Abstract
Since the introduction of negative pressure wound therapy in combination with reticulated open cell foam (NPWT/ROCF) in 1997, the clinical and economic benefits of this therapy have been showed in several randomised‐controlled studies. This article describes the clinical application of a new portable NPWT unit. The V.A.C.Via™ Therapy System (KCI USA, Inc., San Antonio, TX) offers continuous negative pressure and dynamic pressure control for wound treatment of low exudating (<80 ml/day), small‐to‐medium size wounds, grafts and flaps in all care settings, including homecare. We describe four cases in which this new device was successfully used.
Keywords: Dynamic pressure control, DPC, NPWT, NPWT/ROCF, VAC therapy, VAC Via
INTRODUCTION
Since the introduction of negative pressure wound therapy with reticulated open cell foam (NPWT/ROCF; V.A.C.® Therapy; KCI USA, Inc., San Antonio, TX) in 1997 (1), NPWT systems have continued to evolve. Portable therapy units, safety features, optional instillation therapy, controlled pressure application, advanced software options and varied wound contact materials are some of the modifications that have occurred over time. With the increase in variety of available NPWT options, wound type, size and severity, as well as cost and patient mobility, have become important considerations when choosing an NPWT system.
In this age of cost containment, reducing length of stay is an important objective for most hospitals. A new single‐patient‐use, portable and virtually silent NPWT/ROCF system with a 7‐day lifespan (V.A.C.Via™ Therapy System, KCI USA, Inc., San Antonio, TX) potentially enables patients to transition faster from acute care to home (2).
Initial clinical applications occurred on a variety of wounds, including surgical, dehisced and traumatic wounds, as well as pressure ulcers and diabetic foot ulcers. We present four patient cases from four different hospitals showing the initial application of the V.A.C.Via™ Therapy System and the results in clinical practice (Table 1).
Table 1.
Patient demographics and wound healing data
| ID | Age (years) | Sex | Wound type | Wound location | Duration of NPWT/ROCF (days) | Initial system setting (mmHg) | Pressure setting at first dressing change (mmHg) | Days to closure from NPWT/ROCF initiation | Type of closure |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | F | Acute compartment syndrome | Wrist | 7 | −125 cont. | −25 to −125 DPC | 11 | Primary |
| 2 | 82 | F | Dehiscence post‐ORIF | Tibia | 21 (3 units) | −125 cont. | −125 cont. | Did not close | Metal plate prevented closure |
| 3 | 72 | M | STSG | Lower leg | 5 | −125 cont. | −125 cont. | 5 | Complete STSG take |
| 4 | 54 | M | Abscess | Thigh | 7 | −125 cont. | −125 cont. | 22 | Secondary |
NPWT/ROCF, negative pressure wound therapy in combination with reticulated open cell foam; STSG, split‐thickness skin graft.
CASE STUDY 1: SEPTIC ARTHRITIS AND ACUTE COMPARTMENT SYNDROME OF WRIST
A 36‐year‐old female was admitted to the hospital with severe pain in her right wrist, which was diagnosed as septic arthritis with acute compartment syndrome. Surgical debridement was performed in the operating theatre to remove all infected tissue. Bacterial culture showed an infection with a haemolytic Group G. Streptococcus, which was treated with intravenous antibiotic treatment (i.e. penicillin). To prepare the wound bed for closure by delayed primary intention, NPWT/ROCF was prescribed. The selection for the new V.A.C.Via™ Therapy device was made to facilitate patient's mobility. The need for i.v. antibiotics was the only reason why she could not be transitioned from hospital to home. By using this portable device, the treating team encouraged her to stay active and move around within the department. When applying the ROCF dressing, tendons and other delicate tissues were protected by applying a protective, non adherent layer (Mepitel®, Mölnlycke Health Care AB, Gothenburg, Sweden) (Figure 1A). A continuous negative pressure of −125 mmHg was applied during the first 3 days. At the first dressing change (Figure 1B), it was decided to cut the second dressing slightly smaller than the total wound to promote wound edge approximation (Figure 1C). To promote extra granulation tissue formation, the mode was switched from continuous −125 mmHg to DPC, oscillating the pressure between −25 and −125 mmHg. After 7 days of treatment with V.A.C.Via™ Therapy, the wound clearly showed healthy granulation tissue and a visible reduction of oedema (Figure 1D). As the new unit only has a working capacity of 7 days, NPWT treatment was continued with a standard larger mobile NPWT unit for another 4 days before the wound was primarily closed in the operating theatre (Figure 1E). No further wound treatment was required, and the patient was discharged with continuation of systemic i.v. antibiotic therapy via a port‐a‐cath system.
Figure 1.
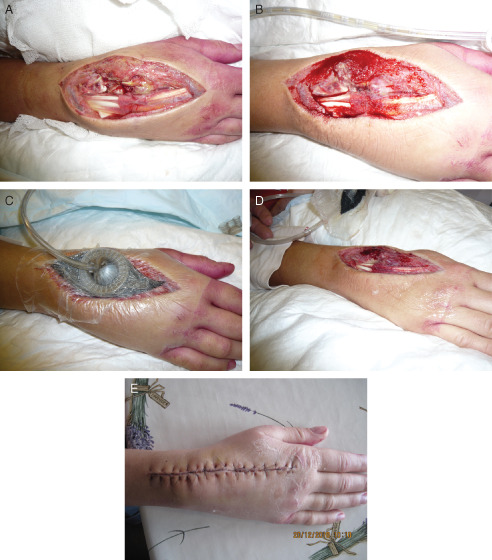
Case study 1: septic arthritis and acute compartment syndrome of wrist. (A) Day 0 – wound after surgical debridement and before application of negative pressure wound therapy in combination with reticulated open cell foam (NPWT/ROCF). (B) Day 3 – wound after removal of first dressing. (C) Day 3 – wound after start of negative pressure with second dressing. (D) Day 7 – wound showed clear reduction of oedema. (E) Day 11 – wound after surgical closure.
CASE STUDY 2: DEHISCENCE AFTER OPEN REDUCTION AND INTERNAL FIXATION OF A TIBIAL FRACTURE
An 82‐year‐old female presented with increasing pain due to an osteoporotic tibial fracture. Eight weeks after initial restoration and fixation of the bone with a metal plate, the surgical wound dehisced. The treating physician performed a combined flap reconstruction and a split‐thickness skin graft (STSG) to close the wound. Standard surgical procedure for these conditions is removal of the metal plate; however, as the fracture was not healed, it was decided to place the flap over the metal plate. Unfortunately, the STSG became infected, placing the flap at serious risk of loss. During admission at the hospital, traditional NPWT/ROCF (ActiV.A.C.® Therapy System, KCI USA, Inc., San Antonio, TX) was started in an attempt to save the flap and systemic antibiotic ciprofloxacin was administered.
After 3 days of NPWT/ROCF (Figure 2A), the patient was transitioned from hospital to home with the new V.A.C.Via™ Therapy System (setting: continuous −125 mmHg) to stimulate her mobility. The patient returned to the hospital for dressing changes (Figure 2B and C). After 1 week, the wound area and flap showed good improvement; therefore, the treatment was continued with a second V.A.C.Via™ Therapy unit. At the end of the second week, flap survival was achieved and the NPWT/ROCF was discontinued. Wound treatment with advanced moist wound dressings was started until a second STSG could be performed. However, the wound did not improve as expected, possibly due to vascular insufficiency. Four weeks after discontinuation, NPWT/ROCF was re‐initiated (Figure 2D). The presence of the metal fixation plate clearly affected wound healing progress (Figure 2E). During the following week, the wound progressed well; however, the metal plate prevented complete wound closure.
Figure 2.
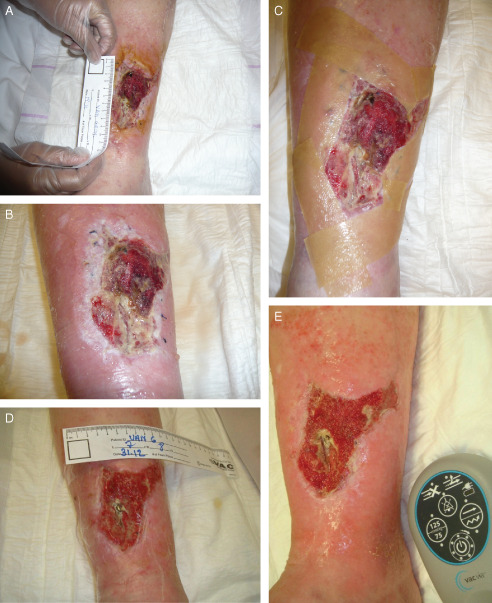
Case study 2: dehiscence after open reduction and internal fixation of a tibial fracture. (A) Dehisced wound after flap reconstruction and skin graft, before application of negative pressure wound therapy in combination with reticulated open cell foam (NPWT/ROCF). (B) Wound immediately after first dressing removal (48 hours after initial NPWT/ROCF application). (C) Wound after cleansing and before application of second dressing. (D) Wound at restart of NPWT/ROCF (6 weeks after first application); the metal fixation plate is still visible. (E) Wound 1 week after restart of NPWT/ROCF (7 weeks after initial start of NPWT/ROCF).
CASE STUDY 3: BILATERAL FASCIOTOMY OF THE LOWER LEG
A 72‐year‐old male patient presented in the hospital with acute ischaemia in the right lower leg. After successful revascularization, bilateral fasciotomies were performed to prevent the development of acute compartment syndrome. To help reduce oedema, traditional NPWT/ROCF was applied to both sides, using an InfoV.A.C.® Therapy System (KCI USA, Inc., San Antonio, TX) at −125 mmHg, continuous negative pressure. Dressing changes were performed every 48–72 hours. On day 7, the left side fasciotomy was closed, while NPWT/ROCF was continued on the right side. On day 14, the remaining fasciotomy wound on the right lateral lower leg was partially closed (Figure 3A), and NPWT/ROCF was continued for another 7 days to prepare the wound bed for closure with an STSG (Figure 3B).
Figure 3.
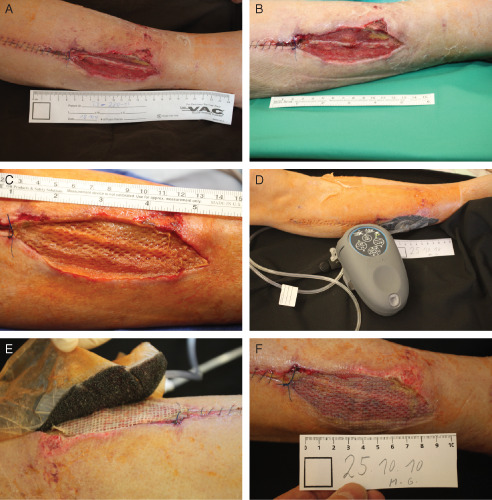
Case study 3: bilateral fasciotomy of the lower leg. (A) Day 14 – wound after partial closure of the right fasciotomy and before application of negative pressure wound therapy in combination with reticulated open cell foam (NPWT/ROCF). (B) Day 21 – clean wound bed prepared for split‐thickness skin graft (STSG). (C) Day 21 – STSG before application of NPWT/ROCF. (D) Day 21 – application of NPWT/ROCF. (E) Day 26 – removal of the foam after 5 days of NPWT/ROCF, a meshed non adherent layer protected the STSG. (F) Day 26 – complete skin graft, taken after NPWT/ROCF.
At day 21 after admission and application of NPWT/ROCF, an STSG was placed over the right remaining fasciotomy wound (Figure 3C). As the patient had to mobilise again, the V.A.C.Via™ Therapy System was chosen for bolstering and protection of the graft (Figure 3D). A non adherent mesh layer was placed over the STSG to provide a protective barrier (Figure 3E). After 5 days of NPWT/ROCF with continuous negative pressure at −125 mmHg, the STSG showed complete graft take (Figure 3F). NPWT/ROCF was discontinued, and the wound was treated with paraffin gauze till complete healing.
CASE STUDY 4: ABSCESS IN RIGHT THIGH
A 54‐year‐old diabetic male presented with pain and swelling in the right thigh. His inflammatory markers and blood sugar levels were increased. An abscess was identified, requiring incision and drainage. A specific cause could not be found; however, the patient's lifestyle and diabetes may have contributed.
The treating surgeon assigned the patient to tissue viability post‐operative care for dressing management. The wound was exudating, requiring gauze dressing changes twice daily. The V.A.C.Via™ Therapy system (continuous −125 mmHg) was applied within 24 hours post‐surgery to promote healing and reduce risk of infection due to multiple dressing changes (Figure 4A). Immediately after negative pressure dressing application, the patient was transitioned to home for further recovery. Dressing changes were performed in the hospital. The first ROCF dressing was changed 3 days later; healthy granulation tissue formation was clearly visible (Figure 4B). After 7days of NPWT/ROCF, the therapy objective of assisting the wound to close was achieved (Figure 4C), and NPWT/ROCF was discontinued. A hydrofibre wound dressing was applied until complete wound closure 15 days later.
Figure 4.
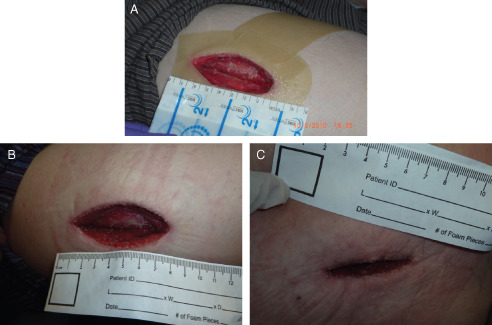
Case study 4: abscess in right thigh. (A) Surgical wound before application of negative pressure wound therapy in combination with reticulated open cell foam (NPWT/ROCF). (B) Wound after first dressing removal; clean wound with healthy granulation tissue. (C) After 7 days of NPWT/ROCF, wound almost closed.
DISCUSSION
On the basis of our initial clinical experience, the V.A.C.Via™ Therapy System is safe to use and assisted in achieving reconstructive goals in all cases in this series.
This new NPWT/ROCF device is designed for ease of use and reduces impact on patient's daily life. The device primarily differs from other NPWT products in its compact size and 7‐day lifespan, which makes it ideal for use in a home (care) situation. For this reason, this system would be best‐suited for grafts, flaps and low exudating (< 80 ml/day), small‐to‐medium size wounds (3).
The one‐button discreet operation and gentle pressure application and modification during DPC make the unit easy to use for caregivers and comfortable for patients. This may lower the impact of NPWT/ROCF on daily life, potentially enabling patients to transition faster from acute care to home. In the authors' views, the enhanced simplicity and portability of this new technology are much needed advancements towards managing wound treatment in low‐emergency care settings. The negative pressure device can be easily concealed underneath clothing, is almost silent, and can be set up with two pushes of a button – factors that can positively affect patient compliance.
Mechanisms of action are those of NPWT/ROCF systems. It is known that mechanisms of NPWT/ROCF as delivered by V.A.C.® Therapy are both fluid‐ and mechanical‐based (4). Applied mechanical forces of negative pressure on ROCF slowly deform tissue and cause cell deformation (5). Meanwhile, removal of excess interstitial fluid pushes the interstitial pressure to below capillary pressure; the capillaries reopen, and flow to the periwound tissue is resumed 6, 7. Cell deformation is followed by stimulation of growth factor pathways, resulting in increased mitosis and production of new granulation tissue 4, 8, 9. Preclinical laboratory studies with the V.A.C.Via™ Therapy system (10) have shown similar results with regard to the mechanisms of action and mechanical properties consistent with previously published scientific outcomes of traditional NPWT/ROCF (11).
Varying negative pressure, termed ‘Dynamic Pressure Control’ (DPC), is a modification of classical intermittent therapy, which has been shown to enhance blood flow and granulation tissue formation, compared with continuous pressure mode (1). Unlike intermittent negative pressure therapy, which returns the negative pressure to 0 mmHg between cycles, DPC returns the negative pressure to −25 mmHg. In this way, the foam dressing remains compressed between cycles, which can be advantageous in reducing the potential for dressing leaks and pain that some patients experience during dressing expansion when negative pressure returns to 0 mmHg (12).
With this new NPWT/ROCF device and the new DPC feature, our patients showed no signs of pain or discomfort as a result of active DPC. Also, no leakage alarms were reported when this therapy setting was used. Further clinical studies are required to confirm that DPC produces similar clinical results as intermittent therapy.
Although this new therapy system makes transition to home and home treatment easier, the patients described here came back to the clinic for dressing changes because there was no previous experience with the new system within the clinical practices of the authors. For this reason they preferred to do the dressing changes in the clinic to evaluate with the patients their experiences at home and to learn about safety and efficacy of the therapy system.
Overall experience showed that achieving the desired vacuum level required a slightly longer time than with other previously used NPWT units. Patients, however, expressed to their treating health care professional this slower induction of negative pressure is experienced as comfortable.
In our initial clinical experience, this new NPWT/ROCF system was used safely on these four patients, and the clinical results appear to be similar to the results we have seen previously using other NPWT/ROCF systems to treat these wound types.
ACKNOWLEDGEMENTS
The authors were involved with clinical treatment, data collection and manuscript preparation. The authors would like to thank Kinetic Concepts, Inc. for providing us product utilized in this evaluation. Also, the authors would like to thank Twan Wackers and Ricardo Martinez of Kinetic Concepts, Inc. for providing medical writing support (editorial assistance).
References
- 1. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997; 38:563–76. [PubMed] [Google Scholar]
- 2. Gabriel A, Thimmappa B, Rubano C, Storm‐Dickerson T. Ultra‐light, single‐patient‐use negative pressure wound therapy device as a bolster over split‐thickness skin grafts. (Presented at the Symposium on Advanced Wound Care Spring Meeting, April 14–17, 2011, Dallas, TX) [Abst 31.2]. Wounds 2011;23:A22–3. [Google Scholar]
- 3. FDA. FDA clearance letter for V.A.C. via negative pressure wound therapy system 3‐10‐2010. Silver Spring, MD, Food and Drug Administration.
- 4. Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum‐assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117:121S–6S. [DOI] [PubMed] [Google Scholar]
- 5. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 6. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 7. Wackenfors A, Sjogren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum‐assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 2004;12:600–6. [DOI] [PubMed] [Google Scholar]
- 8. Argenta A, Webb K, Simpson J, Gordon S, Kortesis B, Wanner M, Kremers L, Morykwas M. Deformation of superficial and deep abdominal tissues with application of a controlled vacuum. Presented at the European Tissue Repair Society, Focus Group Meeting, Topical Negative Pressure (TN) Therapy, Museum of London, 4–6 December 2003.
- 9. Armstrong DG, Lavery LA. Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005; 366: 1704–10. [DOI] [PubMed] [Google Scholar]
- 10. McNulty A, Schmidt M, Sanchez L, Flores I, Garcia S. The delivery of negative pressure using high frequency sound waves. Presented at Symposium on Advanced Wound Care, April 14–17 2011, Dallas, TX.
- 11. McNulty A. The consistent delivery of negative pressure to wounds using reticulated, open cell foam and regulated pressure feedback. [Abst P 081]. Presented at the Clinical Symposium on Advances in Skin & Wound Care, October 22–25, 2009, San Antonio, TX.
- 12. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. [DOI] [PubMed] [Google Scholar]


