques have the disadvantage to be efficient only in the presence of granulating wound bed, hastening a yet active process.
Figure 2.
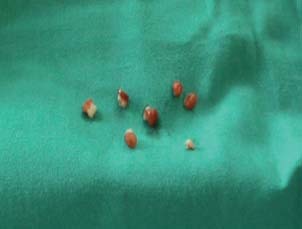
Pieces of the ulcer bed removed.
We propose a new type of graft whose aim is not only to cover a yet granulating wound but to trigger ab initio wound healing: we improve granulation through a pro‐inflammatory stimulus and, at the same time, provide an immediate and complete cellular source. So we practice some punch holes inside chronic wound bed and then we nest full‐thickness graft taken from a healthy donor site.
This surgical approach combines different characteristics of other techniques previously elaborated for ulcers treatment (20). In this paper, we present our preliminary yet interesting results.
MATERIALS AND METHODS
Patients affected by stable venous chronic ulcers unresponsive to traditional dressing were selected for the study. Exclusion criteria were the presence of infection. Informed consent was obtained from all patients. Five patients with chronic wound ulcers were recruited for the treatment; patients (one man and four women) aged 65–79 (mean: 72·4 years) had mean ulcer dimension of 46·4 cm2 (dimensional evaluation has been performed with Visitrak system, Smith & Nephew) and were treated with an average number of 7·4 punches (Table 1). Preparation of ulcers: on presentation most ulcers were oedematous and contained slough. Compression bandaging was usually required over 2 weeks before the application. The donor site was prepared using povidone iodine and a local anaesthesia was achieved using 1% lidocaine. Full‐thickness explants, without hypodermal fat, were taken from the donor site (usually on non cosmetically important sites like upper arm) using a 6‐mm punch biopsy and immediately closed primarily with a simple suture. The graft was deposited in a Petri dish containing physiological saline. The receiving site (ulcer bed) was prepared with soft curettage and then full‐thickness circular fragments of ulcer were removed by using a 5‐mm punch biopsy (1, 2). The chambers were created at a distance of 1–2 cm from each other and from the margins. The explants were taken from the donor site using a slightly larger punch than the one used for the recipient area because of the physiological retraction of the graft. A non adhesive dressing (paraffin gauze) kept the graft in place with suitable compressive bandage. Postoperative immobilisation was not required and patients usually do not need hospitalisation. Dressing was removed after 7–8 days in order to avoid the necessity of medication at home (Figure 3). After the first clinical evaluation we put a silver‐impregnated wound dressings (Aquacell Ag, Convatec) and a standard inelastic bandage. The patients were called weekly in order to assess the extent of re‐epithelialisation and to change dressing. Furthermore, in order to valuate the real efficacy of our technique, we treat one patient affected by a large ulcer involving the anterior inter malleolar zone (55 cm2) with a small spare area upon the Achilles' tendon in two different ways: in the medial zone only curettage of wound bed was made, while in the lateral zone nested graft was used alternate to classic Reverdin graft. Then a bandage was made for the leg.
Table 1.
Results.
| Patient | Age | Ulcer dimension (cm2) | Number of punches | Time of re‐epithelialisation (weeks) |
|---|---|---|---|---|
| 1 | 65 | 120 | 18 | 12 |
| 2 | 72 | 16 | 4 | 7 |
| 3 | 68 | 9 | 4 | 9 |
| 4 | 79 | 15 | 3 | 12 |
| 5 | 78 | 72 | 8 | 9 |
Figure 3.

Clinical appearance at week 1 post‐surgery.
At 2 weeks of follow‐up the lateral zone showed that the nested grafts were vital, helping the re‐epithelisation while epidermal skin graft was rejected. The medial zone was unchanged.
RESULTS
No side effects like infection or pain were present after the treatment. The grafts directly covered a small area of ulcer (4·46%). Of the 37 grafts nested in the 5 patients, all 37 were vascularised in 1 week (100%). The mean initial size of treated ulcer was 46·4 cm2. All treated wounds healed (4, 5). The mean time of closure was 68·6 days (9·8 weeks), translated in a speed of re‐epithelisation of 4·1 cm2/week. All patients showed a complete wound closure after a 16‐month period of follow‐up (Table 1).
Figure 4.
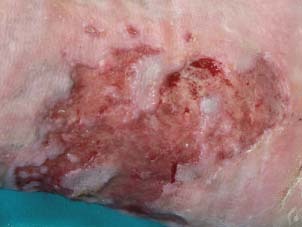
Active borders of the ulcer at week 6.
Figure 5.
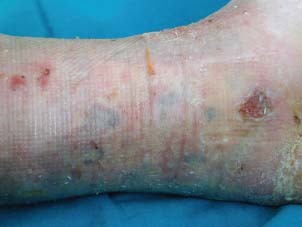
Complete re‐epithelisation at week 12.
DISCUSSION
In the last years research has led to a better understanding of the role of a large number of cytokines, transcriptional factors, cell receptors, chemokines, adhesion molecules and the involvement of all cellular types resident or transiting in the skin 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13. Recent publications have underlined both the importance of each single component of this network and their capacity of reciprocal interaction which can regulate other components but need regulation itself 10, 11. On the basis of such findings, the use of different molecules or cells was proposed for wound healing such as haematic cells, growth factors and cytokines. Although these approaches showed interesting but limited results they often require complex and expensive laboratory procedures that make it not easily applicable 13, 14, 15, 16, 17, 18, 19. Furthermore, traditional surgical approaches based on complete or partial coverage of wounds, generally because of autologous/heterologous direct or cultured skin grafts, have the disadvantage of hastening only the healing of yet granulating wounds.
Starting from the main limitations of traditional surgical approaches and from recent findings concerning the cellular and molecular mechanisms of wound healing, we combined well‐known techniques that aim to provide depleting cells (punch graft, pinch graft and minced graft) with other techniques that aim in stimulating and enhancing granulation tissue. The aim of the nested graft is to repopulate chronic ulcers with healthy cell populations and create an intimate communication between ulcer cells and functional cells taken from normal areas. Nested graft acts both as a fount of all different cells types involved in the repair process and as a producer of intracellular signals (so full‐thickness grafts reproduce a complete and functional skin unit). The method starts with the creation of chambers inside the ulcer not only to contain explants. The stress deriving from surgery treatment in fact induces in both, graft tissue and receiving chambers tissue, an inflammatory stimulus able to trigger a new wound healing process (20). Re‐epithelialisation rapidly starts from the graft and stimulates the border wound cells. Re‐epithelialisation mean speed for a single punch (0·28 cm) is 0·68 cm/week, which means it can triplicate itself every 10 days. We observed a progressive degree of the wound healing speed starting from 4 weeks after grafting conducing to growth arrest in about 10–12 weeks (4, 5). This data suggests a limited regenerative capacity of the graft islands and secondly the necessity to repeat the graft if the wound healing is incomplete after 3 months. The technique is easy and does not require patient immobilisation and hospitalisation, so the health costs are reduced, in comparison with the traditional surgical graft. Furthermore, the donor site is sutured in order to achieve a complete healing in 7 days, with very cheap management. Finally, nested graft does not involve different or more frequent medications compared with the normal wound management (hydrofibre and compression bandage). It can be performed with local anaesthesia and has very little patient impairment. This technique does not present side effects like infection in the donor site or recipient site. Further large‐scale patient studies are required, but these preliminary data showed satisfactory results, with a 100% of wound healing, in relatively short periods of time and in an inexpensive way.
REFERENCES
- 1. Braiman‐Wiksman L, Solomonik I, Spira R, Tennenbaum T. Novel insights into wound healing sequence of events. Toxicol Pathol 2007;35:767–79. [DOI] [PubMed] [Google Scholar]
- 2. Otrock ZK, Mahfouz RA, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis 2007;39:212–20. Epub 6 June 2007. [DOI] [PubMed] [Google Scholar]
- 3. Leahy PJ, Lawrence WT. Biologic enhancement of wound healing. Clin Plast Surg 2007;34:659. [DOI] [PubMed] [Google Scholar]
- 4. Raja SK, Garcia MS, Isseroff RR. Wound re‐epithelialization: modulating kerationcyte migration in wound healing. Front Biosci 2007;12:2849–68. [DOI] [PubMed] [Google Scholar]
- 5. Werner S, Krieg T, Smola H. Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol 2007;127:998–1008. [DOI] [PubMed] [Google Scholar]
- 6. Agren MS. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int J Low Extrem Wounds 2007;6:82–97. [DOI] [PubMed] [Google Scholar]
- 7. Ishida Y, Gao JL, Murphy PM. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J Immunol 2008;180:569–79. [DOI] [PubMed] [Google Scholar]
- 8. Naik TU, Naik MU, Naik UP. Junctional adhesion molecules in angiogenesis. Front Biosci 2008;13:258–62. [DOI] [PubMed] [Google Scholar]
- 9. Ochoa O, Torres FM, Shireman PK. Chemokines and diabetic wound healing. Vascular 2007;15:350–5. [DOI] [PubMed] [Google Scholar]
- 10. Debus ES, Schmidt K, Ziegler UE, Thiede A. The role of growth factors in wound healing. Zentralbl Chir 2000;125 (Suppl 1):49–55. [PubMed] [Google Scholar]
- 11. Grazul‐Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, Abdullah KM. Wound healing: the role of growth factors. Drugs Today (Barc) 2003;39:787–800. [DOI] [PubMed] [Google Scholar]
- 12. Bhat S, Milner S. Antimicrobial peptides in burns and wounds. Curr Protein Pept Sci 2007;8:506–20. [DOI] [PubMed] [Google Scholar]
- 13. Macedo L, Pinhal‐Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88‐deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol 2007;171:1774–88. Epub 1 November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldberg MT, Han Y‐P, Yan C, Shaw MC, Garner WL. TNF‐alpha suppressor alpha‐smooth muscle actin expression in human dermal fibroblasts: an implication for abnormal wound healing. J Invest Dermatol 2007;127:2645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rolfe KJ, Richardson J, Vigor C, Irvine LM, Grobbelaar AO, Linge C. A role for TGF‐beta 1‐induced cellular responses during wound healing of the non‐scarring human fetus? J Invest Dermatol 2007;127:2656–67. [DOI] [PubMed] [Google Scholar]
- 16. Neub A, Houdek P, Ohnemus U, Moll I, Brandner JM. Biphasic regulation of AP‐1 subunits during human epidermal wound healing. J Invest Dermatol 2007;127:2453–63. [DOI] [PubMed] [Google Scholar]
- 17. Barnabei R, Landi F, Lambiase A, Pola R, Aloe L. Effect of topical application of nerve‐growth factor on pressure ulcers. Lancet 1999;24:307. [DOI] [PubMed] [Google Scholar]
- 18. Carter K. Growth factors: the wound healing therapy of the future. Br J Community Nurs 2003;8:S15–6, S18–9, S22–3. [DOI] [PubMed] [Google Scholar]
- 19. Giuggioli D, Magistro R, Colaci M, Franciosi U, Caruso A, Ferri C. The treatment of skin ulcers in systemic sclerosis; use of G‐CSF in 26 patients. Reumatismo 2006;58:26–30. [DOI] [PubMed] [Google Scholar]
- 20. Sullivan TP, Kirsner RS. Surgical pearl: punch technique to improve granulation over exposed tendons in chronic wounds. J Am Acad Dermatol 2002;47:439–40. [DOI] [PubMed] [Google Scholar]


