Abstract
Hypertrophic scars (HSs) and keloids are commonly seen as two different diseases by both clinicians and pathologists. However, as supported by histological evidence showing they share increased numbers of fibroblasts and accumulate collagen products, HS and keloid might be different forms of the same pathological entity, rather than separate conditions. To test this hypothesis, keloids from patients who underwent scar excisions (n = 20) in Nippon Medical School from 2005 to 2010 were examined histologically. The proportion and distribution of cellular and matrix collagen components were evaluated at the centre and periphery of each sample. In keloid samples, coexistence of hyalinised collagen, which is the most important pathognomonic characteristic of a keloid and dermal nodules that are considered to be characteristic of HS, was found. Moreover, hyalinised fibres appeared to initiate from the corner of the dermal nodules. Key features of inflammation such as microvessels, fibroblasts and inflammatory cells all decreased gradually from the periphery to the centre of keloids, indicative of reduced inflammation in the centre. Thus, we hypothesise that HS and keloid can be considered as successive stages of the same fibroproliferative skin disorder, with differing degrees of inflammation that might be affected by genetic predisposition.
Keywords: Fibroproliferation, Hypertrophic scar, Inflammation, Keloid, Scar pathology
Introduction
Traditionally, hypertrophic scars (HSs) and keloids are diagnosed as separate clinical and pathological entities, although they both feature prolonged and aberrant extracellular matrix accumulation. ‘Typical’ keloids grow beyond the confines of their original wounds, with accumulation of dermal hyalinised collagens visible under the microscope. In contrast, HSs generally grow within the boundaries of the original wounds and appear histologically as dermal nodules. However, even senior clinicians sometimes have difficulty in differentiating the two conditions, particularly with atypical cases. Pathologists traditionally rely on the thick oeosinophilic collagen bundles in keloids to distinguish them from HSs, but the diagnosis is not always straightforward. On the basis of a number of observations, including the growth of keloids from mature scars 1 and the induction of HSs by mechanical forces 2, it has been speculated that HSs and keloids may represent different stages of the same underlying pathological lesion. Therefore, we analysed keloids histologically and compared their histological characteristics with those of HSs. We found simultaneous existence of the pathognomonic characteristics of keloid and HS, and their transition within keloid samples closely related to topical inflammation. Thus, we put forward the hypothesis that HS and keloid can be considered as successive stages of the same fibroproliferative skin disorders, distinguished by different degrees of inflammation.
Patients and methods
The study included 20 patients with chest keloids who were treated at Nippon Medical School between 2005 and 2010. All the cases met the clinical diagnostic criteria of keloid as ‘invasive’ growth, in the shape of a dumbbell or butterfly, and beyond the confines of the original wound. The patients were 30·3 years old on average and 50% were females. None of the patients could tell the exact cause of the keloids during the histories of long duration but recalled that it ‘might’ be acne. None of the patients received medical treatments prior to surgery.
Keloid samples were collected after excision, fixed in 4% paraformaldehyde, embedded in paraffin and cut into 4‐µm‐thick sections on a microtome. Sections were made perpendicular to the anterior–posterior axis of the wound and stained with haematoxylin–eosin (H‐E). We measured the area percentage of hyalinised keloidal collagens present in the centre of keloids per high‐power field (100×) and the area percentage of hyalinised keloidal collagens within dermal nodules.
The research complied with the Declaration of Helsinki and was approved by the ethics committee of Nippon Medical School.
Results
General histological characteristics
The epidermis of all keloid specimens was of normal thickness, but lacked the normal rete ridges. The dermis, however, was greatly expanded. In the papillary dermis, the Grenz zone had relatively normal collagen demarcating the boundary between epidermis and dermis. In the upper reticular dermis, a horizontal cellular fibrous band was found, together with increased numbers of microvessels, fibroblasts and inflammatory cells. Hair follicles, sebaceous glands and sweat glands were reduced, although each structure could still be found scattered throughout the dermis.
In the peripheral area of the keloid, fibroblasts dominated the field. Wavy collagen fibres were dispersed around the fibroblasts, sometimes demarcating the dermal nodules. Microvessels were generally dispersed parallel to the epidermis. In the central area, strongly oeosinophilic hyalinised collagen dominated the field, mainly running parallel to epithelial surfaces. Cells and microvessels were scarcely seen within the centre of the lesion.
Hyalinised collagen
Hyalinised collagen was traditionally considered as the most important pathognomonic characteristic of a keloid. In our specimens, central hyalinised collagens were large and thick, strongly oeosinophilic and ran parallel to epithelial surfaces. The average area percentage of hyalinised keloidal collagens present in the centre of keloids per high‐power field (100×) was 38·21% (n = 20). The inter‐collagen fibres were thin and sparsely dispersed. In contrast, collagens in the peripheral part of keloid were not hyalinised and were thin in diameter, low in density and random in orientation, demonstrating less organisation with a tendency towards a wavy, or even fragmented, shape. Moreover, inter‐collagen fibres were more common than in the central region.
Dermal nodules
Dermal nodules, which are considered to be characteristic of HS, were composed of focal fibroblast aggregates and randomly oriented collagen fibres. The nodules were located in both the central and peripheral parts of the keloid lesions. Large nodules in the periphery were dominated mainly by cellular ingredients surrounded by fine collagen bands at the lesion edge, although the demarcation was unclear because of the intimate relationship between collagen fibres and fibroblasts. In contrast, nodules in the central part of the lesion were smaller, with well‐demarcated borders consisting of thick keloidal collagen. With regard to nodule size, quantity of cellular ingredients and quantity and thickness of collagen, nodules in the zone between the periphery and centre of the lesion generally had an intermediate morphology.
Both hyalinised keloidal collagens and dermal nodules were found within the same samples. The character of the hyalinised collagen fibres changed with location. At the base the fibres were short, wavy, randomised in relation to the epidermis and non oeosinophilic, whereas at the top they were long, straight, parallel to the epidermis and strongly oeosinophilic (Figure 1C). The dermal nodules also changed with location. At the periphery they were large, poorly demarcated and highly cellular, whereas at the centre they were small, clearly demarcated by surrounding keloidal collagens and were less cellular (Figure 2). Hyalinised collagens were first apparent at the corner of the dermal nodule (Figure 3). The average area percentage of hyalinised keloidal collagens within dermal nodules was 5·88% (n = 10).
Figure 1.
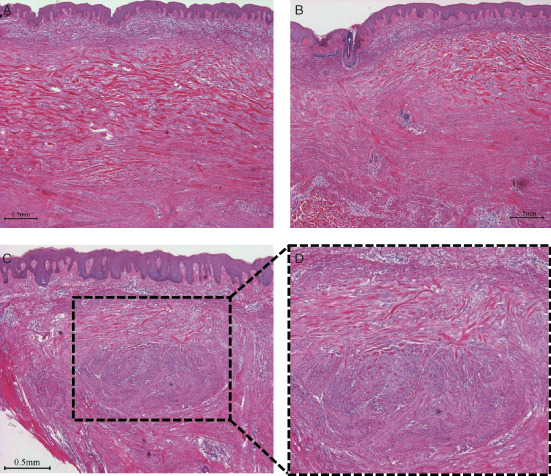
Haematoxylin–eosin stain for case 1. (A) Central area (40×). The expanded dermis was occupied by large and highly oeosinophilic hyalinised collagen bundles running parallel to the superficial epidermis. (B) Peripheral area (40×). The hyalinised keloidal collagens were mainly located in the upper reticular dermis, and were somewhat fragmented and shortened, and interrupted and surrounded by cellular components. The boundaries of the hyalinised keloidal collagens were clearer in the epidermal and central sides than in the dermal and peripheral sides. R side: central side; L side: peripheral side. (C) Coexistence of keloidal collagen and dermal nodules (40×) and its topical enlargement in panel (D) (100×). The dermal nodules, comprising focal fibroblast aggregates together with randomly oriented collagen fibres, had hyalinised collagens scattered on their tops. The character of the hyalinised collagen fibres changed with location. At the base the fibres were short, wavy, randomised in relation to the epidermis and non‐oeosinophilic, whereas at the top they were long, straight, parallel to the epidermis and strongly oeosinophilic.
Figure 2.
Haematoxylin–eosin pictures for case 2. Hyalinised keloidal collagen fibres occupy the expanded reticular dermis in the central area, and the cellularity decreased from the central to the peripheral areas. (A) Panorama (40×). (B) Topical enlargement of central area (100×). (C) Topical enlargement of peripheral area (100×).
Figure 3.
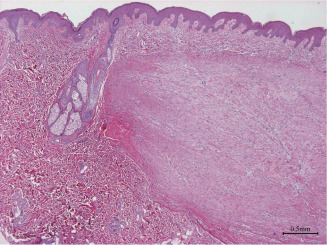
Dermal nodule (40×) in case 2. A typical dermal nodule in the dermis, together with a residual hair follicle and sebaceous gland. Hyalinised collagens first appear at the left corner of the nodule.
Typical cases
Case 1. A 23‐year‐old female with chest keloid.
The expanded dermis was occupied by large, highly oeosinophilic hyalinised collagen bundles running parallel to the superficial epidermis (Figure 1A). The Grenz zone was clearly located in the papillary dermis, with relatively normal collagen demarcating the boundary between epidermis and dermis.
In Figure 1B, the left side shows the peripheral area of the lesion. The hyalinised keloidal collagens were mainly located in the upper reticular dermis, and were somewhat fragmented and shortened, and interrupted and surrounded by cells. The boundaries formed by the hyalinised keloidal collagens were clearer in the superficial epidermal and central areas (right side) than in the dermal and peripheral areas.
Dermal nodules, comprising focal fibroblast aggregates together with randomly oriented collagen fibres, had scattered hyalinised collagens at the top of the nodule (Figure 1C). The collagen fibres changed depending on location: they were short, wavy, random in distribution and minimally oeosinophilic at the base, and long, straight, parallel to the epidermis and highly oeosinophilic at the top of the nodule (Figure 1D).
Case 2. A 28‐year‐old female with chest keloid.
Hyalinised keloidal collagen fibres occupied the expanded reticular dermis in the central area (Figure 2). The ratio of collagen to cells decreased from the central to the peripheral areas. A typical dermal nodule was located in the dermis, together with residual hair follicle and sebaceous gland (Figure 3). Hyalinised collagens were located in the left corner of the nodule.
Case 3. A 19‐year‐old male with a chest keloid.
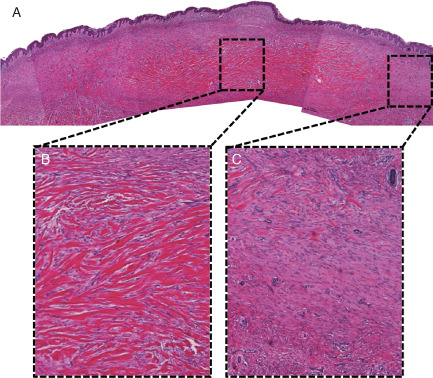
Hyalinised keloidal collagens dominated the expanded reticular dermis in the central area, whereas large dermal nodules were predominant in the peripheral dermis (Figure 4). With increasing distance from the central area, the dermal nodules became larger, contained fewer fibres and had less well‐defined outlines.
Figure 4.

Combined haematoxylin–eosin pictures for case 3. Hyalinised keloidal collagens dominate the expanded reticular dermis in the central area, whereas large dermal nodules dominate the peripheral dermis. With increasing distance from the central area, the dermal nodules become larger, with fewer fibres. (A) Panorama (40×). (B) Topical enlargement of peripheral area (40×). (C) Topical enlargement of central area (100×).
Discussion
The diagnostic distinction between keloid and HS has long been challenging for both clinician and pathologist. Keloid is typically characterised by ‘invasive’ behaviour from the original wound into the neighbouring healthy skin, with a leading edge that is often erythematous and pruritic 1; in contrast, HS undergoes limited growth and remains within the boundaries of the original wounds. However, there are a significant number of cases that do not fall within these discrete classifications, and the diagnosis of these cases can be challenging even for experienced clinicians. For pathologists, pathognomonic histological characteristics of keloid include keloidal hyalinised collagen, a tongue‐like advancing edge underneath normal‐appearing epidermis and papillary dermis, horizontal cellular fibrous bands in the upper reticular dermis and prominent fascia‐like fibrous bands 3. However, such hyalinised collagen is found in only 55% of keloid specimens 3. Dermal nodules have traditionally been a key feature in the histological diagnosis of HS, because they were believed to be absent in keloid 4. However, other studies indicated that dermal nodules were present in both HS and keloids, with well‐demarcated borders in the former and less distinct borders in the latter 5. Indeed, there are many cases in which the scars bear the growth and histological features of both HSs and keloids. On the basis of these features, we speculated that inflammation may initiate, and be a characteristic feature of, keloids, distinguishing them from HSs. This speculation is supported by the inflammation that occurs at the leading edge of a keloid and by the ability of keloids to arise from a mature scar, sometimes years later 1. According to the hypothesis, the differing clinical capacities of HSs and keloids may simply reflect the transition between differing degrees of inflammation.
H‐E staining of the keloid showed that the extent of inflammation decreased from the periphery to the centre, whereas the demarcation of hyalinised collagen increased. These results suggest the involvement of inflammation in keloid formation. Key features of inflammation such as microvessels, fibroblasts and lymphocytes all decreased gradually from the periphery to the centre of keloids, indicative of reduced inflammation in the centre. This was consistent with previous studies showing that peripheral areas of keloid were characterised by angiogenesis, fibroblast proliferation and collagen production, whereas central areas were characterised by hypoxia, apoptosis and abundant collagen 6, 7. Previous studies provided further supporting evidences demonstrating the increased numbers of inflammatory cells in keloids, mainly macrophages and T lymphocytes with a higher (CD4+ T helper : CD8+ T suppressor) Th : Ts ratio 8, together with an absence of B lymphocytes 9. Moreover, the demarcations between hyalinised collagen and the surrounding tissues were clearer in the superficial Grenz zone in the papillary dermis than in the deep reticular dermis, and were increasingly blurred the closer they were to the peripheral area. These changes indicated that the increases in the extent of inflammation were accompanied by reduced demarcation of hyalinised collagen. Thus, we speculate that the invasive behaviour of a keloid might be the result of sustained inflammation in the advancing peripheral area and the subsequent accumulation of collagens in the centre.
Dermal nodules containing abundant fibroblasts with long processes, with fine fibrillar collagen attached to the fibroblasts, were traditionally considered as histological characteristic of HSs and to be absent from keloid 4. However, we found dermal nodules with hyalinised collagen in each keloid sample. In fact, there seemed to be a transition in the characteristics of the keloid from the centre to the periphery, with increased nodular size and cellularity, less well‐defined nodular outlines, and decreased quantity and thickness of collagens (Figure 4). Moreover, hyalinised fibres appeared to initiate from the corner of the dermal nodules (Figure 3), and the continuing growth and maturation of those keloidal fibres could be clearly seen from the bottom to the top of the dermal nodule (Figure 1C). The coexistence of hyalinised collagen and dermal nodules implied that these lesion types may have similar origins, whereas the gradual change in the hyalinised collagen fibres inside the dermal nodule from minimal to fully mature suggested a transition from HS to keloid. Furthermore, the changing features of dermal nodules from the periphery to the centre of the keloid suggested a role of inflammation in initiating the two conditions (and differentiating between them).
On the basis of the histological analyses of keloids, we hypothesise that HS and keloid can be considered as successive stages of the same fibroproliferative skin disorder with differing degrees of inflammation. The fibroproliferation is characterised by accumulation of mesenchymal cells and their connective tissue products 10; while the extent of inflammation determines the nature of the conditions. This hypothesis may shift the diagnostic focus to the early phase of inflammation in wound healing and to the epithelial/epithelial–dermal junction. It also provides a new perspective to explain the coexistence of horizontal invasion of keloid and vertical limits of depth to the dermis because the inflammation is stronger in the periphery than in the centre in the horizontal direction. Actually, inflammation is indispensable in the initial stage of normal wound healing. Nonetheless, excessive inflammation in extent or duration, which may start out as an acute reaction, may lead to chronic and destructive pathological scarring. The role of inflammation in excessive dermal scarring may involve extensive and complex mechanisms such as mechanobiology 11, 12, angiogenesis 13, neurogenic inflammation 14, lipid metabolism 15 and their active crosstalk, which still remain to be clarified. In this study, we have outlined the different degrees of inflammation associated with HSs and keloids, and explained their aetiology under the fibroproliferative skin disorder hypothesis. Future studies will be directed towards obtaining molecular evidence for this hypothesis.
Conclusion
We hypothesise that HS and keloid could be successive stages of the same condition, namely fibroproliferative skin disorders, and can be differentiated by differing degrees of inflammation. The current diagnostic focus on the hyalinised collagen bundle may be contributing to a misunderstanding of keloid pathogenesis.
References
- 1. Bran GM, Goessler UR, Hormann K, Riedel F, Sadick H. Keloids: current concepts of pathogenesis (review). Int J Mol Med 2009;24: 283–93. [DOI] [PubMed] [Google Scholar]
- 2. Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J 2007;21:3250–61. [DOI] [PubMed] [Google Scholar]
- 3. Lee JY, Yang CC, Chao SC, Wong TW. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol 2004;26:379–84. [DOI] [PubMed] [Google Scholar]
- 4. Ehrlich HP, Desmoulière A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, Kapanci Y, Gabbiani G. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol 1994;145:105–13. [PMC free article] [PubMed] [Google Scholar]
- 5. Clark JA, Turner ML, Howard L, Stanescu H, Kleta R, Kopp JB. Description of familial keloids in five pedigrees: evidence for autosomal dominant inheritance and phenotypic heterogeneity. BMC Dermatol 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kischer CW. The microvessels in hypertrophic scars, keloids and related lesions: a review. J Submicrosc Cytol Pathol 1992;24:281–96. [PubMed] [Google Scholar]
- 7. Appleton I, Brown NJ, Willoughby DA. Apoptosis, necrosis, and proliferation: possible implications in the etiology of keloids. Am J Pathol 1996;149:1441–7. [PMC free article] [PubMed] [Google Scholar]
- 8. Boyce DE, Ciampolini J, Ruge F, Murison MS, Harding KG. Inflammatory‐cell subpopulations in keloid scars. Br J Plast Surg 2001;54:511–6. [DOI] [PubMed] [Google Scholar]
- 9. Martin CW, Muir IF. The role of lymphocytes in wound healing. Br J Plast Surg 1990;43:655–62. [DOI] [PubMed] [Google Scholar]
- 10. Hertz MI, Henke CA, Nakhleh RE, Harmon KR, Marinelli WA, Fox JM, Kubo SH, Shumway SJ, Bolman RM 3rd, Bitterman PB. Obliterative bronchiolitis after lung transplantation: a fibroproliferative disorder associated with platelet‐derived growth factor. Proc Natl Acad Sci U S A 1992;89:10385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogawa R, Okai K, Tokumura F, Mori K, Ohmori Y, Huang C, Hyakusoku H, Akaishi S. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20: 149–57. [DOI] [PubMed] [Google Scholar]
- 12. Ogawa R. Mechanobiology of scarring. Wound Repair Regen. 2011; 19(Suppl 1): s2–s9. [DOI] [PubMed] [Google Scholar]
- 13. Le AD, Zhang Q, Wu Y, Messadi DV, Akhondzadeh A, Nguyen AL, Aghaloo TL, Kelly AP, Bertolami CN. Elevated vascular endothelial growth factor in keloids: relevance to tissue fibrosis. Cells Tissues Organs 2004;176:87–94. [DOI] [PubMed] [Google Scholar]
- 14. Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses 2008;71:32–8. [DOI] [PubMed] [Google Scholar]
- 15. Louw L. Keloids in rural black South Africans. Part 1: general overview and essential fatty acid hypotheses for keloid formation and prevention. Prostaglandins Leukot Essent Fatty Acids 2000;63: 237–45. [DOI] [PubMed] [Google Scholar]


