Abstract
An ex vivo porcine skin explant biofilm model that preserves key properties of biofilm attached to skin at different levels of maturity (0–3 days) was used to assess the efficacy of commercially available antimicrobial dressings and topical treatments. Assays were also performed on the subpopulation of antibiotic tolerant biofilm generated by 24 hours of pre‐treatment with gentamicin (120× minimal inhibitory concentration) prior to agent exposure. Five types of antimicrobial agents (iodine, silver, polyhexamethylene biguanide, honey and ethanol) and four types of moisture dressings (cotton gauze, sodium carboxymethylcellulose fibre, calcium alginate fibre and cadexomer beads) were assessed. Time‐release silver gel and cadexomer iodine dressings were the most effective in reducing mature biofilm [between 5 and 7 logarithmic (log) of 7‐log total], whereas all other dressing formulations reduced biofilm between 0·3 and 2 log in 24 or 72 hours with a single exposure. Similar results were found after 24‐hour exposure to silver release dressings using an in vivo pig burn wound model, demonstrating correlation between the ex vivo and in vivo models. Results of this study indicate that commonly used microbicidal wound dressings vary widely in their ability to kill mature biofilm and the efficacy is influenced by time of exposure, number of applications, moisture level and agent formulation (sustained release).
Keywords: Biofilm, Cadexomer iodine, In vitro model, Pseudomonas aeruginosa, Wound dressing
Introduction
It has been hypothesised that four main causative factors, which can occur alone or in combinations, contribute to the pathogenesis of chronic wounds: tissue hypoxia, repetitive ischaemia/reperfusion injury, age‐impaired stress response and elevated bacterial levels 1, 2. Microbial biofilms have been recognised as a common cause of chronic and persistent infections 3 by acting as a microbial reservoir. More recently, the presence of bacteria in the form of biofilms was proposed to be a major cause of impaired skin wound healing, contributing to the development of chronic wounds 4, 5, 6, possibly irrespective of total bacterial levels. James et al. 4 showed that 60% of chronic wounds and 6% of acute wounds contained biofilm structures. This proposed link of bacterial biofilm to the chronic state of wounds has led us to investigate and assess the efficacy of antimicrobial agents and dressings against bacterial biofilm commonly used in the treatment of chronic wounds. Determining the antimicrobial efficacy of dressings and agents usually begins with in vitro assays that allow highly specific control over environmental conditions. Because most biofilm research has focused on in vitro assays that use abiotic surfaces for microbial attachment to answer these questions, there is a need to establish alternate models to study biofilms expressing molecular characteristics more relevant to tissue infections while maintaining the relative ease of an in vitro assay for rapid screening of antimicrobial agents of interest 7.
It is generally understood that microbial biofilm consists of microorganisms embedded in a self‐synthesised secreted exopolymeric substance (EPS), forming dense microbial communities with complex architecture and providing substantial protection for bacteria from host antibodies, phagocytic inflammatory cells, antibiotics, antiseptics and disinfectants while facilitating waste removal and uptake of nutritional requirements 8, 9, 10. Regardless of their location or diversity, biofilms have a common pattern of development including attachment, microcolony formation, maturation and dispersion. Despite these common features, biofilm development is a complex process and its composition is greatly influenced by the identity of the microflora involved, the environmental conditions, both chemical and physical, its maturity, source of nutrition and the substrate to which it attaches 11, 12, 13. Furthermore, the structure, EPS and metabolic status of biofilms provide substantial protection for bacteria and produce extreme tolerance to antimicrobial agents.
We previously developed an ex vivo model of mature biofilm cultured on porcine skin explants 7 and used this model in a pilot study to assess the efficacy of a few commercial antimicrobial agents 14. While the ex vivo porcine skin explant biofilm model used in this study is incapable of accounting for the contribution of host factors (immune response, wound fluid, etc.) found in skin wounds, its use as a dermal substrate for attachment and the primary source of nutrition provides an alternative cost‐effective method that more closely mimics in vivo conditions, with the advantages of an in vitro system, to assess the antimicrobial efficacy of these treatments against mature biofilms. This ex vivo porcine skin explant biofilm model was further validated against data generated from an in vivo pig burn wound model 10, 15 that was used to assess the efficacy of silver dressings against PAO1 biofilms.
Many antimicrobial dressings have been designed to prevent infection and reduce bacterial load while promoting wound healing. Moisture dressings designed to absorb wound exudates and/or maintain a moist wound environment are generally changed every few days, depending on the level of wound drainage. In this study, five types of antimicrobial agents [i.e. iodine, silver, polyhexamethylene biguanide (PHMB), honey and ethanol (ETOH)] and four types of moisture dressings [i.e. cotton gauze, sodium carboxymethylcellulose (NaCMC) fibre, calcium alginate fibre and cadexomer beads] were assessed. The purpose of these experiments was to perform a more in‐depth analysis of various antimicrobial agents in their ability to reduce PAO1 biofilms using a porcine skin explant biofilm model and to identify any significant differences in the bacterial load following prolonged exposure to various moisture dressings.
Materials and methods
Bacterial culture
Pseudomonas aeruginosa PAO1 was streaked for isolation on tryptic soy agar (TSA; Difco™, Becton, Dickinson and Company, Sparks, MD) plates from frozen stock cultures and incubated overnight at 37°C for 16–18 hours. A culture tube containing 5 ml of tryptic soy broth (TSB; Difco™, Becton, Dickinson and Company) was inoculated with an isolated colony and incubated overnight at 37°C. A flask containing 50 ml of TSB was inoculated with 150 µl of overnight culture and incubated at 37°C in a water bath (Becton, Dickinson and Company) at 150 rpm until the culture reached an optical density (OD640) between 0·2 and 0·4 (early‐log phase). The optical density was determined using a spectrophotometer (UNICO 1100, United Products & Instrument INC, Dayton, NJ) and the viable colony‐forming units (CFUs) per millilitre were verified by spread plate cultured colony counts.
Dressings and antimicrobial agents
Five types of commonly used wound dressings or agents were assessed for their antimicrobial efficacy against P. aeruginosa PAO1 biofilm grown using the ex vivo porcine skin model: iodine, silver, PHMB, active Leptospermum honey and ETOH. In addition, several non‐antimicrobial moisture dressings were used to collect relevant control data to compare with the antimicrobial treatment outcomes. The dressings and agents tested in this study, including commercial and generic names with brief background information about the design and relevant application in wound care for each, are listed in Table 1. Based on this information, all dressings (other than antimicrobial solutions) were completely hydrated with sterile water rather than buffered saline solutions to prevent possible salt deactivation while maximising agent release and availability. Complete hydration is important because the majority of the dressings tested were designed to be applied to exudating wounds, and per manufacturer's recommendation, several of the dressings should be activated by pre‐hydration prior to wound application. Furthermore, because the majority of the wound dressings tested were designed to be used continuously for at least 72 hours, each product was evaluated at 24 and 72 hours of continuous exposure. A more complete analysis of the functional relevance of the agents and dressings used will be addressed in the discussion.
Table 1.
Dressings and antimicrobial agents used in this study: Brand name, descriptions and manufacturer recommendations
| Dressing/agent | Brand name and description |
|---|---|
| Cadexomer | Cadesorb® ointment (Smith & Nephew): Moisture dressing of cadexomer beads is designed to absorb wound exudates and modulate (reduce) protease activity by controlling local wound pH and promote healing. Cadesorb consists of water‐soluble cadexomer polymer (dextran and epichlorhydrin) cross‐linked to form a 3D matrix in the form of 0·1–0·3‐mm diameter spheres. 1 g Cadexomer can absorb 6 ml of fluid. |
| Cadexomer iodine | Iodoflex™ pad and Iodosorb® ointment (Smith & Nephew): Antimicrobial moisture dressings designed to absorb wound exudate (of ulcers, sores and infected wounds), lower wound pH and time‐release iodine from cadexomer beads at a rate to maintain iodine availability at 1 ppm to kill microbes without harming healthy tissue. Dry cadexomer beads contain 0·9% iodine. Iodosorb ointment contains 50% cadexomer iodine and 50% ointment base. |
| Calcium alginate (Ca Alginate) | Algisite®‐M (Smith & Nephew): A moisture dressing made of calcium alginate fibres and is designed to absorb wound exudates (of full‐ and partial‐thickness wounds) for up to 3 days (or changed daily for heavily exuding or infected wound). |
| Calcium alginate–nanocrystalline silver (Ca Alginate‐NanoAg) | Acticoat® Absorbent (Smith & Nephew): Antimicrobial moisture dressing made of calcium alginate fibres coated in SILCRYST™ nanocrystalline silver and is designed to absorb wound exudates (of full‐ and partial‐thickness wounds) and steadily release silver ions (70–100 ppm continuously available) for up to 7 days (with an antimicrobial effect minimum of 3 days). |
| Calcium alginate–Leptospermum honey (Ca Alginate‐L. honey) | Medihoney®‐impregnated calcium alginate pad (Derma Sciences): An absorbent wound and burn dressing with 95% active Manuka Leptospermum honey and 5% calcium alginate fibre designed for moderate to heavy exudating wounds. It is used to maintain a moist wound environment and provide high osmolarity for wound cleansing and debridement and low pH for healing of exudating full‐ and partial‐thickness wounds. |
| Cotton gauze | Medline 12 or 16 ply USP Type VII: 100% cotton woven gauze sponge. |
| Cotton gauze–polyhexamethylene biguanide (PHMB gauze) | Curity™ AMD™ (Kendall) gauze: A 100% cotton fine mesh gauze packing strip for exudating wounds, surgical wounds or wound packing, impregnated with the microbicide PHMB (0·2%) and designed to resist bacterial colonisation within the dressing and act as a prophylactic barrier to prevent penetration of external invading bacteria through the dressing. |
| Cotton gauze–silver sulphate (Ag sulphate gauze) | Tegaderm® AgMesh (3M): Antimicrobial non‐woven 100% cotton dressing that contains silver sulphate and is designed to release ionic silver on moist or moistened wounds for up to 7 days. |
| Ethanol (ETOH) | Absolute ethanol (Sigma‐Aldrich ‐E7023): 200 proof, for molecular biology. ETOH is an antimicrobial antiseptic. |
| Hydrocolloid–silver (Ag) | Contreet‐H/Biatain Ag (Coloplast Pty Ltd.): Hydrocolloid moisture dressing designed to release ionised silver (hydroactivated) in moderate to high exudating wounds for up to 7 days. |
| Leptospermum honey (L. honey) | Medihoney ointment (Derma Sciences): An absorbent wound and burn dressing with 100% active Manuka Leptospermum honey for dry to lightly exudating wounds, designed to maintain a moist wound environment and provide high osmolarity for wound cleansing and debridement and low pH for healing of exudating full‐ and partial‐thickness wounds. |
| Polyacrylate–silver chloride (polyacrylate–AgCl) | Silvasorb® Gel (Medline): An antimicrobial wound hydrogel using Microlattice® technology consisting of polyacrylate hydrophilic matrix particles suspended in glycerol and polysaccharides forming a scaffold that stabilises silver chloride salt [AgCl(n)] designed to release microbicidal silver ions at an average rate of 1·5 ppm (continuously available for at least 3 days and can potentially be left in place for up to 7 days) without harming healthy tissue in partial‐ or full‐thickness acute and chronic wounds. The unique Microlattice hydrogel was designed to allow oxygen to penetrate to the wound, minimise the moisture escaping to the surface, while simultaneously controlling the amount of exudate. |
| Polyhexamethylene biguanide (PHMB) | Prontosan™ Wound Gel (B. Braun Medical): A gel designed for moistening absorbent wound dressings and for cleansing and decontaminating partial‐ or full‐thickness acute and chronic wounds to reduce bioburden, inhibit microbial colonisation and prevent infection. It contains glycerol, hydroxyethylcellulose, 0·1% undecylenamidopropyl betaine surfactant and microbicidal 0·1% polyaminopropyl biguanide polymeric cationic antiseptic that kills microbes by disrupting electrostatic interactions without harming healthy tissue. It is designed to be applied to the wound cavity and covered with a secondary dressing and changed every 3 days (±1 day). |
| Polyethylene–nanocrystalline silver (polyethylene‐NanoAg) | Acticoat and Acticoat‐7 (Smith & Nephew): An antimicrobial barrier dressing for partial‐ and full‐thickness wounds judged to be critically colonised with bacteria or at risk from infection. Acticoat has three ultrasonically welded layers consisting of a central apertured non‐woven fabric made from rayon and polyester sandwiched between two outer layers of nanocrystalline silver‐coated polyethylene mesh. Acticoat‐7 has five ultrasonically welded layers consisting of a central nanocrystalline silver‐coated mesh of high‐density polyethylene sandwiched between two core layers of an apertured non‐woven fabric made from rayon and polyester, sandwiched between two outer layers of nanocrystalline silver‐coated polyethylene mesh. It is recommended that Acticoat and Acticoat‐7 be moistened with sterile water prior to use and that a secondary dressing be applied to maintain moisture balance. Acticoat and Acticoat‐7 may be left in place for a maximum of 3 and 7 days, respectively, although it may be necessary to replace it more frequently for heavily exuding wounds. |
| Povidone‐iodine (PVP‐I) | Triadine™ PVP‐I (USP): An antiseptic prep solution containing a 10% topical solution of PVP‐I with 1% available iodine. |
| Sodium carboxymethylcellulose–ionic silver (NaCMC‐Ag) | Aquacel®‐Ag (ConvaTec): An antimicrobial hydrofibre® moisture dressing of NaCMC containing 1·2% ionic silver was designed to absorb wound exudates and steadily release silver ions (1 ppm continuously available) for up to 7 days. |
| Sodium carboxymethylcellulose (NaCMC) | Aquacel (ConvaTec): A hydrofibre® moisture dressing of sodium carboxymethylcellulose fibres designed to absorb wound exudates for up to 7 days. |
Biofilm porcine explant model
The ex vivo model of biofilm on porcine skin explants used in this study 7 consisted of 12‐mm biopsied explants (3–4 mm thick) prepared from freshly harvested, shaved and cleaned porcine skin obtained from a local abattoir (Chiefland Custom Meat, Trenton, FL). The mechanically created ‘wound bed’ (3‐mm high speed, round, cutter bit; Dremel®, Robert Bosch Tool Corporation, Racine, WI) was 3 mm in diameter and approximately 1·5 mm in depth at the centre of each explant. The chlorine gas (45 minutes)‐sterilised explants were placed on soft TSA plates containing 0·5% agar and 50 µg/ml gentamicin. The addition of 50 µg/ml gentamicin (˜30× minimal inhibitory concentration) functions to limit bacterial growth to the explant and inhibits penetration of PAO1 biofilm through the bottom of the explant for up to 5–6 days, depending on the thickness of the explant. The partial‐thickness ‘wound bed’ of the explants was inoculated with 10 µl early‐logarithmic (log)‐phase PAO1 suspension culture (106 CFU) and cultured at 37°C with 5% CO2 and saturated humidity. Explants were transferred daily to fresh soft TSA plates containing 0·5% agar and antibiotic (to maintain moisture) until the desired biofilm maturity was achieved. They were submerged in TSB media containing 200 µg/ml gentamicin for 24 hours to kill planktonic PAO1 in studies used to assess antimicrobial efficacy of test agents specifically against the highly antibiotic tolerant biofilm subpopulation attached to the porcine explants, described in more complete detail below. For clarity, exposure times to the test agents were expressed in hours and the length of biofilm culture incubation prior to treatment was expressed in days.
The bacterial load of the explants was determined in each of the assays of this study as follows: each explant was aseptically placed into a 15‐ml sterile tube (on ice) containing cold 7‐ml sterile phosphate‐buffered saline (PBS) with 5 µl/l Tween‐80. The explants in the tubes were sonicated with a 23‐kHz ultrasonic dismembrator (Model 100, Fisher Scientific, Pittsburgh, PA) probe for 30 seconds at approximately 20 W on ice, which liberated bacteria from the biofilm into the suspension. The setting on the dismembrator probe tip was adjusted to maintain the target watt output. The sonication probe was disinfected between samples using cold 70% ETOH and rinsed with cold sterile PBS (on ice). Serial dilutions of the bacterial suspension were plated in triplicate on TSA plates and incubated overnight at 37°C with 5% CO2 and saturated humidity. Colonies were counted from the plates to determine the CFU/ml of the sonicated explant bacterial suspension.
Assessment of the efficacy of antimicrobial dressings against PAO1 biofilm
24‐ and 72‐Hour continuous exposure
Because several antimicrobial wound dressings used in this study were designed to be used for at least 72 hours continuously (Table 1), antimicrobial efficacy assays against mature PAO1 biofilm attached to the skin were performed with 24‐ and 72‐hour continuous exposure. PAO1 biofilms cultured 3 days on porcine skin explants were transferred to sterile 24‐well microtiter plates and each explant was treated for 24 hours by submersion in 2 ml TSB media containing 200 µg/ml gentamicin. This level of antibiotic was used because it was capable of restraining the PAO1 biofilm to the surface of the explant 7. The media in the wells remained clear and no viable bacteria were detected in the media or the microtiter wells during or after treatment of the explants. As stated previously, pre‐treatment with high antibiotics allows subsequent assessment of the antimicrobial efficacy of the dressing agents directly against the antibiotic tolerant biofilm subpopulation. The antibiotic pre‐treated explants, containing only mature PAO1 biofilm, were each rinsed thrice with 2 ml of sterile PBS, washed in 2 ml PBS for 10 minutes and then rinsed thrice with 2 ml PBS to remove unattached bacteria. The rinsed biofilm explants were transferred to soft TSA plates containing 0·5% agar and 50 µg/ml gentamicin (three or four explants per plate).
The biofilm explants that were used to determine the ‘standard’ baseline total microbial load were covered with sterile ddH2O‐saturated (5 ml) ‘wet’ cotton gauze sponge (2″ × 2″). The rest of the biofilm explants were covered with either (a) liquid antimicrobial agent‐saturated cotton gauze (5 ml) or (b) ointments and gels (3 g) spread on a cotton gauze sponge (2″ × 2″) and hydrated with sterile ddH2O (5 ml) or (c) prepared on commercial wound dressing strips (1″ × 2″) hydrated with ddH2O (3 ml). The dressings that were placed on the biofilm explants were weighed with sterile glass slides (1″ × 3″ × 1·0 mm) to ensure complete dressing contact and incubated for 24 and 72 hours at 37°C with 5% CO2 and saturated humidity. Continuous exposure proceeded for 24 or 72 hours using either a single application or multiple applications of fresh dressing at specified time points. The treated biofilm explants were each processed by sonication in 7 ml PBS with 5 µl/l Tween‐80, as previously described. Bacterial suspensions were immediately serially diluted and plated in triplicate on TSB, and the average CFU/ml was determined for the 7‐ml bacterial suspension from each explant. A minimum of three separate trials, typically using nine explants per treatment group per trial, were performed for each antimicrobial dressing reported in this study. Wet gauze, no dressing and before dressing treatment (0 hour) control groups were included in every trial.
Time‐course assay
The time‐course studies were performed to determine if the antimicrobial efficacy of certain agents depended on biofilm maturity. Several intermediate exposure times were also assessed in conjunction with varying biofilm maturity to determine if bacterial load observed after 24‐hour (or 72‐hour) exposure reflected maximal antimicrobial activity or possibly early biofilm knockdown with subsequent recovery. Sterile porcine skin explants were prepared, inoculated and cultured as previously described in the 24‐ and 72‐hour exposure assays but were not pre‐treated with 24‐hour submersion in antibiotic media prior to dressing exposure. This modification was made in order to assess a wide range of biofilm maturity levels. The explants were dressed as previously described at specific time points of biofilm maturity post‐inoculation: 0‐day biofilm (2 hours post‐inoculation with 106 CFU planktonic bacteria), 1‐day biofilm, 2‐day biofilm and/or 3‐day biofilm. The biofilm explants were continuously exposed to dressing for 1, 12 and 24 hours and in some experiments for 48 and 72 hours. The treated explants, containing various levels of biofilm exposed to test agents for various times, were each processed by sonication in 7 ml PBS with 5 µl/l Tween‐80 as previously described. Bacterial suspensions were immediately serially diluted and plated in triplicate on TSB, and the average CFU/ml was determined for the 7‐ml bacterial suspension from each explant.
Cadexomer iodine—additional assessment
To determine if the bacterial load after 100% cadexomer iodine (Iodoflex™) treatment observed in the 24, 72 hours and time‐course assays reflected complete biofilm kill, reduction of microbial load below the plating method sensitivity or residual iodine active, additional assays were performed. Biofilm explants treated with 100% cadexomer iodine were transferred to 5 ml TSB media, with or without 100 mg/ml sodium thiosulphate (iodine neutralisation agent), and cultured overnight. The presence of growth, indicated by overnight culture turbidity, was recorded. To determine if residual iodine in the bacterial suspension was sufficiently neutralised by dilution during the plating procedure, biofilm explants were treated with 100% cadexomer iodine. Bacterial suspensions of sonicated explants, with or without addition of 100 mg/ml sodium thiosulphate, were spiked with planktonic PAO1 suspension culture of known concentration (0–108 CFU) and incubated for additional 20 minutes. The bacterial suspensions were serially diluted, and the average CFU/ml was determined for all test conditions.
In vivo porcine model
Detailed methods and procedures for animal wounding, wound inoculation, bacterial biofilm formation and bacterial recovery have been previously described by Davis et al. and Mertz et al. 10, 15. The animal protocol and procedures were approved by the University of Miami Institutional Animal Care and Use Committee.
In brief, second‐degree burn wounds were made on the paravertebral area of three animals using brass rods heated in boiled water. The wounds were inoculated (inoculum 6·79 log CFU/ml) with a burn wound isolate of P. aeruginosa (ATCC 27317) and covered with polyurethane films for 72 hours to allow for biofilm formation. Wounds were assigned to one of the following treatment groups: (i) polyethylene‐NanoAg dressing (Acticoat®), (ii) hydrocolloid–Ag dressing (Contreet‐H®) or (iii) untreated control. The NanoAg dressing was activated with sterile water prior to application to wounds per package instruction. Three wounds from each treatment group and animals (n = 9) were recovered on selective media, and P. aeruginosa counts (log CFU/ml) were determined as previously described 15.
Statistics
All bacterial counts (CFU/ml) were log‐transformed prior to analysis in order to normalise the sample distributions. All 24‐ and 72‐hour antibacterial treatment regimens were compared to a ddH2O‐saturated cotton gauze as well as to a no dressing control. For each comparison, one‐way analysis of variance (ANOVA) was first performed on all samples to detect the presence of treatment differences (P < 0·05) between groups. Dunnet's multiple comparison post hoc tests were used to identify treatment versus control group differences (P < 0·05). Additional comparisons were performed to evaluate whether manufacturers' refinements to the standard cadexomer‐, alginate‐ and NaCMC‐based products (vehicles) resulted in any significant (P < 0·05) enhancement to their antibacterial efficacy. Student's t‐tests were used to compare cadexomer (Cadesorb®) against cadexomer iodine pad (Iodoflex) and NaCMC (Aquacel®) against NaCMC‐Ag (Aquacel‐Ag). A one‐way ANOVA followed by Tukey's multiple comparison post hoc test was used to evaluate Ca alginate (Algisite®‐M) versus Ca alginate‐NanoAg (Acticoat‐Absorb) versus Ca alginate‐L. honey (Medihoney®‐Ca Alginate Pad).
Results
24‐ and 72‐Hour continuous exposure
All the data are shown in Figures 1 and 2. The numerical data and statistical comparisons are provided in Tables 2 and 3. Explants containing 3‐day PAO1 biofilms were pre‐treated for 24 hours with high antibiotics in order to assess the specific antimicrobial efficacy of a single application of various dressings against the subpopulation of antibiotic‐tolerant mature biofilm. This subpopulation was labelled as before treatment (0 hour) control and was compared to the microbial load after wet gauze treatment or no dressing control (undressed explants) after 24‐ and 72‐hour exposure. PAO1 bacterial loads after 24‐ and 72‐hour wet cotton gauze treatment were not significantly different (P > 0·05) from the average initial bacterial load before dressing treatment (before treatment 0‐hour control). The bioburden found in the no dressing control (undressed explants) was significantly lower (P < 0·01) than that in wet cotton gauze dressed explants after 24 hours but was similar after 72 hours. Although less than 1 log, statistical analysis indicates a significantly lower bioburden after an additional 24‐hour incubation with no dressing application compared with before treatment (0 hour) control. These results were interpreted as reflecting differences in relative moisture, with biofilm growth or recovery occurring in the presence of moisture dressing without antimicrobial agents, which is supported by the data collected from the other moisture or vehicle dressings tested. An interesting observation is that pre‐hydrated calcium alginate moisture dressing (Algisite‐M) increased total microbial bioburden compared with wet gauze treatment, 1·2 and 2·8 logs after 24 hours (P < 0·05) and 72 hours (P < 0·001), respectively.
Figure 1.
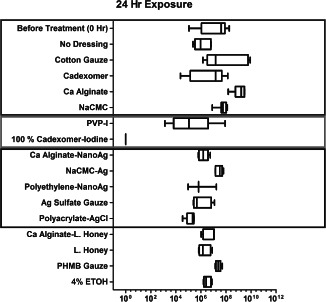
Bacterial load recovered after 24‐hour continuous exposure to a single application of antimicrobial and moisture dressings on mature 3‐day PAO1 biofilm cultured on 12‐mm porcine explants (after 24‐hour pre‐treatment with antibiotics prior to exposure to dressings). The data is reported as CFU/ml found in the bacterial suspension after sonication of each explant in 7 ml phosphate‐buffered saline (PBS) with 5 µl/l Tween‐80. Top blue box: non‐antimicrobial treatments; middle red box: iodine treatment and bottom black box: silver treatment.
Figure 2.
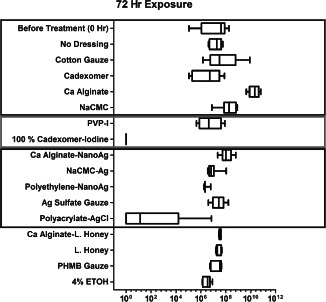
Bacterial load found after 72‐hour continuous exposure to a single application of antimicrobial and moisture dressings on mature 3‐day PAO1 biofilm cultured on 12‐mm porcine explants (after 24‐hour pre‐treatment with antibiotics prior to exposure to dressings). The data is reported as CFU/ml found in the bacterial suspension after sonication of each explant in 7 ml phosphate‐buffered saline (PBS) with 5 µl/l Tween‐80. Top blue box: non‐antimicrobial treatments; middle red box: iodine treatment and bottom black box: silver treatment.
Table 2.
Antimicrobial efficacy after 24 hours of continuous exposure to dressings on mature PA01 biofilms*
| A: 24‐Hour exposure | Mean log CFU/ml | 95% CI log CFU/ml | Log reduction† | Significance |
|---|---|---|---|---|
| Before treatment (0 hour) | 7·076 | 6·221–7·931 |
0·751a −1·001b |
ns ns |
| No dressing | 6·075 | 5·439–6·711 |
1·752a –b |
** – |
| Wet gauze | 7·827 | 7·072–8·583 |
–a −1·752b |
– ** |
| Algisite®‐M (Ca Alginate) | 9·051 | 8·650–9·451 |
−1·223a −2·976b |
* *** |
| Aquacel® (NaCMC) | 7·719 | 7·447–7·992 |
0·108a −1·644b |
ns * |
| Aquacel‐Ag (NaCMC‐Ag) | 7·453 | 7·168–7·739 |
0·374a −1·379b |
ns ns |
| Curity™ AMD (PHMB gauze) | 7·388 | 7·171–7·604 |
0·439a −1·313b |
ns ns |
| Medihoney®‐Ca alginate pad | 6·733 | 6·228–7·237 |
1·095a −0·658b |
ns ns |
| 4% Ethanol (ETOH) | 6·476 | 6·012–6·941 |
1·351a −0·401b |
ns ns |
| Medihoney ointment | 6·272 | 5·806–6·737 |
1·555a −0·197b |
* ns |
| Cadesorb® (cadexomer) | 6·549 | 5·054–8·044 |
1·279a −0·474b |
ns ns |
| Acticoat®‐Absorb (Ca Alginate‐NanoAg) | 6·206 | 5·924–6·487 |
1·622a −0·131b |
*** ns |
| Tegaderm®‐AgMesh (Ag Sulphate gauze) | 6·018 | 5·412–6·623 |
1·81a 0·057b |
*** ns |
| Acticoat 7 (Polyethylene‐NanoAg) | 6·005 | 3·164–8·846 |
1·822a 0·07b |
* ns |
| Silvasorb® gel (Polyacrylate–AgCl) | 5·161 | 4·833–5·488 |
2·666a 0·914b |
*** ns |
| Povidone‐iodine (PVP‐I) | 5·257 | 4·287–6·226 |
2·571a 0·818b |
*** ns |
| Iodoflex™ (100% cadexomer iodine) | 0 | 0–0 |
7·827a 6·075b |
*** *** |
| B: 24‐Hour exposure | Mean log CFU/ml | 95% CI log CFU/ml | Log reduction ‡ | Significance |
| Cadesorb (cadexomer)a | 6·549 | 5·054–8·044 | – | – |
| Iodoflex (100% cadexomer iodine) | 0 | 0–0 | 6·549a | *** |
| Algisite‐M (Ca Alginate)b | 9·051 | 8·650–9·451 | – | – |
| Acticoat‐Absorb (Ca Alginate‐NanoAg) | 6·206 | 5·924–6·487 | 2·845b | *** |
| Medihoney alginate pad | 6·733 | 6·228–7·237 | 2·318b | *** |
| Aquacel (NaCMC)c | 7·719 | 7·447–7·992 | – | – |
| Aquacel‐Ag (NaCMC‐Ag) | 7·453 | 7·168–7·739 | 0·266c | ns |
CFU, colony‐forming unit; CI, confidence interval.
All biofilms were cultured for 3 days on porcine explants and pre‐treated for 24 hours with high antibiotic immersion treatment to kill planktonic bacteria before dressing application, including the before treatment (0 hour) condition. Microbial load reduction due to antimicrobial dressings (single application) was compared to (A) wet gauze or no dressing control or (B) companion (vehicle alone) dressing control.
Compared to awet (ddH20) gauze control or bno dressing control.
Compared to companion dressing as indicated in each demarcated set a,b,c.
* = (0·01 ≤ P< 0·05), ** = (0·001 ≤ P < 0·01), *** = (P < 0·001) and ns = (P ≥ 0·05).
Table 3.
Antimicrobial efficacy after 72 hours of continuous exposure to dressings on mature PA01 biofilms*
| A: 72‐Hour exposure | Mean log CFU/ml | 95% CI log CFU/ml | Log reduction† | Significance |
|---|---|---|---|---|
| Before treatment (0 hour) | 7·076 | 6·221–7·931 |
0·694a 0·079b |
ns ns |
| No dressing | 7·155 | 6·616–7·694 |
0·615a –b |
ns – |
| Wet gauze | 7·770 | 7·154–8·386 |
–a −0·615b |
– ns |
| Algisite®‐M (Ca Alginate) | 10·270 | 9·838–10·71 |
−2·505a −3·12b |
*** *** |
| Aquacel® (NaCMC) | 8·193 | 7·565–8·820 |
−0·423a −1·038b |
ns ns |
| Acticoat®‐Absorb (Ca Alginate‐NanoAg) | 8·024 | 7·524–8·524 |
−0·254a −0·869b |
ns ns |
| Medihoney®‐Ca alginate pad | 7·554 | 7·460–7·648 |
0·216a −0·399b |
ns ns |
| Medihoney ointment | 7·418 | 7·164–7·671 |
0·352a −0·263b |
ns ns |
| Curity™ AMD (PHMB gauze) | 7·305 | 6·861–7·749 |
0·465a −0·15b |
ns ns |
| Tegaderm‐AgMesh (Ag Sulphate gauze) | 7·376 | 6·812–7·939 |
0·394a −0·221b |
ns ns |
| Aquacel‐Ag (NaCMC‐Ag) | 6·969 | 6·511–7·427 |
0·801a 0·186b |
ns ns |
| Povidone‐iodine (PVP‐I) | 6·657 | 5·952–7·363 |
1·113a 0·498b |
ns ns |
| 4% Ethanol (ETOH) | 6·485 | 6·162–6·807 |
1·285a 0·671b |
ns ns |
| Acticoat 7 (Polyethylene‐NanoAg) | 6·460 | 5·741–7·178 |
1·31a 0·695b |
ns ns |
| Cadesorb® (cadexomer) | 6·470 | 5·324–7·616 |
1·3a 0·685b |
ns ns |
| Silvasorb® gel (Polyacrylate–AgCl) | 2·137 | 0·3237–3·95 |
5·633a 5·018b |
*** *** |
| Iodoflex™ (100% cadexomer iodine) | 0 | 0–0 |
7·77a 7·155b |
*** *** |
| B: 72‐Hour exposure | Mean log CFU/ml | 95% CI log CFU/ml | Log reduction ‡ | Significance |
| Cadesorb (cadexomer)a | 6·470 | 5·324–7·616 | – | – |
| Iodoflex (100% cadexomer iodine) | 0 | 0–0 | 6·470a | *** |
| Algisite‐M (Ca Alginate)b | 10·27 | 9·838–10·71 | – | – |
| Acticoat‐Absorb (Ca Alginate‐NanoAg) | 8·024 | 7·524–8·524 | 2·251b | *** |
| Medihoney alginate pad | 7·554 | 7·460–7·648 | 2·716b | *** |
| Aquacel (NaCMC)c | 8·193 | 7·565–8·820 | – | – |
| Aquacel‐Ag (NaCMC‐Ag) | 6·969 | 6·511–7·427 | 1·223c | ** |
CFU, colony‐forming unit; CI, confidence interval.
All biofilms were cultured for 3 days on porcine explants and pre‐treated for 24 hours with high antibiotic immersion treatment to kill planktonic bacteria before dressing application, including the before treatment (0 hour) condition. Microbial load reduction due to antimicrobial dressings (single application) was compared to (A) wet gauze or no dressing control or (B) companion (vehicle alone) dressing control.
Compared to awet (ddH20) gauze control or bno dressing control.
Compared to companion dressing as indicated in each demarcated set a,b,c.
* = (0·01 ≤ P < 0·05), ** = (0·001 ≤ P < 0·01), *** = (P < 0·001) and ns = (P ≥ 0·05).
All dressing treatments were compared to wet gauze and no dressing in Tables 2A and 3A, respectively. Among all the dressings containing antimicrobial agents, only pre‐hydrated NanoAg‐impregnated calcium alginate (Acticoat‐Absorb, P < 0·001), pre‐hydrated polyethylene‐NanoAg composite (Acticoat‐7, P < 0·05), povidone‐iodine (PVP‐I, P < 0·001) saturated cotton gauze, polyacrylate–AgCl gel (Silvasorb®, P < 0·001) covered with wet cotton gauze and 100% cadexomer iodine (Iodoflex, P < 0·001) showed a significant reduction in bioburden after 24‐hour exposure when compared with wet gauze. The latter three had the largest mean log reduction after 24‐hour exposure, whereas only the last two maintained this mean log reduction after 72‐hour exposure. Furthermore, comparing 24‐ to 72‐hour microbial load results, it was apparent that the efficacy of the agents in certain formulations was not sustained, resulting in some recovery (bacterial growth) by 72 hours with a single application (Tables 2 and 3).
Antimicrobial treatments were also compared, more appropriately, to vehicle dressings in Tables 2B and 3B. These results are likely to more strictly reflect antimicrobial efficacy of the agent without the contribution of additional variables such as relative moisture content. After 24‐hour exposure all pairs were found to be significantly different except NaCMC moisture dressing (Aquacel) compared with silver‐impregnated NaCMC (Aquacel‐Ag). After 72‐hour exposure, all pairs were found to be significantly different.
In summary, single applications of time‐released silver (Silvasorb; polyacrylate–AgCl gel) and time‐released iodine (Iodoflex; 100% cadexomer iodine) were relatively effective antimicrobial agents against mature 3‐day PAO1 biofilm with 24‐ and 72‐hour exposure, of which cadexomer iodine was the most effective, showing complete mature PAO1 biofilm knockdown.
Time‐course study of the antimicrobial efficacy of silver or iodine gels or ointments on 3‐day PAO1 bacteria (planktonic and biofilm) cultured on porcine explants
A time‐course study showed no significant reduction of PAO1 bioburden after 0·1% PHMB gel (Prontosan®)‐saturated cotton gauze or cadexomer bead ointment covered with wet cotton gauze treatment of 3‐day cultured PAO1 on explants compared with wet gauze alone over the course of 72 hours (Figure 3). Polyacrylate–AgCl gel (Silvasorb) covered with wet cotton gauze showed increasing reduction (up to approximately 3 logs) in PAO1 bioburden after 24‐hour exposure that was maintained for 72 hours. The 50% cadexomer iodine (Iodosorb®) ointment covered with wet cotton gauze treatment was observed to have up to a 2 log reduction in bacterial load within 12 hours and as much as a 5 log reduction after 24 hours of exposure. The 100% cadexomer iodine (Iodoflex) treatment showed increasing reduction in bacterial load to undetectable levels after 24‐hour exposure.
Figure 3.
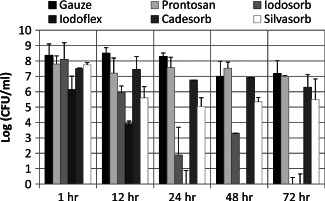
Time‐course study of the antimicrobial efficacy of antimicrobial ointments/gels on PAO1 cultured for 3 days on porcine explants (without antibiotic pre‐treatment) prior to dressing exposure. Single application of dressing with bacterial load determined after 1, 12, 24, 48 and 72 hours of exposure to Silvasorb ® gel (polyacrylate–AgCl), PHMB gel (Prontosan®), 50% cadexomer iodine (Iodosorb ®), 100% cadexomer iodine (Iodoflex™), cadexomer (Cadesorb ®) or cotton gauze. Pairwise statistical comparisons of wet gauze verses the following: Iodoflex P ≤ 0·001 at all time points; Prontosan P ≤ 0·001 at 12 hours; Cadesorb P ≤ 0·001 at 12 and 24 hours; Iodosorb P ≤ 0·001 at all times points except 1 hour; Silvasorb P ≤ 0·001 at all time points except 1 hour.
Time‐course study of PHMB gel bactericidal/bacteriostatic efficacy on PAO1 bacteria (planktonic and biofilm) cultured for 0–3 days on porcine explants
The time‐course study showed that single application of 0·1% PHMB gel (Prontosan)‐saturated gauze reduced the planktonic PAO1 bacterial load by approximately 1 log on porcine skin explants inoculated with 106 planktonic PAO1 cells, as indicated by the bacterial load recovered from treated 0‐day (biofilm maturity) PAO1 explants compared with wet gauze control (Figure 4). No statistical difference was observed between treatments; however, an overall trend of approximately 1 log lower average bioburden for PHMB‐saturated cotton gauze‐treated explants compared with wet cotton gauze was observed for all test conditions. These results suggest that 0·1% PHMB was effective neither in killing PAO1 biofilm nor inhibiting PAO1 biofilm development. However, the trend suggests that there may be some persistent bacteriocidal/bacteriostatic effect on planktonic PAO1 bacteria.
Figure 4.
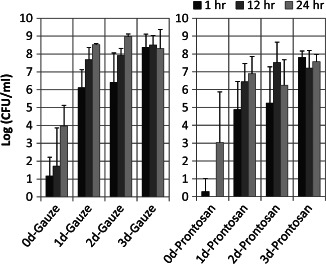
Time‐course study of PHMB gel (Prontosan®) bactericidal/bacteriostatic efficacy on PAO1 that was cultured for 0–3 days on porcine explants (without antibiotic pre‐treatment) prior to dressing exposure. Single application of Prontosan or cotton gauze with bacterial load determined after 1, 12 and 24 hours of exposure. No statistically significant difference was found between exposure times within each level of biofilm maturity. No statistical difference was found between treatments at each level of biofilm maturity.
Time‐course study of iodine bactericidal efficacy on PAO1 bacteria (planktonic and biofilm) cultured for 0–3 days on porcine explants
The time‐course study showed that single application of PVP‐I‐saturated gauze inhibits PAO1 growth on porcine skin explants inoculated with 106 planktonic PAO1 cells, as indicated by the bacterial load recovered from treated 0‐day (biofilm maturity) PAO1 explants compared with wet gauze control (Figure 5A). A single application of PVP‐I‐saturated gauze neither appeared to persist nor inhibited biofilm development, as indicated by the treated 1‐, 2‐ and 3‐day (biofilm maturity) PAO1 explants compared with wet gauze (Figure 5A). Daily application of PVP‐I on mature 3‐day PAO1 biofilm experiment (Figure 5B) indicated that PVP‐I was capable of reducing PAO1 biofilm levels between 1 and 2 logs within 12 hours of exposure with a single application, with bacterial load recovery after 24 hours. This experiment also showed that PVP‐I was capable of significantly reducing PAO1 biofilm with three applications after 72‐hour exposure (>4 log on average) (Figure 5B). This experiment indicates that the antimicrobial efficacy of iodine is dose‐dependent. The 100% cadexomer iodine (Iodoflex) appears to kill planktonic bacteria (between 1 and 12 hours) and significantly reduces PAO1 biofilm (3–5 log) within 12 hours and is capable of reducing mature biofilm levels below detectable levels with 24 hours of continuous exposure (Figure 5A). The antimicrobial efficacy of cadexomer iodine appears to depend on both the biofilm maturity and time of exposure. Considering the previous results (above) showing a dose‐dependent response to multiple applications of PVP‐I, the significantly improved antimicrobial efficacy of iodine provided by cadexomer iodine dressing may be attributed to the vehicle formulation that results in a sustained release maintaining iodine availability at approximately 1 ppm (parts per million, equivalent to µl/l, mg/kg or µmol/mol) (Table 1).
Figure 5.
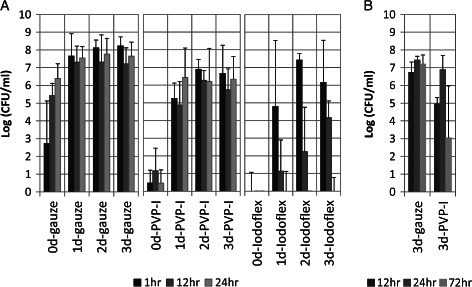
Time‐course study of povidone‐iodine (PVP‐I) and cadexomer iodine bactericidal efficacy on PAO1 that was cultured for 0–3 days on porcine explants (without antibiotic pre‐treatment) prior to dressing exposure. (A) Single application of cotton gauze, PVP‐I or 100% cadexomer iodine (Iodoflex™) with bacterial load determined after 1, 12 and 24 hours of exposure. (B) Daily application of PVP‐I with bacterial load determined after 12, 24 and 72 hours of exposure. Single application: 0‐day gauze P‐values were ≤0·001 between all time points and significantly lower at each exposure time compared with the corresponding exposure times of greater biofilm maturity; no significant difference observed between time points within each biofilm maturity level after 1 day with wet gauze. No significant difference observed between time points within each biofilm maturity level of PVP‐I; Iodoflex P‐values were ≤0·001 between time points within each biofilm maturity level after 1 day. No significant difference between corresponding time points at 1 day or greater biofilm maturity with Iodoflex. Daily application of PVP‐I significantly reduced PAO1 (P ≤ 0·05) at 12‐ and 72‐hour exposure compared with wet gauze.
Validation of iodine bactericidal efficacy against PAO1 biofilm attached to porcine skin and methodology assessment
To determine if the 0 CFU observed after cadexomer iodine treatment reflects total microbial kill rather than a methodology sensitivity limitation (explant sonication in 7 ml PSB and spread plating of the bacterial suspension), iodine‐treated biofilm explants were incubated in TSB culture media. The presence of bacterial growth after overnight incubation of PVP‐I‐treated or 100% cadexomer iodine (Iodoflex)‐treated explants in 5 ml TSB correlated to the CFU/ml data (Figure 5 and Table 4A). One point of variability between the experiments shown in Figure 5 and Table 4A needs clarification; less than half of the 0‐day biofilm explants (2 hours post‐inoculation) treated with a single application of PVP‐I had a detectable bacterial load in TSB culture at each time point of exposure (Figure 5A): two of six explants had growth after 1‐hour exposure; three of six explants had growth after 12‐hour exposure; and two of six explants had growth after 24‐hour exposure. In contrast, all the 12‐ and 24‐hour exposure PVP‐I‐treated 0‐day biofilm explants that were cultured overnight in TSB showed no growth (Table 4A).
Table 4.
Methodology assessment of iodine bactericidal efficacy on PAO1 cultured for 0–3 days on porcine explants (without antibiotic pre‐treatment)*
| A | Exposure (hours) | Biofilm maturity (days) | No. of explants | Growth† |
|---|---|---|---|---|
| Iodoflex™ | 1 | 0 | 3 | Y |
| 12 | 0 | 3 | N | |
| 24 | 0 | 3 | N | |
| 12 | 1 | 3 | Y | |
| 24 | 1 | 3 | N | |
| 12 | 2 | 3 | Y | |
| 24 | 2 | 6 | N | |
| 1 | 3 | 3 | Y | |
| 12 | 3 | 3 | N | |
| 24 | 3 | 6 | N | |
| 72 | 3 | 3 | N | |
| PVP‐I | 1 | 0 | 3 | Y |
| 12 | 0 | 3 | N | |
| 24 | 0 | 3 | N | |
| 12 | 1 | 3 | Y | |
| 24 | 1 | 3 | Y | |
| 12 | 2 | 3 | Y | |
| 24 | 2 | 6 | Y | |
| 1 | 3 | 3 | Y | |
| 12 | 3 | 3 | Y | |
| 24 | 3 | 6 | Y | |
| 72 | 3 | 3 | Y | |
| B | Exposure (hours) | Biofilm maturity (days) | No. of explants | Growth |
| Iodoflex | 24 | 3 | 7 | N |
| 24 | 3 | 2 | Y+ | |
| PVP‐I | 24 | 3 | 9 | Y++ |
To determine if cadexomer iodine bacterial load reduction reflects complete kill or residual bactericidal activity after dilution. (A) Single application treated explants incubated for 24 hours at 37°C in 5 ml TSB; explant inoculum: ˜106 CFU (0‐day biofilm). (B) Single application treated explants incubated for 24 hours at 37°C in 5 ml TSB with 100 mg/ml sodium thiosulphate to neutralise iodine.
Growth indicated by turbidity of overnight culture (the same outcome was observed for each explant).
Three‐day biofilm explants treated for 24 hours with 100% cadexomer iodine (Iodoflex) and then incubated overnight in TSB without addition of neutralising agent showed no growth, whereas treated explants cultured in TSB containing 100 µg/ml sodium thiosulphate neutralising agent showed no growth only in seven of nine cases (Table 4B). These results indicate that 100% cadexomer iodine in Iodoflex is capable of completely killing all PAO1 bacteria within 24 hours in our model. These results also suggest the possibility of some residual iodine activity in the bacterial suspensions and/or reflect the detection limits of serial diluting and plating. This possibility was assessed by spiking 100% cadexomer iodine‐treated explant bacterial suspensions with a known number of planktonic bacteria.
Overnight culture of bacterial suspension (in 5 ml TSB) from 100% cadexomer iodine (Iodoflex)‐treated explants spiked with planktonic bacteria showed that residual cadexomer iodine without either dilution or neutralisation was capable of killing up to 104 susceptible planktonic (log phase) PAO1 cells overnight (data not shown). However, because bacterial biofilm suspensions are diluted and immediately plated in all assays and not incubated for an additional 24 hours, additional assays were performed to determine if residual iodine activity would significantly reduce spiked planktonic bacteria after plating under the standard methods used in the study. To further assess residual iodine activity in order to determine if iodine activity is sufficiently neutralised by the plating method used, bacterial suspensions from 100% cadexomer iodine‐treated explants were spiked with various concentrations of planktonic (log phase) PAO1 culture (0–108 CFU), with or without addition of neutralisation agent, and then incubated for an additional 20 minutes before serial dilution and plating. Each condition was tested in triplicate. The average CFU/ml was not significantly different (<1 log) between samples neutralised by 100 µg/ml sodium thiosulphate or not, despite the additional 20‐minute incubation before plating, indicating that any bacteriocidal activity from residual iodine is effectively neutralised by immediate processing and plating. On the basis of these results, it is presumed that the methods of determining the bioburden after antimicrobial dressing treatment reported in this study accurately express the antimicrobial efficacy of each agent tested against mature PAO1 biofilm (under antibiotic pressure) cultured on porcine skin explants after the specified exposure time.
In vivo porcine model
To further validate the porcine skin explant biofilm model and the results observed in the ex vivo biofilm study performed at the University of Florida, an established in vivo porcine burn biofilm model was used in an independent study performed at the University of Miami. Wounds treated with polyethylene‐NanoAg dressing (Acticoat) group showed no statistical difference in P. aeruginosa counts when compared with those left untreated (after 24 hours of exposure). When comparing data from the hydrocolloid–Ag (Contreet‐H) dressing versus the untreated group, there was a statistically significant increase in P. aeruginosa counts (P ≤ 0·05) (Figure 6).
Figure 6.
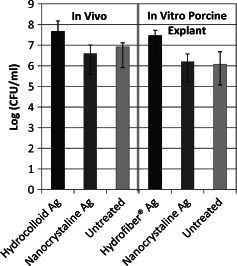
Comparison of Pseudomonas aeruginosa biofilm cultured on an ex vivo porcine skin partial‐thickness ‘wound’ explants to P. aeruginosa biofilm cultured on an in vivo pig burn model after 24‐hour exposure to silver dressings: Hydrocolloid–Ag (Contreet‐H®‐Ag), Hydrofibre–Ag (Aquacel®‐Ag) and Nanocrystalline–Ag (Acticoat®). No statistically significant difference was found between any dressing treatments.
Discussion
Multiple bacterial species, both Gram positive and Gram negative, have been found to reside in chronic wounds. Recent studies analysing bacterial biofilms found in chronic infections (chronic otitis media, cystic fibrosis, infected permanent tissue fillers and chronic wounds) point towards low bacterial diversity and dominant monospecies biofilms within a multispecies background 16, 17, 18. It has been suggested that the presence of P. aeruginosa species in biofilms is the reason why chronic wounds do not heal 19. P. aeruginosa biofilm infections are of particular clinical relevance in skin burns and have been studied using an in vivo pig burn model 10, 15. P. aeruginosa is not only an important opportunistic pathogen and causative agent of emerging nosocomial skin and soft tissue infections but can also be considered a model organism for the study of diverse bacterial mechanisms that contribute to bacterial persistence 20. The primary bacterial strain used in this study was the wound isolate PAO1 of P. aeruginosa.
In this study, dressings of various moisture retention and antimicrobial characteristics were compared to each other, to a vehicle dressing (when available) and an untreated control. The increased bacterial loads recovered from explants that were covered with moisture dressings when compared with the no dressing group support the general observation that certain bacteria (e.g. PAO1) thrive in a moist environment. The results of this study suggest that certain dressings appear to promote PAO1 biofilm development, indicated by increased bacterial load compared with wet gauze treatment. Calcium alginate dressing (Algisite‐M), in particular, significantly increased and maintained PAO1 biofilm (i.e. extremely antibiotic tolerant subpopulation) attached to porcine skin explants. Insoluble calcium alginate fibre polymer is created by a process of combining water‐soluble sodium alginate and calcium chloride. It is a common food additive as well as wound dressing component. Interestingly, P. aeruginosa synthesises and secretes alginate, a major component of its biofilm extracellular matrix, as well as alginate lyase that functions to cleave this polysaccharide into short oligosaccharides 21. These dual functions regulate the development and growth of P. aeruginosa biofilm 21. Studies have shown that when P. aeruginosa is deprived of energy‐rich carbon sources (e.g. cystic fibrosis lung infections) or any environmental stress that leads to a reduction in the growth rate below maximum potential, production of alginate is induced in order to maintain an optimal growth rate 22 and consequently modify biofilm development. For future work, it would be interesting to address the biological and molecular effects of an outside source of alginate on P. aeruginosa metabolism and biofilm development.
Some dressings, such as those containing silver, rely to some degree on the amount of moisture that is present (either from wound fluid or supplemental) in order to be effective. The release of silver from some materials is proportional to the rate of fluid uptake according to the manufacturers. To maximise antimicrobial agent availability, water was used to pre‐hydrate all moisture dressings in this study. Silver dressings should not be moistened with saline owing to precipitation of silver ions, according to the manufacturer's recommendation. Ointments and gels (Iodosorb, Cadesorb, Medihoney and Silvasorb) were covered with a water‐saturated secondary gauze dressing. Liquid PVP‐I, 4% ETOH and 0·1% PHMB (Prontosan™) gels were used alone without addition of water because these liquids completely saturated the gauze. All treated explants were incubated at saturating humidity to maintain moisture levels during exposure.
Iodine is an essential trace element and has been used, depending on the formulation, as a wound antiseptic, disinfectant and water sanitiser. At physiological pH and low concentrations, the only relevant reactive species are I−, I2 and I3 − 23. Hydrated molecular iodine (I2) has the highest antimicrobial potential 23. Iodophors, composed of iodine complexed with organic compounds, are less irritating and allergenic than iodine solutions owing to slow release (dispersion) of free iodine from the aggregates into aqueous solutions 24, 25. The concentration of free iodine, and therefore activity, depends on the type of iodophor. The most common iodophor in clinical use is polyvinyl‐pyrrolidone (PVP‐I) with an available concentration of iodine between 8 and 12% (w/v) 23. Historically, iodine has been considered cytotoxic and use in open wounds had been discouraged. More recent evaluations indicate that iodine does not impair wound healing and is an effective antiseptic 26. Newer slow‐release iodine formulations, such as from cadexomer beads (Iodoflex), have been approved for use in open wounds and have been reported to accelerate epithelialisation of partial‐thickness wounds in a porcine model 27.
This study showed that a single application of PVP‐I partially reduced PAO1 biofilm CFUs, whereas a single application of 100% cadexomer iodine was capable of completely killing PAO1 biofilm attached to porcine skin explants. Cadexomer iodine was designed for use on exudating wounds (Table 1), requiring moisture to release the iodine. Cadexomer beads alone, without the application of water‐saturated secondary gauze dressing, significantly reduced PAO1 biofilm bacterial levels (data not shown), which presumably was due to contact dehydrative effect of the cadexomer beads. The ability of the cadexomer vehicle alone to reduce P. aeruginosa infection has been documented in an in vivo porcine study showing a significant reduction in P. aeruginosa CFUs (2·3 log) after 48 hours of treatment compared with an untreated control 27. Using the same in vivo model, a sustained release of PVP‐I that was incorporated into a hydrogel dressing showed reduction of 2·5 log (compared with hydrogel alone) and 2·9 log (compared with untreated control wounds) after 24 hours of exposure 28. It is important to note that 50% cadexomer iodine (Iodosorb ointment) was capable of reducing 3‐day mature PAO1 biofilm at increasing levels (5–6 log in 24 hours) but was not as effective as 100% cadexomer iodine (Iodoflex pad) in the time‐course study (Figure 3). The source of the observed variability (within 1–2 log) after 48‐ and 72‐hour exposure to the 50% cadexomer iodine ointment is unknown. Perhaps, it may be attributed to some variation in the initial bacterial load between explants between trials. The overall difference in efficacy between Iodosorb ointment and Iodoflex pad was likely owing to the difference in availability of active iodine. The result of daily application of PVP‐I (Figure 5B) indicates that its antimicrobial activity did not persist and suggests that the efficacy of iodine depends on the vehicle used and availability of active iodine.
Silver has been recognised for its antimicrobial properties for centuries. Silver‐resistant bacteria have been reported since the 1950s, including P. aeruginosa strains, but are considered relatively rare 23, 29. Silver is a broad‐spectrum antimicrobial agent and is generally recognised as safe with minimal side effects 29, 30. The silver ion concentration required to kill bacteria is still debated; however, the literature supports bactericidal activity at certain levels of ppm 29, 30. Silver in the form of silver nitrate is more astringent and irritating and used rarely today 30. One strictly molecular study conducted by Milliken & Company (the makers of SelectSilver™) showed that regardless of the dressing type or the form of silver on the dressing (including Acticoat‐7, Aquacel‐Ag and Tegaderm® AgMesh), without organic loading, silver release in simulated wound fluid (142 mM NaCl and 2·5 mM CaCl2) maintained an equilibrium concentration of only 0·5 ppm 31. However, with organic loading (5% BSA) of the simulated wound fluid, the silver dressings were reported to release significantly greater amounts of silver that varied with dressing and time: Acticoat‐7 released >80 ppm after 24‐hour exposure, which reduced to ˜60 ppm after 48‐ and 72‐hour exposure; Aquacel‐Ag released ˜40 ppm after 24‐hour exposure, which reduced to ˜10 ppm after 48‐ and 72‐hour exposure; Tegaderm® AgMesh released >20 ppm after 24‐hour exposure, which reduced to ˜0 ppm after 48‐ and 72‐hour exposure 31. Because the biofilms in our study were cultured on porcine skin explants, providing organic material on contact, the water‐saturated silver dressings were expected to release silver at near maximal levels. Polyacrylate–AgCl gel (Silvasorb) appeared to be the most effective form tested in this study, despite the advertised low levels of silver ions released, with continuous exposure for 72 hours. Because the vehicle component of the Silvasorb dressing was not tested, it is unknown if the hydrogel (advertised to control wound moisture) alone has an antimicrobial dehydrative effect despite the application of a water‐saturated secondary cotton gauze dressing. The wide variability of the Silvasorb data generated in this study from the 24‐ and 72‐hour continuous exposure assessment of mature (antibiotic pre‐treated) PAO1 biofilm indicates the possibility of a vehicle effect (Table 1). The more consistent 2–3 log reduction in bacterial levels observed in the time‐course study (Figure 3) on total PAO1 is likely a reflection of the strictly controlled dressing application methodology to produce a more uniform coverage and maximise hydration of the vehicle (optimised in the time‐course studies and the later trials of the 24‐ and 72‐hour exposure assays).
The in vivo pig burn biofilm studies performed at the University of Miami showed similar limited efficacy results with silver dressings as observed in the in vivo porcine model studies performed at the University of Florida. After establishing two P. aeruginosa bacterial groups, planktonic bacteria and biofilm bacteria, wounds were treated with NanoAg dressing (Acticoat), hydrocolloid–Ag (Contreet‐H) dressings or untreated. The hydrocolloid–Ag dressing significantly reduced planktonic bacteria counts when compared with untreated and NanoAg dressing (data not shown). However, biofilm‐associated bacteria counts for both dressings were similar to untreated control at all sample points (Figure 6) as observed in previous in vivo studies 32.
Data from the ex vivo porcine explant model and the in vivo porcine model in this study appear to correlate well (Figure 6) with both showing that neither the nanocrystalline silver nor the hydrocolloid dressings that were examined appear to be effective in reducing P. aeruginosa counts after biofilms were formed. These results further validate the porcine explant biofilm model used in this study. The use of silver dressings has not been approved for infected wounds that are colonised with biofilm‐associated bacteria and are thought to be ‘barriers’ to prevent the entrance of exogenous pathogenic bacteria.
PHMB is a broad‐spectrum (bacterial and fungal) biocidal antimicrobial agent and disinfectant with no known microbial resistance or negative effects on wound healing 25, 33. It is not considered cytotoxic (at the manufactured concentrations) and is commonly used on skin, wounds and contact lens for cleansing and disinfection. Its mechanism of action is reported to be association of PHMB molecules with the acidic lipid components of microbial membranes to cause phase separation and disruption of microbial membranes at high concentrations 33. Increasing length of the PHMB polymer correlates with increasing antimicrobial efficacy 33. Cazzaniga et al. 33 reported the barrier effects of a gauze containing 0·2% PHMB, which was challenged with a P. aeruginosa suspension above the gauze. PHMB gauze dressing was shown to reduce recovery of invading P. aeruginosa from wounds by 2 log when compared with control gauze. In this study, 0·2% PHMB gauze (Curity™ AMD™)‐ and 0·1% PHMB wound gel (Prontosan)‐saturated gauze did not significantly reduce PAO1 bacterial biofilm levels. The former is a packing dressing intended to be used to inhibit bacterial growth within the dressing and act as a microbial barrier for acute wounds; the latter is designed as a wound cleansing agent to inhibit growth within the agent when used to moisten gauze dressings. The time‐course study showed a trend of reduced bioburden of 0·1% PHMB wound gel (Prontosan)‐saturated gauze treatment compared with wet gauze treatment of porcine explants with various levels of PAO1 biofilm (Figure 4), indicating a small inhibitory effect of PHMB on PAO1 biofilm development. PHMB dressings were not effectively bactericidal or bacteriostatic on infected (106 PAO1) porcine tissue (0‐day biofilm explants) but were likely effective against planktonic bacteria for their intended use as an alternative to water‐moistened cotton gauze in uninfected tissue as a barrier dressing to inhibit infection.
The low pH (˜3·2–4·5) and high osmolarity of honey (˜80%) have been historically used for their bacteriostatic/bactericidal effect on bacteria and fungus 34. Similar to salt, it is a commonly used food preservative against spoilage through a mechanism of dehydration. Honey is known for producing hydrogen peroxide when dissolved in water, proving a slow release of this oxidative microbicide (˜1 mmol/l) 35. Medihoney has Leptospermum honey which contains additional florally derived antimicrobial agents such as methyglyoxal (an α‐oxoaldehyde that reacts with nucleotides and proteins) 36. Studies have shown that antimicrobial activity of honey against P. aeruginosa is primarily due to hydrogen peroxide activity and that the minimum bactericidal concentration is 12·5% v/v 37. P. aeruginosa (planktonic) has been shown to be susceptible to killing by osmotic pressure within 72 hours in sucrose‐saturated nutrient‐rich media 38. In this study, pre‐hydrated Ca alginate‐L. honey dressing (Medihoney‐Ca alginate pad) reduced mature 3‐day PAO1 biofilm 2·3 log after 24 hours and 2·7 log on average after 72 hours compared with calcium alginate dressing (Algisite‐M) (corresponding to no reduction compared with wet gauze) (Figures 1 and 2). These results suggest that L. honey (Medihoney) has bactericidal activity for 24 hours and was relatively bacteriostatic for 72 hours against mature PAO1 biofilm on porcine skin explants.
ETOH is a commonly used topical antiseptic and surface disinfectant at approximately 70% v/v as well as a solvent for substances intended for human contact or consumption. Its bactericidal mode of action includes denaturing proteins, dissolving lipids and dehydration. A recent study showed that coagulase‐negative staphylococci (CoNS) biofilm was effectively eradicated when used as a catheter lock solution 39. The effective range of concentration and exposure times to eradicate CoNS biofilm cultured for 24 hours on polystyrene coupons (˜0·5 × 0·5 cm) was found to be at 40% ETOH after 1 hour and 20% ETOH after 4 hours 39. In contrast, biofilm formation by various strains of CoNS in microwell culture plates has been shown in several studies to depend on the presence of 4% ETOH or 4% NaCl 39. In this study, a single application of gauze saturated with 4% ETOH was neither effective against PAO1 biofilm attached to porcine skin nor appeared to promote biofilm formation.
Because this biofilm model uses porcine skin for attachment and primary source of nutrition, there is some inherent variability between explants with the same treatment within the same trial and particularly for the average results for each treatment type between trials because they used different skin lots. As one would expect for biological systems, there was variability in the observed bioburden; therefore, the sample number was increased per trial and multiple trials (at least three) were performed in order to generate conservative statistical data.
A critical consideration when analysing log reduction of microbial biofilm load is the recovery rate. For example, one log reduction from 1 × 109 to 1 × 108 is a loss of 9 × 108 bacteria or can be expressed in simple mathematical terms as 90% reduction. This is an insignificant reduction in a biological sense when bacteria such as P. aeruginosa have an average generation doubling time of ˜40–50 minutes, thus full recovery could be achieved in less than 3 hours. This factor is evident for several dressings when comparing the 24‐ and 72‐hour exposure bioburden observed in this study. Alternatively, a 5 log reduction of PAO1 biofilm would require at least 17 generations to recover (˜12 hours) in this study. At this level of antimicrobial efficacy against bacteria/biofilms attached to skin wounds, multiple applications of non‐specific microbicide treatment would be feasible and likely effective. The majority of the antimicrobial wound dressings tested in this study were selected based on broad‐spectrum antimicrobial activity and claims of sustained efficacy for a minimum of 72 hours. Based on the data in this study, within the constraints of this model, a dressing with greater than 5 log reduction in bacterial biofilm is most likely an effective antimicrobial dressing. A dressing with greater than 3 log reduction in bacterial biofilm may be an effective antimicrobial dressing for planktonic infections, low‐load microbial biofilms or as a barrier dressing. Dressings containing antimicrobial agents showing less than 3 log reduction are likely better used as barrier dressings. The application of barrier dressings to properly prepared wound beds has considerable value in wound care. It is interesting to note that some dressings, even without antimicrobial activity, can act as barriers to infection and can actually reduce the incidence of infection 40.
The lack of a host immune system to assist the antimicrobial efficacy of the wound dressings as well as the high bacterial biofilm load of a monospecies biofilm must be considered when interpreting the results of this study. The model used in this study was intended to be interpreted as an advanced biofilm model. The objective of this study was to determine the efficacy of several commercially available wound dressings and/or antimicrobial agents against PAO1 biofilm with all the yet undefined molecular characteristics of biofilm attached to skin. Studies have shown or suggested that the molecular characteristics of microbial biofilm depend on the chemical and physical cues provided by the environment 8, 9, 10, 11, 12, 13. Using porcine skin as the substrate for attachment and as the primary source of nutrition, while under antibiotic pressure, would be expected to produce attached biofilms that are specifically adapted to these environmental cues, thus simulating several key factors of chronic wounds. Furthermore, assessment of agents that may react to constituents found in the skin would benefit from using this rapid, reproducible and inexpensive ex vivo biofilm model. In conclusion, the use of the porcine explant biofilm model would be helpful in the study of effective antimicrobial therapeutics and was shown here to produce results similar to those of in vivo PAO1 biofilm infection pig burn wound studies. The translation of ex vivo and in vivo studies from the laboratory into the clinic is necessary to obtain successful antimicrobial therapeutics 41.
Acknowledgements
The study was funded in part by unrestricted research grant from Smith & Nephew. There are no conflicts of interest to report for this study.
References
- 1. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(Suppl 1):S1–28. [DOI] [PubMed] [Google Scholar]
- 2. Falanga V, Brem H, Ennis WJ, Wolcott R, Gould LJ, Ayello EA. Maintenance debridement in the treatment of difficult‐to‐heal chronic wounds—recommendations of an expert panel. Ostomy Wound Manage 2008; Suppl:2–13. [PubMed] [Google Scholar]
- 3. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–22. [DOI] [PubMed] [Google Scholar]
- 4. James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 5. Percival SL, Bowler PG. Biofilms and their potential role in wound healing. Wounds 2004;16:234–40. [Google Scholar]
- 6. Jones SG, Edwards R, Thomas DW. Inflammation and wound healing: the role of bacteria in the immuno‐regulation of wound healing. Int J Low Extrem Wounds 2004;3:201–8. [DOI] [PubMed] [Google Scholar]
- 7. Phillips P, Yang Q, Sampson E, Progulske‐Fox A, Antonelli P, Shouquang J, Schultz G. Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen 2013. DOI: 10.1111/wrr.12074. [DOI] [PubMed] [Google Scholar]
- 8. Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2003;2:114–22. [DOI] [PubMed] [Google Scholar]
- 9. Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to staphylococcus aureus biofilms. Infect Immun 2002;70:6339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM. Microscopic and physiologic evidence for biofilm‐associated wound colonization in vivo. Wound Repair Regen 2008;16:23–9. [DOI] [PubMed] [Google Scholar]
- 11. Ren D, Bedzyk LA, Thomas SM, Ye RW, Wood TK. Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol 2004;64:515–24. [DOI] [PubMed] [Google Scholar]
- 12. Luppens SB, Ten Cate JM. Effect of biofilm model, mode of growth, and strain on streptococcus mutans protein expression as determined by two‐dimensional difference gel electrophoresis. J Proteome Res 2005;4:232–7. [DOI] [PubMed] [Google Scholar]
- 13. Cho KH, Caparon MG. Patterns of virulence gene expression differ between biofilm and tissue communities of streptococcus pyogenes. Mol Microbiol 2005;57:1545–56. [DOI] [PubMed] [Google Scholar]
- 14. Phillips PL, Yang Q, Sampson EM, Schultz GS. Effects of antimicrobial agents on an in vitro biofilm model of skin wounds. In: Sen CK, editor. Advances in wound care. Wound Healing Society Yearbook Publication. New Rochelle: Mary Ann Liebert, Inc. Publishers, 2010:299–304. [Google Scholar]
- 15. Mertz PM, Alvarez OM, Smerbeck RV, Eaglstein WH. A new in vivo model for the evaluation of topical antiseptics on superficial wounds. The effect of 70% alcohol and povidone‐iodine solution. Arch Dermatol 1984;120:58–62. [PubMed] [Google Scholar]
- 16. Fazli M, Bjarnsholt T, Kirketerp‐Moller K, Jorgensen B, Andersen AS, Krogfelt KA, Givskov M, Tolker‐Nielsen T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol 2009;47:4084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirketerp‐Moller K, Jensen PO, Fazli M, Madsen KG, Pedersen J, Moser C, Tolker‐Nielsen T, Hoiby N, Givskov M, Bjarnsholt T. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol 2008;46:2717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burmolle M, Thomsen TR, Fazli M, Dige I, Christensen L, Homoe P, Tvede M, Nyvad B, Tolker‐Nielsen T, Givskov M, Moser C, Kirketerp‐Moller K, Johansen HK, Hoiby N, Jensen PO, Sorensen SJ, Bjarnsholt T. Biofilms in chronic infections ‐ a matter of opportunity ‐ monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol 2010;59:324–36. [DOI] [PubMed] [Google Scholar]
- 19. Bjarnsholt T, Kirketerp‐Moller K, Jensen PO, Madsen KG, Phipps R, Krogfelt K, Hoiby N, Givskov M. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008;16:2–10. [DOI] [PubMed] [Google Scholar]
- 20. Sriramulu DD, Lunsdorf H, Lam JS, Romling U. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol 2005;54(Pt 7):667–76. [DOI] [PubMed] [Google Scholar]
- 21. Boyd A, Chakrabarty AM. Pseudomonas aeruginosa biofilms—role of the alginate exopolysaccharide. J Ind Microbiol 1995;15:162–8. [DOI] [PubMed] [Google Scholar]
- 22. Schlictman D, Kavanaughblack A, Shankar S, Chakrabarty AM. Energy‐metabolism and alginate biosynthesis in Pseudomonas aeruginosa—role of the tricarboxylic‐acid cycle. J Bacteriol 1994;176:6023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooper RA. Iodine revisited. Int Wound J 2007;4:124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaye KS, Kaye D. Multidrug‐resistant pathogens: mechanisms of resistance and epidemiology. Curr Infect Dis Rep 2000;2:391–8. [DOI] [PubMed] [Google Scholar]
- 25. White RJ, Cutting K, Kingsley A. Topical antimicrobials in the control of wound bioburden. Ostomy Wound Manage 2006;52:26–58. [PubMed] [Google Scholar]
- 26. Vermeulen H, Westerbos SJ, Ubbink DT. Benefit and harm of iodine in wound care: a systematic review. J Hosp Infect 2010;76:191–9. [DOI] [PubMed] [Google Scholar]
- 27. Mertz PM, Davis SC, Brewer LD, Franzen L. Can antimicrobials be effective without impairing wound‐healing—the evaluation of a cadexomer iodine ointment. Wounds 1994;6:184–93. [Google Scholar]
- 28. Mertz PM, Marshall DA, Kuglar MA. Povidone‐iodine in polyethylene oxide hydrogel dressing—effect on multiplication of staphylococcus‐aureus in partial‐thickness wounds. Arch Dermatol 1986;122:1133–8. [PubMed] [Google Scholar]
- 29. Brett DW. A discussion of silver as an antimicrobial agent: alleviating the confusion. Ostomy Wound Manage 2006;52:34–41. [PubMed] [Google Scholar]
- 30. White R. Efficacy of silver‐containing dressings. J Wound Care 2006;15:417–8 author reply 18. [DOI] [PubMed] [Google Scholar]
- 31.Canada TA, Wiencek KM, Cowan ME, Lindsey BJ. Challenging silver‐influence of extraction medium on the release of silver from commercial silver dressings. Spartanburg: Milliken & Company, 2007. URL http://healthcare.milliken.com/en‐us/Documents/Silver_Release_White_Paper_2007.pdf [accessed on 11 Aug 2013].
- 32. Davis SC, Cazzaniga A, Ricotti CA, Mertz P. Silver dressings: effective antimicrobial therapies? J Am Acad Dermatol 2004;50:P168. [Google Scholar]
- 33. Cazzaniga A, Serralta V, Davis S, Orr R, Eaglstein W, Mertz MP. The effect of an antimicrobial gauze dressing impregnated with 0.2‐percent polyhexamethylene biguanide as a barrier to prevent Pseudomonas aeruginosa wound invasion. Wounds 2002;14:169–76. [Google Scholar]
- 34. Blair SE, Cokcetin NN, Harry EJ, Carter DA. The unusual antibacterial activity of medical‐grade Leptospermum honey: antibacterial spectrum, resistance and transcriptome analysis. Eur J Clin Microbiol Infect Dis 2009;28:1199–208. [DOI] [PubMed] [Google Scholar]
- 35. White JW, Schepartz AI, Subers MH. Identification of inhibine, antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose‐oxidase system. Biochim Biophys Acta 1963;73:57. [DOI] [PubMed] [Google Scholar]
- 36. Adams CJ, Boult CH, Deadman BJ, Farr JM, Grainger MNC, Manley‐Harris M, Snow MJ. Isolation by hplc and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr Res 2008;343:651–9. [DOI] [PubMed] [Google Scholar]
- 37. Sherlock O, Dolan A, Athman R, Power A, Gethin G, Cowman S, Humphreys H. Comparison of the antimicrobial activity of ulmo honey from Chile and manuka honey against methicillin‐resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa . BMC Complement Altern Med 2010;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chirife J, Herszage L, Joseph A, Kohn ES. In vitro study of bacterial‐growth inhibition in concentrated sugar solutions—microbiological basis for the use of sugar in treating infected wounds. Antimicrob Agents Chemother 1983;23:766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qu Y, Istivan TS, Daley AJ, Rouch DA, Deighton MA. Comparison of various antimicrobial agents as catheter lock solutions: preference for ethanol in eradication of coagulase‐negative staphylococcal biofilms. J Med Microbiol 2009;58(Pt 4):442–50. [DOI] [PubMed] [Google Scholar]
- 40. Hutchinson JJ, Lawrence JC. Wound‐infection under occlusive dressings. J Hosp Infect 1991;17:83–94. [DOI] [PubMed] [Google Scholar]
- 41. Davis SC, Bouzari N. Development of antimicrobials for wound care: in‐vitro and in‐vivo assessments. Wounds 2004;16:344–7. [Google Scholar]


