Abstract
This study compared wound healing efficacy of two silver dressings, AQUACEL® Ag and Urgotul® Silver, against venous ulcers at risk of infection, over 8 weeks of treatment. The primary objective was to show non inferiority of AQUACEL® Ag to Urgotul® Silver. Patients (281) were randomised into two groups. The AQUACEL® Ag group had 145 patients treated with AQUACEL® Ag for 4 weeks followed by AQUACEL for another 4 weeks. TheUrgotul® Silver group had 136 patients treated with Urgotul® Silver for 4 weeks followed by Urgotul® for another 4 weeks. In both groups, ulcer size and depth, safety events and ulcer healing were compared. After 8 weeks of treatment, the AQUACEL® Ag group had a relative wound size reduction (49·65% ± 52·53%) compared with the Urgotul® Silver group (42·81% ± 60·0%). The non inferiority of the AQUACEL® Ag group to the Urgotul® Silver group was established based on the difference between them (6·84% ± 56·3%, 95% confidence interval −6·56 to 20·2) and the pre‐defined non inferiority margin (−15%). Composite wound healing analysis showed that the AQUACEL® Ag group had statistically higher percentage of subjects with better wound progression (66·9% versus 51·9%, P = 0·0108). In general, both dressings were effective at promoting healing of venous ulcers.
Keywords: Antimicrobial, Moist wound healing, Non inferiority, Silver, Venous leg ulcer
INTRODUCTION
Venous leg ulcers occur in 1–3% of the adult population and account for the majority of lower extremity ulcerations (1), and are an important patient health and safety concern. The prevalence of venous leg ulcers ranges from 0·6 to 1·6 per 1000 for the total adult population, increasing between 10 and 30 per 1000 in the population over the age of 85 years 2, 3, 4. Despite recent advances in wound care, ulcers can take months to heal, have frequent complications (e.g. infections and cellulitis), often recur, and are costly to treat (5). The refractory nature of venous leg ulcers can affect a patient's quality of life and productivity at work, causing significant morbidity (6).
The chronic nature of venous leg ulcers is characterised by three measures: (i) the duration of the ulceration, (ii) ulcer recurrence and (iii) the period of time since the onset of the first ulceration to the time of the survey 2, 3. Reports from studies in Western countries on the duration of leg ulcers indicate that more than 50% last longer than 1 year 2, 7, 8. Results of data collected on the ulcer prevalence indicate that between 28% and 45% of individuals with leg ulcers experience episodes of leg ulcers for more than 10 years 2, 3.
Currently, the standard of care consists of local wound management combined with compression bandaging to reduce oedema and promote venous return (5). The principles of moist wound healing 9, 10, 11 coupled with graduated compression bandaging 12, 13, 14 have become the cornerstone of treatment for leg ulcers. Modern wound dressings have an increased capacity for managing exudate and its potentially pathogenic bioburden: reducing the need for frequent dressing changes, protecting the surrounding skin, decreasing odour from wounds and diminishing pain between and during dressing changes. When a patient has an effective regimen of care that addresses these problems, this individual may be able to resume their normal activities in a timely manner.
It is widely known that silver contains broad‐spectrum antimicrobial properties 15, 16, 17. AQUACEL® dressing, when combined with ionic silver, is indicated for the management of wounds that are becoming infected or are infected. There has been a debate on the indication of the use of silver products in non infected leg ulcers, but it has to be emphasised that leg ulcers which have the potential of becoming infected or are infected is an indication for using silver products (18).
It is generally accepted that wounds heal faster in a moist wound environment (19). Modern wound dressings have been designed to promote these conditions. This type of dressing interacts with the wound exudate, providing a non adherent layer between the wound and the dressing. This moist layer not only protects the wound from mechanical damage, but also sequesters and immobilises bacteria (20), and provides an optimal environment for the wound to heal (21).
Urgotul® Silver is developed from a lipidocolloid technology. This sterile, non adhesive and non occlusive dressing is composed of a polyester textile mesh impregnated with hydrocolloid particles and vaseline. Silver is incorporated within the structure as silver sulphate that gradually releases over the course of 7 days, and is released as silver ion when the dressing enters into contact with wound fluids. Urgotul® is the non silvered version of this dressing.
Recently, a multi‐centre, open label randomised controlled trial found that Urgotul® Silver worn under compression bandaging promoted healing of critically colonised venous leg ulcers, when compared with a non silver control (Urgotul®). A total of 102 patients from 24 centres were included in the efficacy analysis with at least three of five following clinical signs present: pain between two consecutive dressing changes, periwound erythema, oedema, malodour and heavy exudation. The treatment period lasted 8 weeks with patients in the treatment group receiving Urgotul® Silver for the first 4 weeks followed by Urgotul® for the following 4 weeks. The control group received Urgotul® for 8 weeks (22).
Previously, two studies evaluated the efficacy and safety of AQUACEL® Ag dressing in subjects with chronic ulcers. Both studies showed wound size reduction after 4 weeks of treatment 23, 24. However, there is minimal comparative data available for existing silver dressings on the market. As a result, this study was conducted using a silver dressing for 4 weeks, followed by the non silver version of the same dressing for 4 weeks, which is the basis for the proposed study design to evaluate the effects of AQUACEL® Ag on the healing of chronic venous leg ulcers. Furthermore, a non inferiority trial was designed to compare the healing effects of AQUACEL® Ag with Urgotul® Silver in venous leg ulcers. The objective of this study was to show the non inferiority of AQUACEL® Ag to Urgotul® Silver.
PATIENTS AND METHODS
This was a two‐arm parallel, multi‐centre, open label, randomised controlled clinical trial conducted in 281 patients across 43 centres in the UK, Germany, France, Denmark and Poland.
Ethics
The study protocol was submitted to the local ethics committees for approval and the clinical trial was conducted in compliance with Good Clinical Practices and with the principles in the Declaration of Helsinki. All patients gave written consent to participate after having received full written information regarding the study objectives and content.
Patient population
Included in this study were male and female subjects over 18 years of age having an ankle to brachial pressure index of 0·8 or greater and a venous leg ulcer (i.e. CEAP classification of C6), with duration less than 24 months and size ranging between 5 and 40 cm2, meeting at least three of the five following clinical signs of bacterial contamination, defined by some as critical colonisation of the wound: pain between dressing changes, perilesional skin erythema, oedema, foul odour and high levels of exudate.
The following patients were excluded from this study: subjects who had current local or systemic antibiotics in the week before inclusion, had leg ulcers that were clinically infected or erysipelas, malignant, recent deep venous thrombosis or venous surgery within the last 3 months, progressive neoplastic lesion treated by radiotherapy or chemotherapy, were receiving ongoing treatment with immunosuppressive agents or high dose corticosteroids.
Sample size determination
Sample size was based on power analysis, which ensured 80% power to detect the non inferiority of AQUACEL®Ag dressing to Urgotul® Silver dressing on 8 week ulcer area relative reduction (RR) from the baseline value. If the mean clinical difference in the 8 week relative wound size reduction between the two dressings is 0% (SD = 49%), based on 5% alpha level, a non inferiority margin of 15%, the sample size needed per group is 133, and hence a total of 266 subjects was required.
Design and procedures
Subjects who met the selection criteria and who gave their written consent to participate in this study were randomly allocated in a 1 : 1 ratio to be treated either with AQUACEL® Ag dressing or with Urgotul® Silver dressing for 4 weeks followed by AQUACEL® or Urgotul®, respectively, for the remaining 4 weeks. Subjects in both groups were followed to complete healing or for a maximum of 8 weeks.
Subject demographics, baseline characteristics, leg ulcer history and the number of pre‐defined local signs were recorded at baseline and an index leg was selected (in the case of bilateral ulceration). A baseline acetate tracing of the wound surface area was also taken. The ulcer was covered on its whole surface by the test dressing followed by a sterile absorbent pad. A (UK) Class III compression system was applied to deliver an appropriately high level of compression. Both dressings were changed based on the clinical condition of the wound and the volume of exudate. Dressing changes were carried out by a health care professional either at the subjects' homes or in the clinic. For subjects wearing compression hosiery, dressing changes could have been performed by the subject at home at the discretion of the investigator. At each dressing change, wounds were inspected and cleaned exclusively with normal saline or warm water. If necessary, mechanical debridement could have been performed to remove slough and necrotic tissue.
Wounds were assessed weekly for the first 4 weeks (±2 days), and then every 2 weeks (±2 days) until the eighth week. At each clinic visit, wound status, perilesional skin appearance and condition of the wound (the presence or absence of the pre‐defined local signs) were recorded and acetate tracings were taken. Photographs could have been taken at the discretion of the investigator. The nature and frequency of all adverse events (AEs) were recorded and an AE profile was generated. To maintain uniformity of the clinical evaluations, all investigators were equivalently trained for all the assessments. The wound assessment tool used in this study is widely accepted in common clinical practice and can be found in published literature (25); therefore the inter‐rater reliability of this wound assessment tool is assured. In addition, all individuals conducting the patient assessments were fully trained before study commencement. Control was maintained by a clinical study manager who audited the study report.
Outcomes/endpoints
The primary efficacy outcome was the wound area RR at the last available evaluation compared with baseline (percent decrease from baseline), which was determined by acetate tracings taken during the weekly visits and at the final evaluation visit. The secondary outcomes included absolute wound area reduction, wound closure rate, chance to reach 40% wound area reduction at the last evaluation, local signs of heavy bacterial colonisation, clinical improvement of the reference ulcer, periulcer skin condition, condition of the reference ulcer and tolerance (occurrence of local AEs).
Randomisation
Subjects were randomly allocated in a 1 : 1 ratio to be treated either with the AQUACEL® Ag dressing or with the Urgotul® Silver dressing for 4 weeks, followed by AQUACEL® or Urgotul® (respectively) for the remaining 4 weeks. Randomisation was carried out using permuted blocks scheme. Randomisation envelopes were provided to sites whereby the next lowest sequential subject number was picked.
Statistical analysis
All randomised subjects and who had at least one exposure to the device were included in the intent‐to‐treat (ITT) population. Subjects were analysed in the treatment groups to which they were randomly assigned to avoid bias in the treatment comparisons. The treatment code was determined by the original randomisation. The per protocol (PP) population consisted of all enrolled subjects who satisfied the entry criteria of this study and who completed the treatment as defined in the protocol, without any major protocol violation.
The test for the non inferiority of AQUACEL® Ag to Urgotul® Silver in area reduction was carried out through a confidence interval (CI) approach. Non inferiority was established if the lower bilateral limit of the 95% CI for the between‐treatment difference (AQUACEL® Ag – Urgotul® Silver) in the mean percentage area change from baseline did not exceed −15%. According to previously developed recommendations 26, 27, the non inferiority test was evaluated on both ITT and PP populations.
Secondary outcomes included the absolute wound area reduction: value W 8w−value W baseline. A t test or Wilcoxon test was used to compare the absolute wound area reduction between the two groups. The wound closure rate was calculated as (value W 8w−value W baseline)/t, where t was the number of days between the two measurements. The results were expressed in cm2/day. A chi‐square test was used and chances to reach a 40% wound area reduction at the last evaluation. A logistic regression model was used to compare the difference in the chance with reach a 40% wound area reduction after adjusting for the following factors: age, body mass index (BMI), ulcer duration and baseline ulcer area.
A post hoc analysis was performed based on a composite wound healing assessment. Two parameters were included as the composite endpoint: wound volume reduction and final overall wound assessment of improvement. A chi‐square test was used to compare the proportions of subjects with the better wound assessment between the two groups.
RESULTS
Between January 2010 and December 2010, a total of 281 subjects were enrolled in this study. One hundred and forty‐five subjects were randomised to AQUACEL® Ag followed by the AQUACEL® regimen, while 136 subjects were randomised to Urgotul® Silver followed by the Urgotul® regimen (Table 1). Of these subjects, 134 subjects (92·4%) completed the study PP in the AQUACEL® treatment group while 120 subjects (88·2%) completed the study PP in the Urgotul® group. Eleven subjects (7·6%) in the AQUACEL® Ag treatment group and 16 subjects (11·8%) in the Urgotul® Silver treatment group discontinued from this study (Table 1). Eight subjects (5·5%) discontinued because of AEs in the AQUACEL® Ag treatment group, whereas 11 subjects (8·1%) discontinued because of AEs in the Urgotul® Silver treatment group.
Table 1.
Subject disposition
| Subject status | AQUACEL® Ag/AQUACEL® (n = 145), N (%) | Urgotul® Silver/Urgotul® (n = 136), N (%) |
|---|---|---|
| Enrolled | 145 | 136 |
| Healed | 24 (16·6) | 21 (15·4) |
| Maximum study participation | 110 (75·9) | 99 (72·8) |
| Discontinued | 11 (7·6) | 16 (11·8) |
| Due to adverse event | 8 (5·5) | 11 (8·1) |
| Withdrew consent | 1 (0·7) | 1 (0·7) |
| Lost to follow‐up | 0 | 2 (1·5) |
| No longer meets criteria | 0 | 1 (0·7) |
| Protocol violation | 1 (0·7) | 0 |
| Investigator discretion | 1 (0·7) | 1 (0·7) |
Baseline characteristics
The mean age of the study population was 68·72 years in the AQUACEL® Ag treatment group and 71·21 years in the Urgotul® Silver treatment group (Table 2). The majority of the study population was female in both treatment groups [94 subjects (64·8%) in the AQUACEL® Ag and 90 subjects (66·2%) in the Urgotul® Silver treatment group]. While the majority of demographic characteristics were comparable between both treatment groups, there was a statistically significant difference in the BMI at baseline between both treatment groups (P = 0·016, Table 2) with a higher mean BMI in the AQUACEL® Ag treatment group.
Table 2.
Subject demographics
| Characteristic | AQUACEL® Ag/AQUACEL® (n =145) | Urgotul® Silver/Urgotul® (n =136) | P‐value | ||
|---|---|---|---|---|---|
| Mean ± SD or N (%) | Minimum/maximum | Mean ± SD or N (%) | Minimum/maximum | ||
| Age (years) | 68·72 ± 13·07 | 34/97 | 71·21 ± 12·13 | 40/99 | 0·10 |
| Height (cm) | 166·82 ± 9·52 | 131/192 | 167·05 ± 9·54 | 144/198 | 0·84 |
| Weight (kg) | 86·91 ± 25·19 | 39·0/230·0 | 81·03 ± 23·61 | 42·7/172·0 | 0·04 |
| Gender | |||||
| Male | 51 (35·2) | 46 (33·8) | |||
| Female | 94 (64·8) | 90 (66·2) | 0·81 | ||
| Body mass index | 31·08 ± 8·05 | 16·41/76·85 | 28·86 ± 7·22 | 15·43/58·35 | 0·016 |
Wound characteristics at baseline were also comparable across both treatment groups (Table 3). However, the majority of subjects in the AQUACEL® Ag treatment group had ulcers on the left leg (83 subjects, 57·2%), whereas the majority of subjects in the Urgotul® Silver treatment group had ulcers on the right leg (78 subjects, 57·4%). There was also a statistically significant difference in the occurrence of recurrent ulcers between both treatment groups (P = 0·026) with more recurrent ulcers in the AQUACEL® Ag treatment group. The majority of ulcers in this study were either deteriorating or had shown no progress in both treatment groups (sum total of ‘Deteriorating’ and ‘No Progress' = 82·6% for AQUACEL® Ag and 78·7% for Urgotul® Silver, Table 3). At baseline, 110 subjects (75·9%) in the AQUACEL® Ag treatment group and 95 subjects (69·9%) in the Urgotul® Silver treatment group were using compression therapy.
Table 3.
Wound characteristics at baseline
| Characteristic | AQUACEL® Ag/AQUACEL® (n = 145) | Urgotul® Silver/Urgotul® (n = 136) | P‐value | ||
|---|---|---|---|---|---|
| Mean ± SD or N (%) | Minimum/maximum | Mean ± SD or N (%) | Minimum/maximum | ||
| Duration of leg ulcer | 0·8 ± 0·6 | 0·01/2·0 | 0·72 ± 0·53 | 0·04/1·83 | 0·24 |
| Ankle to brachial pressure index | 1·03 ± 0·15 | 0·8/1·52 | 1·06 ± 0·17 | 0·8/2·05 | 0·17 |
| Leg ulcer is present | |||||
| Left | 83 (57·2) | 58 (42·6) | |||
| Right | 62 (42·8) | 78 (57·4) | 0·01 | ||
| Primary location of ulcer | |||||
| Foot | 3 (2·1) | 2 (1·5) | |||
| Ankle | 63 (43·4) | 70 (51·5) | 0·61 | ||
| Calf | 51 (35·2) | 42 (30·9) | |||
| Gaiter | 28 (19·3) | 22 (16·2) | |||
| Ulcer recurrent or new | |||||
| Recurrent | 69 (47·6) | 47 (34·6) | |||
| New | 76 (52·4) | 89 (65·4) | 0·03 | ||
| Depth of leg ulcer | |||||
| Superficial | 15 (10·3) | 16 (11·8) | |||
| Shallow | 119 (82·1) | 111 (81·6) | 0·64 | ||
| Deep | 11 (7·6) | 9 (6·6) | |||
| Current status of ulcer | |||||
| Unavailable | 0 | 1 (0·7) | |||
| Improving | 25 (17·2) | 28 (20·6) | 0·41 | ||
| No progress | 63 (43·3) | 57 (41·9) | |||
| Deteriorating | 57 (39·3) | 50 (36·8) | |||
Relative wound area reduction
By week 4, ulcer area decreased on average by 38·24% ± 40·63% in the AQUACEL® Ag treatment group and by 32·47% ± 48·93% in the Urgotul® Silver treatment group (Figure 1). After week 4, when all subjects in the AQUACEL® Ag group switched to AQUACEL® and all Urgotul® Silver subjects switched to Urgotul®, further reduction in ulcer area from baseline was noted in both the ITT and PP populations (Figure 1). A larger difference was observed in the RR of wound area in the AQUACEL® Ag treatment group as compared with the Urogtul® Silver treatment group at week 8, in both ITT and PP populations (ITT: 49·65% ± 52·53% AQUACEL® Ag versus 42·81% ± 60·0% Urogtul® Silver; PP: 51·73% ± 52·94% AQUACEL® Ag versus 45·08% ± 63·93% Urogtul® Silver, Table 4). The mean difference between the AQUACEL® Ag and Urgotul® Silver groups was 6·84% ± 56·28% with a bilateral 95% CI (−6·56% to 20·23%) in the ITT population and 6·64% ± 58·41% with a bilateral 95% CI (−9·1% to 22·38%) in the PP population. As a result, non inferiority could be established in both the ITT as well as the PP populations, based on the pre‐defined non inferiority margin (−15%).
Figure 1.
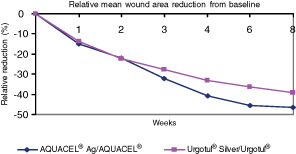
Relative mean wound area reduction from baseline (differences not significant).
Table 4.
Statistical analysis for the reduction in ulcer area
| Treatment | Mean ± SD | SE | Minimum/maximum | 95% confidence interval mean | P‐value | |
|---|---|---|---|---|---|---|
| Change from baseline – LOCF intent‐to‐treat population | ||||||
| AQUACEL® Ag/AQUACEL® (n = 141) | 49·65 ± 52·5 | 4·42 | −212·3/100 | 40·9 | 53·4 | |
| Urgotul® Silver/Urgotul® (n = 133) | 42·81 ± 60·0 | 5·2 | −217·7/100 | 32·5 | 53·1 | |
| Difference (1–2) | 6·84 ± 56·3 | 6·8 | −6·56 | 20·2 | 0·3158 | |
| Change from baseline – LOCF per protocol population | ||||||
| AQUACEL® Ag/AQUACEL® (n = 115) | 51·7 ± 52·9 | 4·9 | −212·3/100 | 41·9 | 61·5 | |
| Urgotul® Silver/Urgotul® (n = 104) | 45·1 ± 63·9 | 6·3 | −217·7/100 | 32·6 | 57·5 | |
| Difference (1–2) | 6·64 ± 58·4 | 7·9 | −9·1 | 22·4 | 0·4062 | |
LOCF, last observation carried forward.
Absolute wound area reduction
The absolute wound area was reduced in both treatment groups at the end of this study at week 8 (Figure 2). While a larger reduction was seen in the AQUACEL® Ag treatment group (8·76 ± 12·84 sq cm) as compared with the Urgotul® Silver treatment group (7·21 ± 9·5 sq cm) at week 8, this reduction was not statistically significant (P = 0·2537). On average, the ulcer area decreased from 17·9 ± 15·19 sq cm at baseline to 8·98 ± 11·3 sq cm at final evaluation (week 8) in the AQUACEL® Ag treatment group and from 17·14 ± 14·03 sq cm at baseline to 9·64 ± 11·62 sq cm at week 8 in the Urgotul® Silver treatment group.
Figure 2.
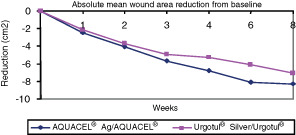
Absolute mean wound area reduction from baseline (differences not significant).
Wound closure rate
The change in overall wound closure rate from baseline to week 8 was similar across both treatment groups (0·17 ± 0·43 in the AQUACEL® Ag treatment group and 0·14 ± 0·19 in the Urgotul® Silver treatment group) and no statistically significant differences were observed (P = 0·4380).
Chance to reach 40% wound area reduction
The majority of subjects in both treatment groups had ulcers that decreased by 40% or more at the end of week 8. While a larger percentage of subjects (66·2%) in the AQUACEL® Ag group had ulcers that decreased by 40% or more in size as compared with Urgotul® Silver (58·5%); this difference was not statistically significant (P = 0·1842).
By logistic regression, including the parameters treatment group, age, BMI, duration of ulcer in years and ulcer size at baseline, in this model, only the duration of ulcer in years was a significant predictive factor for this endpoint with an odds ratio of 0·55 (P = 0·0076, 95% CI 0·35 to 0·85).
Local signs of heavy bacterial colonisation
At week 4, no clinical signs of heavy bacterial colonisation were reported in 17·91% of the ulcers in the AQUACEL® Ag group as compared with 15% in the Urgotul® Silver group. This number increased to 39·5% and 32·5% at week 8 in the AQUACEL® Ag and Urgotul® silver groups, respectively. There was no significant difference.
Clinical improvement of reference ulcer
The majority of subjects showed a marked improvement in the condition of their ulcer at the final evaluation in the AQUACEL® Ag treatment group (81 subjects, 57%). Twenty‐four subjects had healed ulcers in the AQUACEL® Ag treatment group and 21 subjects (14·8%) showed mild improvement. In comparison, ulcers in 60 subjects (45·5%) showed marked improvement in the Urgotul® Silver treatment group and 21 subjects (15·9%) had healed wounds. Similar results were seen across both treatment groups in overall change in condition of the ulcer at final evaluation, and there was no statistically significant difference (P = 0·0889) between both treatment groups.
Post hoc analysis
It is well accepted that both wound surface area and wound depth are important objective wound healing trajectories. In practice, the physician's general impression of the wound status change after treatment is also critical, and semi‐quantitative evaluations have been used to assess wound healing (25). Therefore, two parameters were identified as an appropriate composite endpoint: wound volume reduction and a final wound assessment of improvement. A post hoc analysis was performed based on a composite wound healing assessment (Table 5). On the basis of this composite endpoint, the AQUACEL® Ag treatment group had a significantly higher percentage of subjects who showed better wound progression (66·9% versus 51·9%, P = 0·0108, Table 5).
Table 5.
Overall wound progression based on composite wound healing assessment
| AQUACEL® Ag/AQUACEL® | Urgotul® Silver/Urgotul® | P‐value | |
|---|---|---|---|
| No | 48 (33·1%) | 64 (48·12%) | |
| Yes | 97 (66·9%) | 69 (51·9%) | 0·0108 |
| Total | 145 | 133+ |
+, 3 missing values.
Safety
A total of 72 subjects (49·7%) in the AQUACEL® Ag treatment group and 57 subjects (41·9%) in the Urgotul® Silver group experienced AEs during this study (Table 6). Of these, only 33 subjects (22·8%) in the AQUACEL® Ag treatment group and 24 subjects (17·6%) in the Urgotul® Silver treatment group had AEs that were related to the study product (Table 6). Six subjects in the AQUACEL® Ag and six in the Urgotul® Silver treatment groups experienced serious adverse events (SAEs), and of these, two deaths occurred in the Urgotul® Silver treatment group. The SAEs and deaths were not considered to be related to treatment. Overall, 9 subjects (6·2%) in the AQUACEL® Ag treatment group and 12 subjects (8·8%) in the Urgotul® Silver treatment group discontinued because of AEs (Table 6).
Table 6.
Adverse events
| AQUACEL® Ag/AQUACEL® (n = 145), N (%) | Urgotul® Silver/Urgotul® (n = 136), N (%) | |
|---|---|---|
| Summary of AEs | ||
| Subjects with events | 72 (49·7) | 57 (41·9) |
| Subjects with related AEs | 33 (22·8) | 24 (17·6) |
| Subjects with SAEs | 6 (4·1) | 6 (4·4) |
| Subjects with death | 0 | 2 (1·4) |
| Related SAEs | 0 | 0 |
| Discontinuations due to AEs | 9 (6·2) | 12 (8·8) |
| Incidence of AEs | ||
| Subjects with events | 72 (49·7) | 57 (41·9) |
| Skin and subcutaneous tissue disorder | 32 (22·1) | 40 (29·4) |
| Eczema | 9 (6·2) | 4 (2·9) |
| Venous ulcer pain | 9 (6·2) | 9 (6·6) |
| Skin ulcer | 8 (5·5) | 16 (11·8) |
| Erythema | 7 (4·8) | 12 (8·8) |
| Excessive granulation tissue | 2 (1·4) | 5 (3·7) |
| General disorders and administration site conditions | 31 (21·4) | 22 (16·2) |
| Deterioration of the wound | 13 (9·0) | 13 (9·6) |
| Pain | 11 (7·6) | 8 (5·9) |
| Oedema peripheral | 10 (6·9) | 6 (4·4) |
| Infections and infestations | 16 (11·0) | 12 (8·8) |
| Infected skin ulcer | 6 (4·1) | 4 (2·9) |
| Injury, poisoning and procedural complications | 21 (14·5) | 17 (12·5) |
| Wound secretion | 9 (6·2) | 6 (4·4) |
AE, adverse event; SAE, serious adverse events.
Deterioration of the wound (13 subjects, 9·0%) was the most frequently reported AE in the AQUACEL® Ag treatment group, followed by pain (11 subjects, 7·6%) and peripheral oedema (10 subjects, 6·9%). Skin ulcer (16 subjects, 11·8%) was the most frequently reported AE in the Urgotul® Silver treatment group, followed by deterioration of the wound (13 subjects, 9·6%) and erythema (12 subjects, 8·8%) (Table 6).
With regard to related AEs, pain and eczema (6 subjects, each 4·1%) were the most frequently reported in the AQUACEL® Ag treatment group, followed by deterioration of wound (5 subjects, 3·4%) and wound secretion (4 subjects, 2·8%). Deterioration of wound (7 subjects, 5·1%) was the most frequently reported related AE in the Urgotul® Silver treatment group, followed by skin ulcer (5 subjects, 3·7%) and pain (4 subjects, 2·9%).
DISCUSSION
This 8‐week, multi‐centre, open, randomised study was designed to test non inferiority of AQUACEL® Ag relative to Urgotul® Silver. All subjects were randomised to AQUACEL® Ag for 4 weeks followed by AQUACEL®, or Urgotul® Silver for 4 weeks followed by Urgotul®.
The primary objective of this study, showing non inferiority of AQUACEL® Ag to Urgotul® Silver, was established in both the ITT as well as the PP populations, based on the pre‐specified margins. Relative wound area reduction from baseline was chosen as the primary efficacy parameter. At the end of week 8, the average percent decrease in wound area from baseline was 49·65% ± 52·53% for AQUACEL® Ag as compared with 42·81% ± 60·00% for the Urgotul® Silver treatment group in the ITT population. The mean difference in the percent decrease in wound from baseline between the two treatment groups, AQUACEL® Ag versus Urgotul® Silver, was 6·84% ± 56·28% (95% CI −6·56% to 20·23%) in the ITT population and 6·64% ± 58·41% (95% CI −9·1% to 22·38%) in the PP population.
Leg ulcers included in this clinical trial can be considered as difficult‐to‐heal wounds. The mean age of the population was more than 68 years, ulcers were present for 7 months on average, and over 40% were recurrent at baseline. All these criteria are known to predict poor healing, which may explain why around 70% of selected wounds were considered as worsening or showing no progression at baseline by experienced physicians, despite adequate local and general management. However, absolute wound area also decreased in both treatment groups, and the majority of subjects in both treatment groups showed 40% decrease or more in ulcer size, with larger decreases seen in the AQUACEL® Ag group as compared with the Urgotul® Silver treatment group. Clinical improvement based on signs of heavy bacterial colonisation was also seen at week 8 where the number of subjects without bacterial colonisation increased from 4·48% and 3·33% at baseline in the AQUACEL® Ag and Urgotul® silver groups, respectively, to 39·5% and 32·5% at week 8 in the AQUACEL® Ag and Urgotul® silver groups, respectively. Moreover, the majority of subjects showed a marked improvement in the condition of the ulcer at the final evaluation in the AQUACEL® Ag treatment group (81 subjects, 57%) and Urgotul® Silver treatment group (ulcers on 60 subjects, 45·5%).
A physician's overall impression of change in wound status after treatment is a critical consideration of assessing wound progression towards healing. To encompass this evaluation, a post hoc analysis was conducted based on composite wound healing assessment, which showed that the AQUACEL® Ag group had a higher percentage of subjects with better wound progression as compared with the Urgotul® Silver treatment group (66·9% versus 51·9%, P = 0·0108). In general, the safety profile was similar between both treatment groups. Both dressings were well tolerated, and there were no dressing‐related SAEs or deaths in either treatment group.
In conclusion, the primary objective of this study, non inferiority, was established based on pre‐specified margins. The results from this study showed that both dressings were efficacious in reducing the ulcer size; however, a larger non significant decrease was seen in the AQUACEL® Ag treatment group as compared with the Urgotul® Silver treatment group. Post hoc analysis conducted based on composite wound healing assessment showed that the AQUACEL® Ag group had a significantly higher percentage of subjects with better wound progression as compared with the Urgotul® Silver treatment group.
ACKNOWLEDGEMENTS
This work was supported by ConvaTec Inc. The sponsor designed this study and reviewed and approved the final article; the authors had full control over the contents of this article and approved the final submission. Dr Kommala is an employee of ConvaTec Inc. The other authors report no conflicts of interest and received no financial support to write this article. AQUACEL is a trademark of ConvaTec Inc. and Urgotul is a trademark of Société de Development et de Recherche Industrielle.
REFERENCES
- 1. Mekkes JR, Loots MA, Van der Wal AC, Bos JD. Causes, investigation and treatment of leg ulceration. Br J Dermatol 2003;148:388–401. [DOI] [PubMed] [Google Scholar]
- 2. Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46:381–6. [DOI] [PubMed] [Google Scholar]
- 3. Baker SR, Stacey MC, Jopp‐McKay AG, Hoskin SE, Thompson PJ. Epidemiology of chronic venous ulcers. Br J Surg 1991;78:864–7. [DOI] [PubMed] [Google Scholar]
- 4. Nelzen O, Bergquist D, Lindhagen A. The prevalence of chronic lower‐limb ulceration has been underestimated: results of a validated population study. Br J Surg 1996;83:255–8. [PubMed] [Google Scholar]
- 5. Finlayson K, Edwards H, Courtney M. Relationships between preventive activities, psychosocial factors and recurrence of venous leg ulcers: a prospective study. J Adv Nurs 2011;67:2180–90. [DOI] [PubMed] [Google Scholar]
- 6. de Araujo T, Valencia I, Federman DG, Kirsner RS. Managing the patient with venous ulcers. Ann Intern Med 2003;138:326–34. [DOI] [PubMed] [Google Scholar]
- 7. Baker S, Stacey M, Singh G, Hoskin S, Thompson P. Aetiology of chronic leg ulcers. Eur J Vasc Surg 1992;6:245–51. [DOI] [PubMed] [Google Scholar]
- 8. Cornwall J, Dore C, Lewis J. Leg ulcers: epidemiology and aetiology. Br J Surg 1986;73:693–6. [DOI] [PubMed] [Google Scholar]
- 9. Atiyeh BS, Ioannovich J, Al‐Amm CA, El‐Musa KA. Management of acute and chronic open wounds: the importance of moist environment in optimal wound healing. Curr Pharm Biotechnol 2002;3:179–95. [DOI] [PubMed] [Google Scholar]
- 10. Benbow M. Exploring the concept of moist wound healing and its application in practice. Br J Nurs 2008;17:S4–S8. [DOI] [PubMed] [Google Scholar]
- 11. Seaman, S. Dressing selection in chronic wound management. J Am Podiatr Med Assoc 2002;92: 24–33. [DOI] [PubMed] [Google Scholar]
- 12. Bradley L. Venous haemodynamics and the effects of compression stockings. Br J Community Nurs 2001;6:165–75. [DOI] [PubMed] [Google Scholar]
- 13. Johnson S. Compression hosiery in the prevention and treatment of venous leg ulcers. J Tissue Viability 2002;12:67–74. [DOI] [PubMed] [Google Scholar]
- 14. O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev 2009;21:CD000265. [DOI] [PubMed] [Google Scholar]
- 15. Edwards‐Jones V. The benefits of silver in hygiene, personal care and healthcare. Surg Infect 2009;10:289–92. [DOI] [PubMed] [Google Scholar]
- 16. Jørgensen B, Price P, Andersen KE, Gottrup F, Bech‐Thomsen N, Scanlon E, Kirsner R, Rheinen H, Roed‐Petersen J, Romanelli M, Jemec G, Leaper DJ, Neumann MH, Veraart J, Coerper S, Agerslev RH, Bendz SH, Larsen JR, Sibbald RG. The silver‐releasing foam dressing, contreet foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J 2005;2:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carter MJ, Tingley‐Kelley K, Warriner RA. Silver treatments and silver impregnated dressings for the healing of leg wounds and ulcers: a systemic review and meta‐analysis. J Am Acad Dermatol 2010;63:668–79. [DOI] [PubMed] [Google Scholar]
- 18. Gottrup F, Apelqvist J. The challenge of using randomized trials in wound healing. Br J Surg 2010;97:303–4. [DOI] [PubMed] [Google Scholar]
- 19. Alvarez O. The effect of occlusive dressings on collagen synthesis and re‐epithelialisation in superficial wounds. J Surg Res 1983;35:142–8. [DOI] [PubMed] [Google Scholar]
- 20. Walker M, Hobot JA, Newman GR, Bowler PG. Scanning electron microscopic examination of bacterial immobilisation in a carboxymethyl cellulose (AQUACEL) and alginate dressings. Biomaterials 2003;24:883–90. [DOI] [PubMed] [Google Scholar]
- 21. Varghese MC, Balin AK, Carter DM, Caldwell D. Local environment of chronic wounds under synthetic dressings. Arch Dermatol 1986;122: 52–7. [PubMed] [Google Scholar]
- 22. Lazareth I, Meaume S, Sigal‐Grinberg ML, Combemale P, Guyadec T, Zagnoli A, Perrot J‐L, Sauvadet A, Bohbot S. The role of a silver releasing lipido‐colloid contact layer in venous leg ulcers presenting inflammatory signs suggesting heavy bacterial colonization: results of a randomized controlled study. Wounds 2008;20:158–66. [PubMed] [Google Scholar]
- 23. Vanscheidt W, Lazareth I, Routkovsky‐Norval C. Safety evaluation of a new ionic silver dressing in the management of chronic ulcers. Wounds 2003;15:371–8. [Google Scholar]
- 24. Coutts P, Sibbald RG. The effect of a silver‐containing hydrofiber dressing on superficial wound bed and bacterial balance of chronic wounds. Int Wound J 2005;2:348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Henanff A, Giraudeau B, Baron G, Ravaud P. Quality of reporting of noninferiority and equivalence randomized trials. JAMA 2006;295: 1147–51. [DOI] [PubMed] [Google Scholar]
- 26. Guidance for Industry Non‐Inferiority Clinical Trials. URL http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf [accessed on 18 October 2011].
- 27. Beele H, Meuleneire F, Nahuys M, Percival SL. A prospective randomised open label study to evaluate the potential of a new silver alginate/carboxymethylcellulose antimicrobial wound dressing to promote wound healing. Int Wound J 2010;7:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


