Dear Editors,
Ulceration constitutes the main complication of necrobiosis lipoidica (NL) and usually occurs after trauma. Ulcerated NL predisposes patients to infections and very rarely is associated with malignant transformation, mainly to squamous cell carcinoma (SCC).
We describe the case of a 68‐year‐old Caucasian male diagnosed with disseminated NL associated with type 2 diabetes mellitus (DM), presenting a SCC on an ulcerated plaque of NL located on the left forearm and chronic osteomyelitis of the radius.
Case report
A 68‐year‐old Caucasian male was examined for the appearance of a friable and painful vegetating tumour on the left wrist next to the distal radius, over a chronic ulcerated plaque of NL.
Since 1995 the patient has been followed up for several dermatology consultations following the appearance of erythematous brown plaques with atrophic centre on the left wrist and anterior aspect of the left thigh, showing slow and progressive centrifuge growth (Figure 1A). Skin and synovial biopsies taken from the left wrist were compatible with tuberculosis. A tuberculin skin test result was positive (15 mm) but smear and cultures were negative for Mycobacterium tuberculosis and mycobacteria. The patient underwent tuberculostatic tritherapy (isoniazid, rifampicin and pyrazinamide) on the basis of histopathological and tuberculin test results, without improvement. In 2000, skin biopsies from two distinct topographic plaques (Figure 1F) confirmed a diagnosis of NL. Type 2 DM was detected 2 years after the NL diagnosis, and the patient is currently under medication with glibenclamide and metformin hydrochloride.
Figure 1.
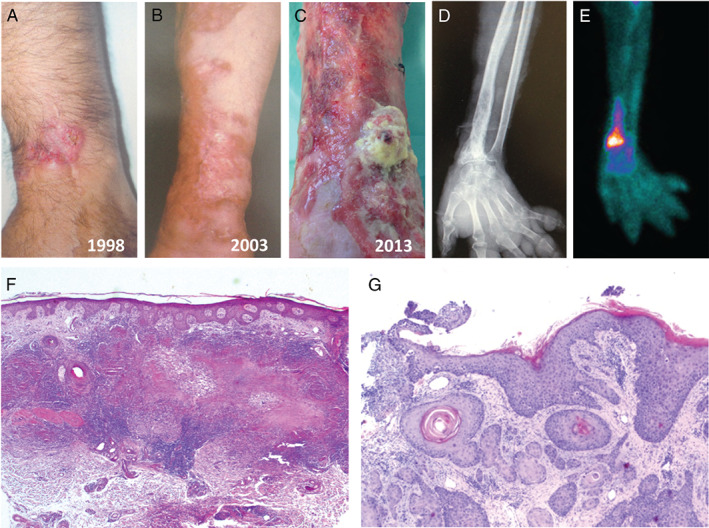
(A) Erythematous round plaque with yellow atrophic centre on the left wrist. (B) Erythematous brown plaque of necrobiosis lipoidica (NL) – centrifuge growth. (C) Friable vegetating tumour lesion on the distal radial area of the left forearm. (D) Digital radiography of the left forearm and hand: honey‐comb pattern in the distal radius. (E) Bone scintigraphy with Technetium‐99m, increased fixation of the isotope in the distal radius. (F) Haematoxylin and eosin (H&E), ×25 NL. (G) H&E, ×40 squamous cell carcinoma.
Meanwhile, NL progressed with the appearance of an atrophic ulcerated plaque with extension to the entire skin surface of the left forearm (Figure 1B).
In 2003, the patient was admitted with the diagnosis of erysipela with compartment syndrome of the left upper limb. He was submitted to surgical decompression incisions and medicated with imipenem/cilastatin (500 mg/day I.V. q8h).
Despite monthly benzathine penicillin prophylaxis, there were several episodes of erysipela of the left upper limb, which required hospitalisations involving intravenous antibiotic therapy.
In the last 4 years, several treatment options have been used, such as betamethasone dipropionate cream twice daily, clofazimine (100–200 mg/day), acetyl salicylic acid (100 mg/day) and pentoxifylline (400 mg twice daily), but without significant healing of the left forearm ulceration. The patient also received 40 daily sessions of hyperbaric oxygen therapy without improvement.
Following the appearance of a friable vegetating tumour lesion (4 × 3 cm2) on the distal radial area of the left forearm (Figure 1C) two biopsies were performed, and histopathology showed a G3, poorly differentiated invasive SCC (Figure 1G).
The digital radiography of the forearm and hand (Figure 1D) showed a honey‐comb pattern in the distal radius, suggestive of osteomyelitis. A computed tomography of the same region was also consistent with the diagnosis of chronic osteomyelitis. A bone scintigraphy with Technetium‐99m showed an increase fixation of the isotope in the distal radius, also suggestive of osteomyelitis (Figure 1E). A chest CT scan showed the presence of lymphadenopathy in the left axillary region.
Amputation was carried out at the proximal third of the left humerus along with axillar lymphadenectomy that showed a positive lymph node with extracapsular extension. The patient was referred for radiotherapy.
Discussion
The development of SCC on chronic ulcerative lesions or scarring in association with multiple dermatological entities is well known. The ulceration is a major complication of NL, occurring in up to one third of cases, often following a trauma 1, 2.
Although NL lesions may be self‐limited and resolve spontaneously, most show a chronic progress and often prove to be challenge to treat. The appearance of skin lesions compatible with the diagnosis of SCC in patients with a history of NL is rare and has been documented only in 13 patients (Table 1). This case corresponds to a chronic ulcerated NL plaque with neoplastic transformation in the upper limb, a feature never before described, which makes this case peculiar and distinctive of all documented to date.
Table 1.
Reported cases of malignant transformation arising in necrobiosis lipoidica
| Age/Gender | Diabetes | Duration of NL | Type of cancer | Site | Metastasis | Treatment | Source |
|---|---|---|---|---|---|---|---|
| 57/F | No | 32 | SCC | Lower limb | Unknown | Unknown | Muller et al., 1966 2 |
| 59/F | IDDM | 36 | SCC | Right outer leg | Inguinal nodes | Below knee amputation and radiotherapy to inguinal region | Clement et al., 1985 3 |
| 39/M | No | 20 | SCC | Bilateral pre‐tibial areas | None | Excision and graft | Kossard et al., 1987 4 |
| 69/F | No | 40 | SCC | Bilateral pre‐tibial areas | Right inguinal nodes | Bilateral amputation | Beljaards et al., 1990 5 |
| 46/F | IDDM | 11 | SCC | Left lower leg | None | Excision and graft | Porneuf et al., 1991 6 |
| 44/M | Unknown | 12 | Leiomyosarcoma | Left pre‐tibial area | None | Excision, graft and postsurgical irradiation | Perrot et al., 1994 7 |
| 52/M | NIDDM | 6 | SCC | Right leg | None | Excision, graft and postsurgical irradiation | Pavithran, 1998 8 |
| 46/F | IDDM | 29 | SCC | Inner left leg | None | Excision and graft | Gudi et al., 2000 9 |
| 28/F | IDDM | 12 | SCC | Left leg | None | Excision and graft | Imtiaz and Khaleeli, 2001 10 |
| 76/F | No | 30 | SCC | Right leg | None | Below knee amputation | Santos‐Juanes et al., 2004 1 |
| 33/M | IDDM | 18 | SCC | Right leg | Disseminated | Radiotherapy | Vanhooteghem et al., 2005 11 |
| 40/F | IDDM | 12 | SCC | Left medial lower leg | None | Excision and graft | McIntosh et al., 2005 12 |
| 53/F | IDDM | 25 | SCC | Right medial leg | None | Excision and graft | Lim et al., 2006 13 |
| 68/M | NIDDM | 18 | SCC | Left distal forearm | Left axilar nodes | Above elbow amputation | Present case, 2013 |
F, female; M, male; IDDM, insulin‐dependent diabetes mellitus; NDDM, non‐insulin‐dependent diabetes mellitus; NL, necrobiosis lipoidica; SCC, squamous cell carcinoma.
All the 14 reported cases of NL associated with cancer were SCCs, except for a case of leiomyosarcoma.
In this case, the latent period of 18 years between the diagnosis of NL and the appearance of SCC is in agreement with other reported cases.
The primary skin cancer appeared an average of 21·5 years after the diagnosis of NL. At the time of diagnosis, three cases presented with regional lymph node metastasis (31%), and one developed disseminated metastasis months later.
In the group of metastatic disease, a mean of 28 years was calculated after the appearance of NL, whereas in the group of non‐metastatic skin cancer, a shorter period of 17 years elapsed from the NL diagnosis.
Risk factors that might be involved in malignant transformation may include melanin loss, facilitating ultraviolet damage, chronic inflammation and hypoxia 3, 4, 5, 6, 7, 8, 9, 11, 12.
Ulcerated NL is a therapeutic challenge, and there are no established treatment regimens. Many treatment options have been recommended either alone or in combination with varying degrees of success but none of them appear to be reliable. Clofazimine in a dose of 100 mg per day has been used in NL treatment with beneficial response 14. Pentoxifylline may exert a beneficial effect in the treatment of ulcerating NL 15. The anti‐TNF monoclonal antibodies infliximab and adalimumab have been shown to be beneficial in chronic cutaneous granulomatous diseases, such as disseminated granuloma annulare and sarcoidosis, as well as in a few cases of ulcerative NL 15. Etanercept was also used in particular cases of NL with improved response 15.
Other treatment options include hyperbaric oxygen therapy. This treatment modality emphasises the role of hypoxia in the pathogenesis of NL and might be an alternative for patients with chronic non‐healing NL who fail to respond to other therapeutic approaches 15.
The development of SCC in association with NL is very rare but should be considered in chronic, non‐healing and recalcitrant ulcers developing in areas of NL. In this case, chronic osteomyelitis may as well play a role as an underlying cause of malignant transformation.
The early detection of and intervention in SCC associated with NL may still allow conservative surgical treatment, thus avoiding amputation.
Luís Uva MD1,2, João Freitas MD1,2, Luis Soares de
Almeida MD, PhD1,2, Hugo Vasques MD3, Cecília Moura
MD4Diana Miguel MD5 & Paulo Filipe MD, PhD1,2
1Faculdade de Medicina de Lisboa
Clínica Universitária de Dermatologia
Lisboa, Portugal
2Instituto de Medicina Molecular
Lisboa, Portugal
3Instituto Português de Oncologia‐Serviço de Cirurgia
Lisboa, Portugal
4Instituto Português de Oncologia‐Serviço de Dermatologia
Lisboa, Portugal
5Clinic of Dermatology
Erfurt, Germany
luisuva@hotmail.com
References
- 1. Santos‐Juanes J, Galache C, Curto JR, Carrasco MP, Ribas A, Sanchez del Rio J. Squamous cell carcinoma arising in long‐standing necrobiosis lipoidica. J Eur Acad Dermatol Venereol 2004;18:199–200. [DOI] [PubMed] [Google Scholar]
- 2. Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. A clinical and pathological investigation of 171 cases. Arch Dermatol 1966;93:272–81. [DOI] [PubMed] [Google Scholar]
- 3. Clement M, Guy R, Pembroke AC. Squamous cell carcinoma arising in long‐standing necrobiosis lipoidica. Arch Dermatol 1985;121:24–5. [PubMed] [Google Scholar]
- 4. Kossard S, Collins E, Wargon O, Downie D. Squamous carcinomas developing in bilateral lesions of necrobiosis lipoidica. Australas J Dermatol 1987;28:14–7. [DOI] [PubMed] [Google Scholar]
- 5. Beljaards RC, Groen J, Starink TM. Bilateral squamous cell carcinomas arising in long‐standing necrobiosis lipoidica. Dermatologica 1990;180:96–8. [DOI] [PubMed] [Google Scholar]
- 6. Porneuf M, Monpoint S, Barneon G, Alirezai M, Guillot B, Guilhou JJ. Carcinoma cuniculatum arising in necrobiosis lipoidica. Ann Dermatol Venereol 1991;118:461–4. [PubMed] [Google Scholar]
- 7. Perrot JL, Poulard F, Soulhiard J, Weschsler AL, Claudy FC. Léiomyosarcome développé au sein d'une plaque de necrobiose lipoidique. Ann Dermatol Venereol 1994;121:S40–1. [Google Scholar]
- 8. Pavithran K. Squamous cell carcinoma in a plaque of necrobiosis lipoidica diabeticorum. Indian J Dermatol 1998;43:26. [Google Scholar]
- 9. Gudi VS, Campbell S, Gould DJ, Marshall R. Squamous cell carcinoma in an area of necrobiosis lipoidica diabeticorum: a case report. Clin Exp Dermatol 2000;25:597–9. [DOI] [PubMed] [Google Scholar]
- 10. Imtiaz KE, Khaleeli AA. Squamous cell carcinoma developing in necrobiosis lipoidica. Diabet Med 2001;18:325–8. [DOI] [PubMed] [Google Scholar]
- 11. Vanhooteghem O, Andre J, de la Brassinne M. Epidermoid carcinoma and perforating necrobiosis lipoidica: a rare association. J Eur Acad Dermatol Venereol 2005;19:756–8. [DOI] [PubMed] [Google Scholar]
- 12. McIntosh BC, Lahinjani S, Narayan D. Necrobiosis lipoidica resulting in squamous cell carcinoma. Conn Med 2005;69:401–3. [PubMed] [Google Scholar]
- 13. Lim C, Tschuchnigg M, Lim J. Squamous cell carcinoma arising in an area of long‐standing necrobiosis lipoidica. J Cutan Pathol 2006;33:581–3. [DOI] [PubMed] [Google Scholar]
- 14. Benedix F, Geyer A, Lichte V, Metzler G, Rocken M, Strolin A. Response of ulcerated necrobiosis lipoidica to clofazimine. Acta Derm Venereol 2009;89:651–2. [DOI] [PubMed] [Google Scholar]
- 15. Reid SD, Ladizinski B, Lee K, Baibergenova A, Alavi A. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol 2013;69:783–91. [DOI] [PubMed] [Google Scholar]


