Abstract
Malignant degeneration of wounds is rare and often misdiagnosed. Delay in diagnosis may result in a worse prognosis. The aim of this study is to determine the number of skin cancers associated with chronic skin ulcers in our facility over a period of 10 years. Between January 2002 and December 2012, a total of about 1000 patients had consulted with us for chronic wounds, especially of vascular, diabetic and traumatic origin and pressure ulcers. Thirteen skin cancers had been detected: seven squamous cell and five basal cell carcinomas and one melanoma. We highlight how important it is to be aware of the signs suggesting a malignant change and the importance of biopsy at regular intervals during the life cycle of any chronic wound.
Keywords: Basal cell carcinoma, Chronic wound, Malignant transformation, Marjolin's ulcer, Squamous cell carcinoma
Introduction
Marjolin's ulcer was first described by Jean‐Nicholas Marjolin, a French surgeon, in 1828, as a malignant transformation of chronic healing processes resulting from burn injuries 1. Today, the term ‘Marjolin's ulcer’ is used to describe cancers arising from scars of old burns, venous insufficiency ulcers, pressure ulcers, traumatic wounds, cystostomy sites, scarring from lupus, amputation stumps, chronic lymphoedema, chronic pilonidal sinuses, hidradenitis suppurativa, chronic ulcers of leprosy, necrobiosis lipoidica and chronic osteomyelitic fistulae 2. Chronic ulcer associated with scarring is the type of precursory lesion most often described in medical literature. The precancerous potential of chronic ulcers of the lower limbs has been investigated and upheld by several case reports 1, 3. Malignant transformation is a rare and often misdiagnosed complication that conveys an ominous prognosis. Delay in diagnosis may result in a worse prognosis, loss of the affected limb or occurrence of metastases 4, 5. In the majority of these cases, the malignancy consists of a rare, but highly aggressive, squamous cell carcinoma (SCC), generally located on the lower limbs. Latency, until malignant transformation, takes on an average three decades 6. Surgery is the treatment of choice and represents the therapeutic option with the highest cure rate and best survival 7. In our facility that handles difficult wounds, in a period of 10 years, from about 1000 patients treated for chronic wounds of several aetiologies, 13 patients with malignant degeneration of long‐standing and non‐healing ulcers have been detected.
Methods
From January 2002 to December 2012, a retrospective study was performed on about 1000 patients who received medical dressings for chronic wounds in our facility. Skin wounds were especially of vascular, diabetic and traumatic origin and pressure ulcers. Data were collected on gender, age, cause of the skin wound, time of onset and variations of wound aspect over the months. The assessments were based on photographs and comparative photography.
Results
A total of 13 patients, with a mean age at diagnosis of 75 ± 2·5 years (mean ± 2 SD) (range: 50–90) and a female:male ratio of 1·2:1, with chronic wounds degenerating to skin cancers, had been seen during the past 10 years. These 13 patients had developed a total of 13 skin carcinomas: 7 (54%) SCCs, 5 (38%) basal cell carcinomas (BCCs), and 1 (8%) melanoma. Four patients (31%) had ulcers of arteriovenous or arterial origin (Figure 1), four (31%) had diabetic ulcers (Figure 2), three (23%) had chronic wounds of traumatic origin (Figure 3) and two (15%) had pressure ulcers. The mean duration of skin ulcer before the diagnosis of carcinoma was 9 ± 3·3 years. Nine tumours developed from chronic wounds on the legs and four on the feet. Three patients had a past history of visceral cancer (breast, colon and melanoma), one of hepatitis C, one of hepatitis B and four of hypertension and cardiopathy.
Figure 1.
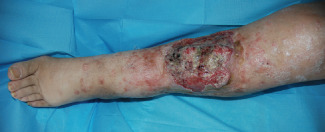
Non‐healing diabetic ulcer on the left leg in a 74‐year‐old woman.
Figure 2.
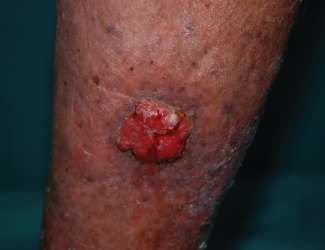
Arteriovenous ulcer of the leg with an increase in size and recurrent bleeding in a 65‐year‐old woman.
Figure 3.
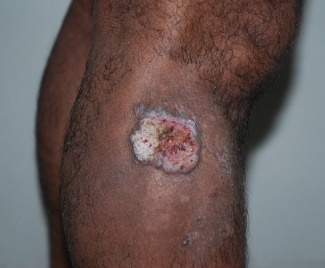
Chronic wounds of traumatic origin on the right leg in 56‐year‐old man.
Eleven of these patients presented a non‐healing ulcer over the right thigh for about 9 years. Over this period of time, before the admission to our facility, some of the patients were treated by their general practitioner and by a home‐care team. Clinically, the ulcers appeared oval, 10 × 6 cm in mean size, of varying depth and involving bone, with elevated margins, sanious wound bed, showing nodules, smelly, not painful and indolent on palpation. Dressing changes were performed every day. The wounds were disinfected with sodium hypochlorite 0·05% (Amukine®, S.p.A., Genoa, Italy) and iodopividone 0·5% (Betadine®, Meda Pharma S.p.A., Milano, Italy). After careful wound disinfection, several types of advanced dressings were applied.
The ulcers, after a slight improvement, underwent sudden changes with a deterioration of their appearance. In two cases, 6 months after healing, a new wound arose on the scar. Local clinical signs or symptoms were noted including abnormal granulation, recalcitrant and progressive increase in size and in pain, recurrent bleeding, protracted course and/or spreading of the ulcer despite appropriate treatment, prompting the clinician to perform a skin biopsy which confirmed the malignant transformation of the chronic wounds.
At least two punch biopsies, one at the wound edge and one in the wound bed, in the most clinically suspicious areas, were systematically performed. Seven SCCs, five BCCs and one melanoma were detected. Eleven patients received a wide local excision of the malignant ulcer and the defect was covered with a split‐thickness skin graft. Three patients were identified to undergo amputation of the leg. One of them refused this type of treatment and did not give more information on his clinical conditions (Figure 4A–D).
Figure 4.
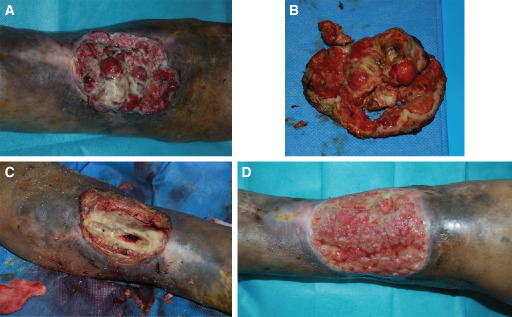
Arteriovenous ulcer of the leg in a 66‐year‐old man. (A) Ulcer of the leg showing shiny granulation tissue, preoperative view. (B) Macroscopic appearance of the lesion. (C) Intraoperative view. (D) Recurrence of the cancer after 2 months.
The excision margins were free of tumour and there were no recurrences of cancer after a year of follow‐up.
Discussion
The precancerous potential of chronic leg ulcers is well established but the number of reported cases overall is small. Hyperkeratotic granulation in the ulcer, alterations or raising of the margin, unusual pain, bleeding or a protracted course despite appropriate treatment are possible indications of malignant transformation of a chronic leg ulcer 4. The mechanism of malignant transformation of chronic ulcers is not completely understood 8. There are several theories. The principal factors involved include exposure to cytotoxic by‐products of chronic inflammation, an impaired mitotic cycle and epidermal implantation resulting in a dermal foreign body reaction 9, 10, 11. Kirsner et al. have estimated that 1·7% of chronic wounds develop malignant properties. This estimate could be low, considering the incidence of misdiagnosis and subsequent failure to report the occurrence 12, 13, 14, 15. A study of 125 cases found that 25% of patients with BCC had concomitant chronic venous stasis, suggesting a relationship between venous disease and BCC 16. The majority of reported cases in literature are SCC, but other types of malignancies such as BCC and melanoma can also be less frequently found 17. Hansson and Andersson, conversely, identified BCC and SCC as the most frequently encountered cutaneous, ulcerative malignancies at 60% and 15%, respectively 10. In this study, patients were affected by BCC. Early treatment is imperative to save the patient because carcinomas originating in these lesions are more aggressive than other primary SCCs and BCCs. Novick et al. reported a 54% incidence of metastases from lower extremity lesions 18. In our study, patients with ulcers and chronic wounds were monitored meticulously which has allowed us to recognise and treat this type of cancer early. Tissue biopsy is the gold standard for diagnosis of cutaneous malignancy. Ackroyd and Young recommend performing a biopsy if a chronic wound failed to respond satisfactorily after 3 months of reasonable treatment, whereas Hansson and Andersson recommend waiting for 4 months 13, 19. In our experience, any sudden and suspicious change in the characteristics of a chronic wound should prompt the suspicion of a malignant transformation and biopsy is the only diagnostic test. For the treatment of Marjolin's ulcer, wide local excision with a margin of at least 1 cm of healthy tissue should be performed. Amputation is indicated when wide local excision is not possible owing to deep invasion, bone or joint involvement, infection or haemorrhage, or when excision would cause major functional disability. Regional lymph node dissection is indicated when nodes are palpable 20. Recurrence incidence ranges from 20% to 50%. Most recurrences happen within 3 years of excision 21. In a study performed during 10 years in Urmia, among 19 cases of Marjolin's ulcer, 47% due to childhood burn, all were SCCs and were treated with amputation 2. In our study, however, early diagnosis allowed a more conservative treatment in most cases of malignant ulcers without recurrences in the follow‐up.
Conclusions
Marjolin's ulcers are not rare and commonly occur in burn scars that are not skin grafted, and are left to heal secondarily but they can develop into several kinds of complicated chronic wounds. Caution and alertness is required in the management of chronic non‐healing ulcers and all suspected lesions should be biopsied. Early recognition and aggressive treatment of Marjolin's ulcers and close follow‐up are needed to improve outcomes.
Acknowledgements
All authors hereby declare not to have any potential conflict of interests and not to have received funding for this work from any of the following organisations: National Institutes of Health (NIH), Wellcome Trust, Howard Hughes Medical Institute (HHMI) and other(s). Each author participated sufficiently in the work to take public responsibility for the content. Special thanks to Dr Franco Bartolomei for his help in preparing this manuscript.
References
- 1. Marjolin JN. Ulcère. In: Adelon NP, editor. Dictionnaire de médecine. Paris: Becher, 1828:31–50. [Google Scholar]
- 2. Shahla A. An overview of heel Marjolin's ulcers in the Orthopedic Department of Urmia University of Medical Sciences. Arch Iran Med 2009;12:405–8. [PubMed] [Google Scholar]
- 3. Bauer T, David T, Rimareix F, Lortat‐Jacob A. Marjolin's ulcer in chronic osteomyelitis: seven cases and a review of the literature. Rev Chir Orthop Reparatrice Appar Mot 2007;93:63–71. [DOI] [PubMed] [Google Scholar]
- 4. Combemale P, Bousquet M, Kanitakis J, Bernard P. Malignant transformation of leg ulcers: a retrospective study of 85 cases. J Eur Acad Dermatol Venereol 2007;21:935–941. [DOI] [PubMed] [Google Scholar]
- 5. Erfurt‐Berge C, Bauerschmitz J. Malignant tumours arising in chronic leg ulcers: three cases and a review of the literature. J Wound Care 2011;20:396–400. [DOI] [PubMed] [Google Scholar]
- 6. Chalya PL, Mabula JB, Rambau P, Mchembe MD, Kahima KJ, Chandika AB, Giiti G, Masalu N, Ssentongo R, Gilyoma JM. Marjolin's ulcers at a university teaching hospital in Northwestern Tanzania: a retrospective review of 56 cases. World J Surg Oncol 2012;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tavares E, Martinho G, Dores JA, Vera‐Cruz F, Ferreira L. Marjolin's ulcer associated with ulceration and chronic osteomyelitis. An Bras Dermatol 2011;86:366–9. [DOI] [PubMed] [Google Scholar]
- 8. Lidell K. Malignant change in chronic varicose ulceration. Practitioner 1975;215:335–9. [PubMed] [Google Scholar]
- 9. Fleming ID, Barnawell JR, Burlison PE, Rankin JS. Skin cancer in black patients. Cancer 1975;35:600–5. [DOI] [PubMed] [Google Scholar]
- 10. Arons MS, Lynch JB, Lewis SR, Blocker TG Jr. Scar tissue carcinoma. I. A clinical study with special reference to burn scar carcinoma. Ann Surg 1965;161:170–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schnirring‐Judge M, Belpedio D. Malignant transformation of a chronic venous stasis ulcer to basal cell carcinoma in a diabetic patient: case study and review of the pathophysiology. J Foot Ankle Surg 2010;49:75–9. [DOI] [PubMed] [Google Scholar]
- 12. Yang D, Morrison BD, Vandongen YK, Singh A, Stacey MC. Malignancy in chronic leg ulcers. Med J Aust 1996;164:718–20. [DOI] [PubMed] [Google Scholar]
- 13. Hansson C, Andersson E. Malignant skin lesions on the legs and feet at a dermatological leg ulcer clinic during five years. Acta Derm Venereol 1998;78:147–148. [DOI] [PubMed] [Google Scholar]
- 14. Voisard JJ, Lazareth I, Baviera E, Priollet P. Leg ulcers and cancer. 6 case reports (in French). J Mal Vasc 2001;26:85–91. [PubMed] [Google Scholar]
- 15. Kirsner RS, Spencer J, Falanga V, Garland LE, Kerdel FA. Squamous cell carcinoma arising in osteomyelitis and chronic wounds. Treatment with Mohs micrographic surgery vs amputation. Dermatol Surg 1996;22:1015–8. [DOI] [PubMed] [Google Scholar]
- 16. Aloi F, Tomasini C, Margiotta A, Pippione M. Chronic venous stasis: not a predisposing factor for basal cell carcinoma on the leg. A histopathological study. Dermatology 1994;188:91–3. [DOI] [PubMed] [Google Scholar]
- 17. Alconchel MD, Olivares C, Alvarez R. Squamous cell carcinoma, malignant melanoma and malignant fibrous histiocytoma arising in burn scars. Br J Dermatol 1997;137:793–8. [PubMed] [Google Scholar]
- 18. Novick M, Gard DA, Hardy SB, Spira M. Burn scar carcinoma: a review and analysis of 46 cases. J Trauma 1977;17:809–17. [PubMed] [Google Scholar]
- 19. Ackroyd JS, Young AE. Leg ulcers that do not heal. Br Med J (Clin Res Ed) 1983;286:207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moises M, Robert A. Marjolin's ulcer: report of two cases. Wounds 2006;18:65–70. [Google Scholar]
- 21. Thio D, Clarkson JH, Misra A, Srivastava S. Malignant change after 18 months in a lower limb ulcer: acute Marjolin's revisited. Br J Plast Surg 2003;56:825–8. [DOI] [PubMed] [Google Scholar]


