Abstract
The treatment of chronic wounds poses a significant challenge for clinicians and patients alike. Here we report design and preclinical efficacy of a novel nitric oxide gas (gNO)‐producing probiotic patch for wound healing. Specifically, a wound healing patch using lactic acid bacteria in an adhesive gas permeable membrane has been designed and investigated for treating ischaemic and infected full‐thickness dermal wounds in a New Zealand white rabbit model for ischaemic wound healing. Kaplan–Meier survival curves showed increased wound closure with gNO‐producing patch‐treated wounds over 21 days of therapy (log‐rank P = 0·0225 and Wilcoxon P = 0·0113). Cox proportional hazard regression showed that gNO‐producing patch‐treated wounds were 2·52 times more likely to close compared with control patches (hazard P = 0·0375, score P = 0·032 and likelihood ratio P = 0·0355), and histological analysis showed improved wound healing in gNO‐producing patch‐treated animals. This study may provide an effective, safe and less costly alternative for treating chronic wounds.
Keywords: Dressing, Nitric oxide, Patch, Probiotic, Wound
INTRODUCTION
The treatment of chronic wounds poses a significant challenge for clinicians and patients alike. Chronic wound healing is often slow or stagnant, causing prolonged patient morbidity. The prevalence of unfavourable wound environments, such as ischaemia, venous stasis or infection further hinders successful treatment of these difficult wounds. In addition, several pathological healing conditions, including diabetes and venous stasis, are associated with dysregulation of wound healing mediators which disrupts wound healing and facilitates the formation of chronic wounds 1, 2, 3.
Currently used methods such as the use of topical antibiotics, as well as other antimicrobial agents such as colloidal silver polymyxins and dye compounds have become increasingly ineffective against common pathogens. In fact, recent reviews support the well‐accepted fact that there is a worldwide increase in drug‐resistant strains of bacteria since the introduction of antimicrobial agents 4, 5, 6, 7. Among these bacteria are methicillin‐resistant Staphylococcus aureus, vancomycin‐intermediate resistant S. aureus, glycopeptide‐intermediate S. aureus and community‐acquired Enterococcus faecium and Pseudomonas aeruginosa. Thus, as the common antimicrobial agents begin to fail alternative treatments which do not rely on conventional antibiotics will be required.
Reduced local and regional circulation is another problem when treating infected chronic wounds with systemic antibiotics (8). Patients with venous stasis ulcers have venous thrombosis, reduced circulation and poor regional blood flow and patients with diabetic foot ulcers suffer from poor microcirculation due to endothelial dysfunction and impaired blood flow and pressure in the microvasculature 9, 10, 11. Topical applications are often more effective at concentrating the antimicrobial agent at the wound site. However, these antimicrobials are often less effective at eliminating infection because of reduced circulation, or the ability of bacteria to form biofilms that restrict the delivery and efficacy of the antibiotics 8, 12. Thus, traditional therapies frequently leave infected wounds untreated and a patient's limb and life in danger.
Nitric oxide (NO) is a biological mediator of wound healing that acts as a signalling molecule in endothelial, neuronal and immunological cells and is involved in many phases of wound healing including inflammation, angiogenesis, collagen deposition, re‐epithelialisation and collagen rearrangement 1, 13. During the inflammatory phase, NO acts as a chemoattractant for monocytes and neutrophils, and stimulates the production of multiple proinflammatory cytokines such as IL‐1 and TGF‐β (14). Throughout re‐epithelialisation NO regulates other chemoattractants, such as RANTES, and is important for the proliferation of keratinocytes (14). During the later stages of wound healing, NO acts in collagen deposition and in the activity of matrix metalloproteinase (MMP) involved in the rearrangement and maturation of the newly deposited collagen at the wound site 14, 15, 16, 17.
It has recently been shown that topical exposure of NO gas (gNO) to wounds such as chronic non healing ulcers can be beneficial in promoting healing and preparing the wound bed for treatment and recovery (18). Application of exogenous gas has been shown to reduce microbial infection downregulating inflammation, manage exudates and secretion, upregulate expression of endogenous collagenase to debride the wound and regulate the formation of collagen (18). Furthermore, treatment regimens have been proposed for chronic wounds which specify high and low gNO treatment periods to first reduce the microbial burden and consequent inflammation, increase collagenase expression in a process of debridement and then restore the balance of NO which induces collagen expression and aids in wound closure (18). The gNO delivery device, however, used cumbersome and expensive components and required that the patient be tethered and remain non ambulatory during the duration of therapy (18). Other methods for delivering gNO have been explored and include adsorbing NO to polymers (19), zeolite (20) or silica particles 21, 22 requiring vast amounts of purified gNO under pressure, or delivering exogenous nitrosothiols (GSNO) (23) which can be expensive and is limited by shelf life (GSNO is thermally and photolytically liable). These methods are each able to deliver some NO; however, the level is often not physiologically relevant or the length of gNO release is inadequate. For this reason we are proposing a probiotic bacteria‐based system for the production of NO so that the control of gNO production relies on the metabolism of bacteria that can be sustained for the longer durations required for treatment of chronic wounds both in a hospital and community setting.
Here we report design of a novel probiotic patch for treating ischaemic and infected full‐thickness dermal wounds and investigation of the preclinical efficacy in an experimental New Zealand white rabbit animal model of chronic wound healing.
MATERIALS AND METHODS
NO producing probiotic patch design
The gNO‐producing dressings were designed using two Tegaderm™ adhesive patches (3M Canada, London, Ontario, Canada), one in contact with the skin and the other acting as a top layer of the device (Figure 1), as described in our previous study (19). The active ingredients of the dressing, namely lyophilised alginate microbeads containing Lactobacillus fermentum 7230 sourced from NCIMB (Bucksburn, Aberdeen, Scotland) and a solution containing glucose (10% w/v), NaCl (0·85% w/v) and sodium nitrite (30 mM), were placed in a heat sealed pocket made between the Tegaderm™ in contact with the skin and a layer of polyethylene (Figure 1) (19). Control patches were constructed similarly, but were prepared with glucose (10% w/v), NaCl (0·85% w/v) and no sodium nitrite. All chemicals used were of analytical grade and were procured from Sigma‐Aldrich (Oakville, Ontario, Canada). The active area of the patch was a square of 5cm × 5 cm (25 cm2).
Figure 1.
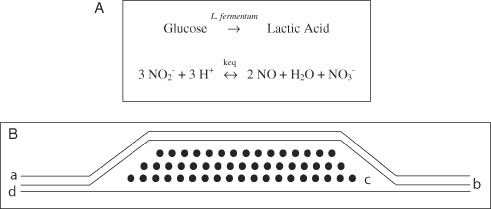
(A) Top: chemistry for novel nitric oxide gas (gNO)‐producing probiotic patch in which gNO is generated from nitrite and protons produced by Lactobacillus fermentum NCIMB 7230 bacteria from glucose. (B) Bottom: cross section of novel gNO‐producing probiotic patch design. From the outermost to innermost layers, the patch is composed of (a) adhesive non occlusive Tegaderm™, (b) polyethylene/nylon laminated film, (c) immobilised bacteria L. fermentum NCIMB 7230 and a solution containing 10% glucose, 30 mM NaNO2 and 0·85% NaCl and (d) a layer of adhesive non occlusive Tegaderm™.
Animals
Male New Zealand white rabbits (1·5–2·5 kg) were obtained from Charles River Laboratories (Wilmington, MA). The animals were housed at the UQAM animal centre in individual cages with food and water ad libitum. The animals were kept on a 12‐h light–dark cycle and were left to acclimatise for 4 weeks prior to surgery.
Surgical procedures
The animals were sedated with glycopyrrolate (0·1 mg/kg SC) and anaesthesia was started with ketamine (35 mg/kg IM) and xylazine (5 mg/kg IM). Once asleep, the anaesthesia was maintained with 4–5% isofluorane and the animals were monitored for heart and breathe rates. All anaesthetics used were of analytical grade and were procured from Sigma‐Aldrich. The surgical procedures for the induction of ischaemia and the creation of full‐thickness wounds were based on a modified method published by Chien et al. (24). Briefly, the ventral side of each ear was shaved as well as the areas at the base of the left ear, where the incisions were made for the induction of ischaemia. The surgical areas were wiped with ethanol and the rabbits were placed on a heated pad during the duration of the procedure.
Three vertical incisions were made about 1 cm from the base of the ear, closely located to the central, cranial and caudal bundles (composed of the artery, vein and nerves). The central artery was surgically divided from the nerve and veins. The central artery was ligated with 5‐0 silk while the venous circulation was preserved. The entire cranial bundle was ligated with 5‐0 silk. The caudal bundle was identified and separated from surrounding tissues, but left untouched. After disruption of the arteries, a subcutaneous tunnel was created between the three incisions. Using a sharp pair of scissors, the tissues under the skin were cut and removed to expose cartilage all around the base of the ear, working through the three incisions. Once the disruption was complete, any bleeding was controlled by applying pressure with a sterile gauze pad before closing the incisions using 4‐0 nylon sutures.
Four full‐thickness wounds were created on the ventral side of the ears using a sterile sharp 6 mm biopsy punch. Very light pressure was applied to avoid breaking through the thin cartilage of the ear. The wounds were created on the upper portion of the rabbit's ears, to allow easy access and to maintain the wounds on a flat surface. Once the skin was cut, it was removed from the cartilage and special care was taken to remove the perichondrial membrane and expose the cartilage. Following the creation of the wounds, the ears were covered with sterile gauze. Cotton was used to pack the ventral side of the ear. Gauze bandages were used to wrap the ears and were immobilised with surgical tape. The wrapping was kept relatively tight to prevent the loss of the dressing but not so tight as to limit blood flow in the non ischaemic control ear. Elizabethan collars were also fitted to the rabbits to prevent damage caused by the rabbits scratching their wounds. An injection of antibiotics (enrofloxacin, Baytril®, 5 mg/kg IM) procured from Bayer Corporation (Toronto, Ontario, Canada), was administered intraoperatively, to reduce the risk of systemic infections. A Fentanyl patch (25 µg/hours) was applied for 3 days following surgery on a shaved area located 10 cm distally from the base of the skull, on the rabbits back.
Infection
Experimental rabbits were infected with S. aureus to assess the effect of gNO‐producing patches on infected wounds. An overnight culture of S. aureus was diluted to approximately 1 × 106 CFU/ml in saline (0·85% NaCl) and applied with sterile cotton tipped applicators to the four wounds on each rabbit ear for 10 minutes before the control or treatment patches were applied. Wound bacterial counts were determined by plating serial dilutions of the wound swab samples in Tryptic Soy Agar after an overnight incubation at 37°C as previously described (25).
Wound treatment
Starting a day after surgery, the wounds were treated with control or gNO‐producing patches designed to produce gNO levels of approximately 200 ppmV for 24 hours (Figure 2). Only one patch per ear was sufficient to cover all four wounds. The patches were replaced daily after gently wiping the wounds using sterile gauze. The wounds were dressed as described at the end of the surgical procedure. The treatment was maintained for 20 or 21 days, irrespective of wound closure.
Figure 2.
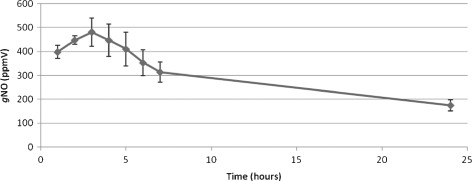
Nitric oxide gas (gNO) production by gNO‐producing probiotic patch containing 2·6 ml of glucose (10% w/v), sodium nitrite (30 mM) and 0·5 g lyophilised alginate microbeads containing 20% w/v immobilised Lactobacillus fermentum NCIMB 7230 by weight. gNO was detected using a Chemilumiescent Nitric Oxide Analyser (Sievers®, GE) in a custom designed high‐density polyethylene chamber.
Wounds of rabbits 1 and 2 were not infected. Wounds on rabbits 3 and 4 were infected with S. aureus. Wounds of rabbits 1 and 3 were treated with vehicle control patches; wounds of rabbits 2 and 4 were treated with gNO‐producing patches.
Data collection
Wound healing was monitored daily and photographic records were kept for morphometric analysis. Starting on the day of surgery, photographs were taken of the wounds on each ear. Morphometric analysis was performed by measuring the wound areas from daily photographs of wounds treated with gNO‐producing or vehicle control patches for each experimental condition.
Euthanasia and sample collection
Rabbits were euthanised under general anaesthesia using ketamine (35 mg/kg IM) and xylazine (5 mg/kg IM) followed by inhalation of isoflurane (5% in 1 l/minutes O2). Cardiac puncture was performed until complete exsanguination. Blood was collected in EDTA collection tubes for haematological analysis and in glass tubes for serum collection after clotting. Urine samples were also collected directly from the bladder after death of the animals. The ears were removed and the four wounds on each ear were cut out and placed in buffered formalin for histopathological analysis.
Histopathology
Histopathology was performed on all the wounds. Tissue sections were left to fix for at least 24 hours in formalin. The samples were bisected, placed in cassettes and processed to paraffin. Sections were sectioned at approximately 5 µm, mounted on glass slides and stained with haematoxylin and eosin and Masson's trichrome stains. Fixation, mounting, staining and analysis of the stained samples were performed by blinded pathologists using a semi‐quantitative grading system. Inflammation was graded from 0 (absent or very few inflammatory cells) to 3 (abundant inflammatory cells). Maturity was graded based on the organisation and quantity of granulation tissue. A grade of 0 indicates the absence of granulation tissue, while a score of 4 indicates a dense granulation tissue with bundles of mature dermal‐type collagen.
Haematology
Haematological analysis was performed with an ADVIA 120 analyser. The following parameters were evaluated: red blood cell counts, haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, platelet count, white blood cells (WBC), WBC differential counts, cell morphology and reticulocyte count. Blood smears were also prepared to evaluate morphology.
Blood chemistry
Serum samples were analysed for sodium (Na+), potassium (K+), chloride (Cl−), bicarbonate (HCO3 −), creatinine, urea, glucose, calcium (Ca2+), phosphate (PO4 3−), aspartate aminotransferase, alanine aminotransferase, lipase and C‐reactive protein using a Hitachi 911 blood clinical chemistry analyser (Roche Diagnostics, Laval, Quebec, Canada). Plasma nitrate and nitrite were determined by chemiluminescence using a Sievers Nitric Oxide Analyser (GE Analytical Instruments, Boulder, CO) as described earlier (26).
Methaemoglobin measurements
Blood samples were haemolysed with distilled water and phosphate buffer pH 7·0. Percentage of blood methaemoglobin in the samples were determined according to a modified Evelyn–Mallow method (16). The assay is based on the fact that haemoglobin can be converted to methaemoglobin after reacting with potassium ferricyanide and that the resulting absorbance maximum at 632 nm disappears after addition of sodium cyanide. Briefly, OD632 of haemolysed blood was measured to determine the concentration of methaemoglobin. Total haemoglobin was determined by OD632 after addition of potassium ferricyanide to haemolysed blood. The baseline correction values were determined by subtracting OD632 values of both solutions after the addition of sodium cyanide. The percentage of methaemoglobin was calculated as the quotient of the corrected OD632 values.
Statistical analysis
Statistical analysis of wound morphometric data was performed by repeated measures ANOVA with mixed models (SAS version 9·2, Cary, NC). Statistical analysis of complete wound closure was performed by Kaplan–Meier curves and Cox proportional hazard regression analysis. Kaplan–Meier curves (survival curves) express the likelihood of survival over time. Here the statistical method was used as a representation of the likelihood of wound closure over time. These data were plotted using time‐to‐closure of each wound separately, on a Kaplan–Meier graph and statistical analysis was performed using each of the variables present in the study: time‐to‐closure, treatment, ischaemia and infection. The Cox proportional hazard model is a statistical method used to determine the multiplicative effect on a subject's hazard rate. The hazard ratio represents the increase (or decrease) in the likelihood of an event occurring (wound closure). Data analysis was performed using Epi info™ from the Center for Disease Control (CDC, Atlanta, GA).
RESULTS AND DISCUSSION
NO producing probiotic patch design
Figure 1 shows the chemistry of the gNO‐producing probiotic device (top) and depicts the patch design (bottom). The release of gNO was evaluated in three representative patches over a 24‐hour period and was measured by chemiluminescence (Sievers®, GE) (Figure 2). There was no gNO detected for control patches (not shown). The patches released a gNO dose of 88·5 nmoles/cm2/hours.
Photographic evaluation and morphometric analysis of wounds treated with gNO‐producing probiotic patches
In general, non infected wounds appeared dry for the duration of the trial, whereas infected wounds presented purulent exudates. Photographs of infected, ischaemic and non ischaemic wounds, treated with either gNO‐producing probiotic patches or vehicle control patches taken at days 1, 13 and 20 are presented (Figure 3). Non ischaemic wounds closed between 13 and 15 days post‐surgery, independent of infection, as previously reported (24).
Figure 3.
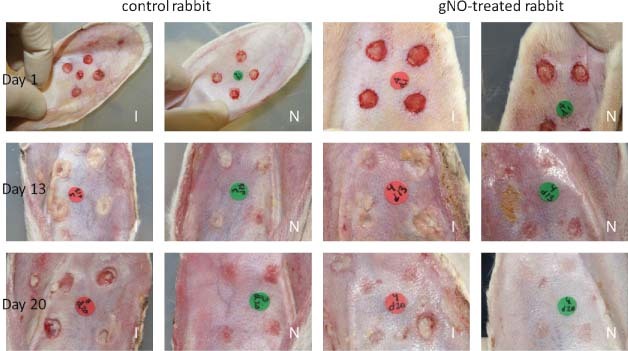
Photographs of infected full‐thickness dermal wounds on ears that are either ischaemic ‘I’ or non ischaemic ‘N’ and treated with nitric oxide gas‐producing probiotic patches or treated with vehicle control patches at days 1, 13 and 20 post‐surgery. Wound healing was monitored daily and photographic records were kept for computer‐aided morphometric analysis.
The efficacy of gNO‐producing probiotic patches versus vehicle control patches was determined by morphometric analysis of wound area under four experimental conditions: (i) ischaemic non infected wounds (Figure 4, upper left); (ii) ischaemic infected wounds (Figure 4, upper right); (iii) non ischaemic non infected wounds (Figure 4, lower left) and (iv) non ischaemic infected wounds (Figure 4, lower right). Repeated measures analysis of the entire data set, across all experimental conditions (n = 32), showed that treatment resulted in a significant decrease in wound area over the duration of this study (P = 0·0001), that the presence of ischaemia significantly slowed wound closure (P = 0·0009) and that the presence of infection did not significantly affect the healing process (P = 0·2578). The statistics for ischaemic wounds only (n = 16) showed that treatment with gNO‐producing patches resulted in a significant decrease in wound area over the duration of this study (P < 0·0001). There was also a significant decrease in wound area for treated ischaemic non infected wounds (P = 0·0006) (Figure 4, upper left) and treated ischaemic infected wounds (P = 0·015) (Figure 4, upper right) compared with the respective vehicle control‐treated wounds. Finally, treatment of non ischaemic non infected wounds with gNO‐producing patches did not significantly accelerate the rate of healing (P = 0·3539) (Figure 4, lower left) and treatment of non ischaemic infected wounds did not significantly accelerate the rate of wound healing (P = 0·1988, through day 11) (Figure 4, lower right) when compared with treatment with vehicle control patches.
Figure 4.
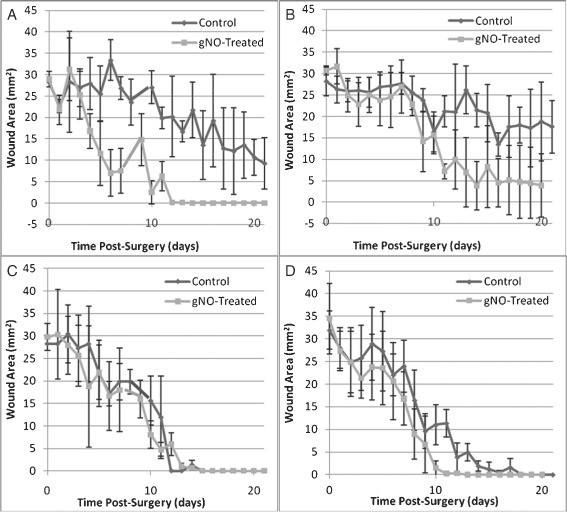
Effect of treatment with a nitric oxide gas (gNO)‐producing probiotic patch compared with a vehicle control patch under four experimental conditions as seen by daily morphometric analysis: (A) ischaemic, non infected wounds treated with active gNO‐producing probiotic patches versus vehicle control patches; (B) ischaemic, infected wounds treated with active gNO‐producing probiotic patches versus vehicle control patches; (C) non ischaemic, non infected wounds treated with active gNO‐producing probiotic patches versus vehicle control patches and (D) non ischaemic, infected wounds treated with active gNO‐producing probiotic patches versus vehicle control is shown. Error bars represent one standard deviation from the mean.
Histopathological analysis of wounds treated with NO producing probiotic patch
The histological results show a trend towards improved wound healing in tissues of the gNO‐producing probiotic patch‐treated animals when compared with those treated with vehicle control patches. Table 1 shows histological analysis of ischaemic wounds on ears of rabbits treated with gNO‐producing probiotic patches or those treated with vehicle control patches. The results show a trend towards improved wound healing as evidenced by less surface depression, less crusting and exudates, less inflammation/infiltration, greater epithelial coverage, reduced hyperplasia, greater dermal thickness and improved overall epidermal and dermal maturity. The results are averages of gNO‐producing patch‐treated (n = 16) and vehicle control patch‐treated wounds (n = 16). In almost every case, there was improvement in the average score (defined in materials and methods) given to histological observations consistent with improved wound healing (Table 1). At the wound surface less depression was observed (mean grade of 0·14 ± 1·68 versus −1·00 ± 1·77) and less crusting and exudates was observed (mean grade of 0·5 ± 1·07 versus 1·63 ± 1·41). The epidermal layer showed improved mean coverage (87·5 ± 31·5% versus 79·4 ± 29·8%), less hyperplasia (mean of 2·29 ± 0·76 versus 2·63 ± 0·74 in multiples of epidermal thickness) and improved epidermal maturity (mean score of 3·13 ± 0·64 versus 2·38 ± 0·91) for gNO‐treated wounds compared with vehicle controls. The granulation tissue and dermal layer showed increased thickness (1·13 ± 0·64 versus 0·84 ± 0·76 multiples of dermal thickness), less inflammation and infiltration of inflammatory cells (2·13 ± 0·83 versus 2·38 ± 0·74) and improved maturity (mean grade of 1·88 ± 0·99 versus 1·13 ± 0·83) for the case of gNO‐treated wounds compared with vehicle controls (Table 1). Figure 5 shows representative Masson's trichrome stained sections for the evaluation of wound healing, inflammation and maturity.
Table 1.
Histological analysis
| Control (n = 16) | Treatment (n = 16) | Improvement | ||
|---|---|---|---|---|
| Wound surface | Wound width (microscopic fields at 4× magnification) | 1·00 ± 0·27 | 1·08 ± 0·19 | N/C |
| Raised (+)/depressed (−) (−3 to 3) | −1·00 ± 1·77 | 0·14 ± 1·68 | Improved | |
| Central protrusion (0–3) | 0·13 ± 0·35 | 0·57 ± 0·98 | ||
| Crusting/exudates (0–3) | 1·63 ± 1·41 | 0·5 ± 1·07 | Improved | |
| Epidermis | Coverage (estimated %) | 79·4 ± 29·8 | 87·5 ± 31·5 | Improved |
| Hyperplasia (multiple of normal epidermal thickness) | 2·63 ± 0·74 | 2·29 ± 0·76 | Improved | |
| Maturity (1–4) | 2·38 ± 0·91 | 3·13 ± 0·64 | Improved | |
| Granulation tissue/dermis | Thickness (multiple of normal dermal thickness) | 0·84 ± 0·76 | 1·13 ± 0·64 | Improved |
| Inflammation/infiltration (0–3) | 2·38 ± 0·74 | 2·13 ± 0·83 | Improved | |
| Maturity (0–4) | 1·13 ± 0·83 | 1·88 ± 0·99 | Improved |
Figure 5.
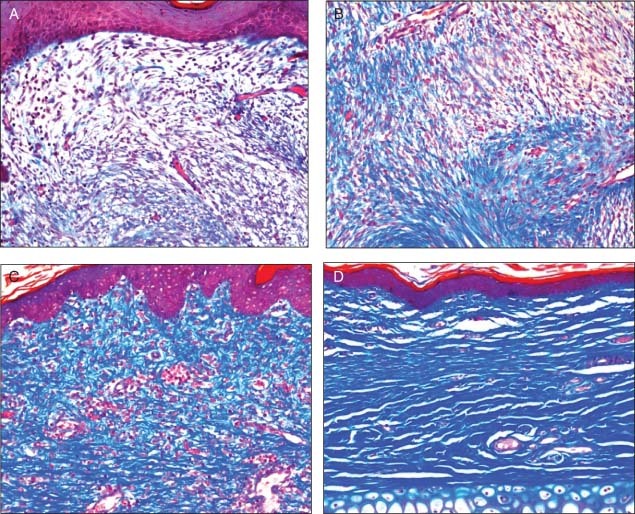
Representative Masson's trichrome stained sections for the evaluation of wound healing, inflammation and maturity by probiotic patch. (A) Section showing with no granulation tissue and almost absent staining for collagen (grade 1). (B) Shows loose granulation tissue with low collagen (grade 2). (C) Dense granulation tissue with a high concentration of disorganised collagen (grade 3). (D) Dense granulation tissue with bundles of dermal‐type collagen (grade 4).
While a larger sample size would be required to show significance, these results show improved scores for surface, epidermal and dermal observations and show a tendency towards improved wound healing in the gNO‐producing probiotic patch‐treated wounds. In addition, these histological results provide growing support for the hypothesis that improved wound healing and maturity because of the topical application of gNO may be due to increased collagen deposition, increased stimulation of endothelial progenitor cells, stimulation of neovascularisation, endothelial cell relaxation and improved keratinocyte migration 1, 27.
It has been showed that while elevated MMP activities in wound fluids impair endogenously produced, or exogenously applied, growth factors from acting as mitogenic agents, NO may circumvent this difficulty by acting as a mitogenic agent much the same way as a protein growth factor (KGF, VEGF, FGF, PDGF) (28). In fact, it is believed that NO may be indistinguishable from protein‐like growth factors, with respect to modulating mitogenic response of cells to external stimuli, and may act to improve wound healing by promoting proliferation of cells in a wound (28). Furthermore, it has been shown that epithelial cell migration (29), wound angiogenesis (30) and granulation tissue formation (31), critical processes in wound repair and new tissue formation, are mediated by NO. First attempts have been made using polymeric NONOates, NO‐donating agents and free NO gas to supplement NO during wound healing and these have shown promising results in wound repair 2, 19, 23, 32, 33. Thus, it is believed that NO deficiency in chronic non healing ulcers can be restored improving: (i) antimicrobial activity; (ii) fibroblast‐regulated collagen deposition; (iii) angiogenesis by endothelial cell progenitor proliferation and (iv) keratinocyte proliferation and migration, circumventing elevated MMP levels and/or low tissue inhibitors of MMPs levels and aiding in wound closure.
Safety of NO producing probiotic patch
Determination of the safety and toxicity profile of the gNO‐producing probiotic patches was performed by blood analysis. Table 2 shows safety and toxicological data of the gNO‐producing probiotic patch‐treated and vehicle control patch‐treated animals. Non significant differences were observed between gNO‐producing probiotic patch‐treated and vehicle control patch‐treated animals in body weight, blood morphology, haematology, blood chemistry or methaemoglobin levels. Analysis of biochemical markers of safety showed that serum samples of gNO‐treated and vehicle control‐treated animals were within normal ranges except for elevated potassium in serum samples for rabbit 3 (9·79 mmol/l) and rabbit 4 (11·44 mmol/l) (treated and control, infected rabbits). These animals were found to be mildly hyperkalemic which was thought to be the result of either a compensated metabolic acidosis, due to infection and/or poor perfusion, or haemolysis of blood samples prior to centrifugation and serum collection.
Table 2.
Safety and toxicological data *
| Rabbit 1 | Rabbit 3 | Rabbit 2 | Rabbit 4 | Finding | ||
|---|---|---|---|---|---|---|
| Body weight (kg) | −0.1 | −0.3 | −0.1 | −0 | No difference | |
| Blood morphology | Normal | Normal | Normal | Normal | No difference | |
| Haematology | White blood cell (×109/l) | 6.16 | 1·54 | 7.81 | 4.66 | |
| Red blood cell (×1012/l) | 5.91 | 6.10 | 6.07 | 5.98 | ||
| Hemoglobin (g/l) | 122 | 121 | 119 | 122 | ||
| Hematocrit (l/l) | 0.35 | 0.34 | 0.35 | 0.36 | ||
| Mean corpuscular volume (fl) | 59.9 | 55.2 | 57.7 | 60.3 | ||
| Mean corpuscular haemoglobin (pg) | 20.6 | 19.8 | 19.7 | 20.5 | ||
| Mean corpuscular haemoglobin concentration (g/l) | 344 | 359 | 341 | 339 | ||
| Platelets (×109/l) | 315 | 444 | 247 | 583 | ||
| Neut (×109/l) | 1.85 | 0.38 | 1.52 | 1.32 | ||
| Lymp (×109/l) | 3.69 | 1.07 | 5.75 | 2.98 | ||
| Mono (×109/l) | 0.06 | 0.01 | 0.07 | 0.05 | ||
| Eos (×109/l) | 0.11 | 0.03 | 0.15 | 0.09 | ||
| Luc (×109/l) | 0.01 | 0.00 | 0.01 | 0.00 | ||
| Baso (×109/l) | 0.43 | 0.05 | 0.31 | 0.22 | ||
| Retic (×1012/l) | 0.211 | 0.086 | 0.163 | 0.137 | ||
| Blood chemistry | Na+ (mmol/l) | 146.8 | 146.3 | 148 | 148 | |
| Electrolytes | K+ (mmol/l) | 5.79 | 9·79 | 5.55 | 11·44 | |
| Cl− (mmol/l) | 101.5 | 102.9 | 107.5 | 109.2 | ||
HCO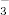 (mmol/l) (mmol/l) |
34.13 | 30.86 | 27.52 | 22.76 | ||
| Kidney | Creat (µmol/l) | 126.84 | 162.61 | 144.78 | 127.26 | |
| Urea (mmol/l) | 6.99 | 6.17 | 7.15 | 6.57 | ||
| Endocrine | Glucose (mmol/l) | 13.73 | 11.22 | 12.56 | 14.74 | |
| Ca2+ (mmol/l) | 3.29 | 3 | 3.26 | 3.16 | ||
| PO4− (mmol/l) | 2.11 | 2.06 | 2.74 | 1.97 | ||
| Liver | Alanine aminotransferase (U/l) | 50.83 | 31.01 | 28.71 | 32.12 | |
| Aspartate aminotransferase (U/l) | 32.89 | 33.3 | 54.95 | 19.32 | ||
| Pancreas | Lipase (U/l) | 190.59 | 158.6 | 148.37 | 194.28 | |
| Inflammatory | C‐reactive protein (nmmol/l) | 0.14 | 1.88 | −0.38 | 1.34 | |
| Methaemoglobin | 0·3 ± 0·2% | 0·1 ± 0·1% | Normal levels | |||
*Bolded values are those outside of the normal range.
Results of a complete haematologic profile showed treated and non treated animals to be comparable (Table 2). One non gNO‐treated animal was found to be leucopenic which may have resulted from the stress from the surgical procedure and daily manipulation, or may simply be a statistical outlier; however, no abnormal values were believed to be associated with gNO‐producing probiotic patch treatment.
One possible consequence of gNO absorption is an increase in the blood level of methaemoglobin which is a haemoglobin molecule in which the iron is oxidised from Fe2+ to Fe3+ (16). Analysis of levels of methaemoglobin, however, showed normal levels in both the gNO‐treated (0·1 ± 0·1%) and vehicle control‐treated rabbits (0·3 ± 0·2%), indicating that the gNO‐induced formation of methaemoglobin was undetectable or that clearance was adequate.
Plasma nitrate and nitrite levels did not present differences between treated and non treated rabbits as previously reported (33). These observations are in agreement with methaemoglobin results and confirm that the topical delivery of gNO did not alter systemic concentrations of nitrates and nitrites.
Thus, the results of blood analysis for safety and toxicity markers corroborate reports that the topical application and inhalation of gNO is non toxic and safe (34).
Wound microbial load
Viabilities from wound swab samples were determined for infected wounds that were treated the gNO‐releasing probiotic patches or controls. Whereas the total viability in the wound swab samples from the controls was 3 × 106 CFU, in the gNO‐treated wound swab samples the viability was less than 1 × 106 CFU. These results are in agreement with the observations previously reported (33).
Kaplan–Meier curve and Cox proportional hazard regression
While improved wound healing (decreased wound area) over time was easily quantifiable, and successfully showed by morphometric analysis, the definitive endpoint for efficacy in chronic wound healing is complete wound closure (35). Thus, log‐rank and Wilcoxon statistics for Kaplan–Meier survival probability plots and Cox proportional hazard regression analyses were performed to determine the increased or decreased likelihood of complete closure.
A Cox proportional hazard survival regression analysis was performed for all wounds (n = 32) for the variables: treatment, ischaemia and infection, and for ischaemic wounds (n = 16) for the variables: treatment and infection. Table 3 shows Cox proportional hazard survival regression analysis for all wounds (n = 32) and for ischaemic wounds (n = 16) under the conditions: treatment, ischaemia and infection. Hazard ratios represent an increase (or decrease) in the likelihood of an event occurring (wound closure). These data were analysed using EpiInfo software (CDC). The hazard ratio represents the increase or decrease in the likelihood of an event occurring (wound closure). Analysis of all wounds showed a 2·45‐fold increase in the likelihood of closure for treated wounds versus control (P = 0·053) and that ischaemic wounds were 0·21 times less likely to close (P = 0·0039). Analysis of ischaemic wounds only (n = 16, 8 treated and 8 non treated) showed a 17·95‐fold increase in the likelihood of closure for treated wounds versus control (P = 0·030), and that infected ischaemic wounds were 0·068 times less likely to close (P = 0·037) compared with non infected ischaemic wounds.
Table 3.
Cox proportional hazard survival regression analysis for all wounds *
| Variable | Hazard ratio | P value | |
|---|---|---|---|
| All wounds | Treatment (n = 32) | 2·45 | 0·053 |
| Ischaemia (n = 16) | 0·21 | 0·0039 | |
| Infection (n = 16) | 0·68 | 0·42 | |
| Ischaemic | Treatment (n = 8) | 17·95 | 0·030 |
| Infection (n = 8) | 0·067 | 0·037 |
*Bolded values indicates a significant difference.
The likelihood of wound closure over time was evaluated in wounds treated with gNO‐producing probiotic patches versus wounds treated with vehicle control patches by a Cox proportional hazard regression plot independent of covariables (Figure 6). The analysis shows that gNO‐treated wounds were 2·52 times more likely to close than vehicle control (hazard P = 0·038, score P = 0·032 and likelihood ratio P = 0·036).
Figure 6.
Cox proportional hazard regression plot investigating likelihood (survival probability) of wound closure over time in nitric oxide gas (gNO)‐producing patch‐treated wounds versus wounds treated with vehicle control patches independent of covariables. These data show that gNO‐producing probiotic patch‐treated wounds are 2·52 times more likely to close compared with wounds treated with vehicle control patches (hazard P = 0·038, score P = 0·032 and likelihood ratio P = 0·036) and that several non treated wounds remained open. The light line represents gNO‐producing probiotic patch‐treated wounds (16 wounds) and the dark grey line represents wounds treated with vehicle control patches (16 wounds). These data were graphed using EpiInfo software (Center for Disease Control).
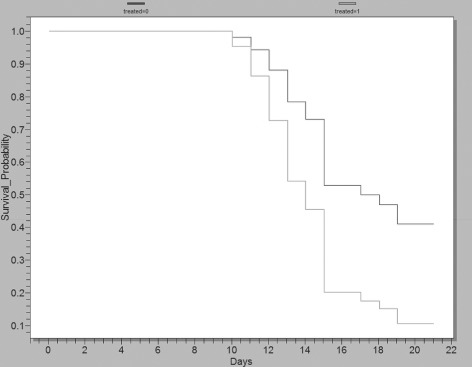
The likelihood of wound closure over time was evaluated in wounds treated with gNO‐producing probiotic patches versus wounds treated with vehicle control patches by a Kaplan–Meier plot and calculating the log‐rank and Wilcoxon statistics (Figure 7). The analysis shows that gNO‐treated wounds closed before vehicle control (log‐rank P = 0·023 and Wilcoxon P = 0·011, 16 wounds per group) and that several of the control‐treated wounds remained open.
Figure 7.
Kaplan–Meier plot showing the likelihood (survival probability) of wound closure in wounds treated with nitric oxide gas (gNO)‐producing probiotic patches versus wounds treated with vehicle control patches. These data show that gNO‐producing probiotic patch‐treated wounds closed before wounds treated with vehicle control patches (log‐rank P = 0·0223 and Wilcoxon P = 0·011) and that several non treated wounds remained open. The light grey line is gNO‐producing probiotic patch‐treated wounds (16 wounds) and the dark grey line represents vehicle control‐treated wounds (16 wounds). These data were graphed using EpiInfo software (Center for Disease Control).
The likelihood of wound closure over time was evaluated for ischaemic and non ischaemic wounds (Figure 8). Results show that ischaemia significantly delays wound closure in this animal model (log‐rank P = 0·005 and Wilcoxon P = 0·0056, 16 wounds per group). Analysing only the infected ischaemic wounds (n = 8, 4 wounds per group) the same analysis shows a significant improvement in wound closing for the gNO‐treated groups as compared with the vehicle controls despite the number of wounds (log‐rank P = 0·04 and Wilcoxon P = 0·046).
Figure 8.
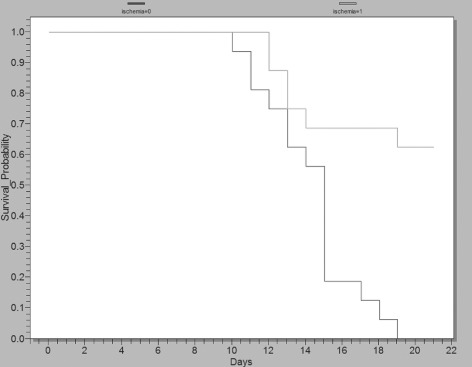
Kaplan–Meier plot showing the likelihood (survival probability) of wound closure over time for ischaemic wounds versus non ischaemic wounds. These data show that ischaemia delays wound closure (log‐rank P = 0·005 and Wilcoxon P = 0·0056) and that some ischaemic wounds remain open at 21 days. The light grey line represents ischaemic wounds (16 wounds), whereas the dark grey line represents non ischaemic (16 wounds). These data were graphed using EpiInfo software (Center for Disease Control).
Although, morphometric analysis did not show a significant effect of gNO on wound healing in infected and non infected non ischaemic wounds, a non significant improvement was seen in treated ischaemic and non ischaemic infected wounds, whereas no such trend was evident in non infected wounds. Thus, while morphometric analysis shows that ischaemia plays a role in the pathophysiology of delayed wound healing, and that gNO‐producing probiotic patches can be used to accelerate wound closure in infected or non infected ischaemic wounds, only a promising trend exists for treating non ischaemic infected wounds with gNO. However, based on the wide spectrum antimicrobial activity of NO(36), the proven antimicrobial and antifungal activity of this gNO‐producing probiotic patch (37), and the known pathologic action of bacterial biofilms in slowing wound closure, a real difference may be apparent in a larger population of wounds.
A total of 32 wounds have been evaluated in this study, including four rabbits. Even though the sample size may be limited, the data analysis is robust and allowed to detect significant differences between gNO‐treated and control groups in some cases, as described. This statistical analysis corroborates results seen in analysis of morphometric data and strengthens the hypothesis that the pathophysiology of non healing ischaemic wounds is closely tied to a dysregulation of endogenous NO production and that the exogenous application of gNO may have a therapeutic effect 13, 17, 19, 23.
CONCLUSIONS
This study shows for the first time, the efficacious and safe use of a gNO‐producing probiotic patch for ischaemic and infected wound healing. Specifically, this study describes the design of a probiotic patch, its fabrication, in vitro testing and investigates the potential of a gNO‐producing probiotic patch on healing of ischaemic and/or infected full‐thickness wounds in New Zealand white rabbits.
The results show that wound healing, as determined by morphometric analysis, was significantly accelerated in gNO‐treated, non infected and infected ischaemic wounds. These results were corroborated by analysis of accelerated complete wound closure (Kaplan–Meier and Cox plots). Analysis of all wounds showed that gNO treatment significantly improved the likelihood of wound closure and that gNO‐treated ischaemic wounds were significantly more likely to close than vehicle control‐treated ischaemic wounds. Finally, histological analysis showed improved wound healing in tissues of gNO‐treated wounds and safety analysis showed no abnormal haematologic and biochemical markers of safety associated with gNO treatment.
While several groups are working on gNO‐releasing wound healing devices, the barriers to commercial use (sustained gNO release, shelf life, ease of use and cost) have not yet been overcome 18, 19, 20, 21, 22, 23. The gNO‐releasing probiotic patch was also efficacious at reducing the wound bacterial load without altering the systemic levels of nitrates, which is in agreement with a previous study in which rabbits with infected dorsal wounds were treated with 200 ppmV gNO delivered by a tank and regulator (33).
This study shows that gNO‐producing probiotic patches can be designed and used for topical applications, including treating ischaemic and infected full‐thickness dermal wounds that approximate chronic non healing ulcers, and that gNO‐producing probiotic patches may provide an elegant solution to the obstacles to commercial development. This study also provides further evidence that the dysregulation of gNO may be intimately associated with the pathophysiology of chronic ischaemic wounds and that exogenous application can accelerate wound healing. Human clinical efficacy is yet to be evaluated.
ACKNOWLEDGEMENTS
We gratefully acknowledge financial support from the Industrial Research Assistance Programme (IRAP) of the National Research Council (NRC) of Canada and research grant from Micropharma Limited. Please note that Satya Prakash and Mitchell Jones have conflict; they are inventor of the technology mentioned in this research that has been patented by Micropharma. None of the other authors have any personal or financial conflict of interest.
REFERENCES
- 1. Blackytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006;23:594–608. [DOI] [PubMed] [Google Scholar]
- 2. Luo JD, Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin 2005;26:259–64. [DOI] [PubMed] [Google Scholar]
- 3. Luo JD, Hu TP, Wang L, Chen MS, Liu SM, Chen AF. Sonic hedgehog improves delayed wound healing via enhancing cutaneous nitric oxide function in diabetes. Am J Physiol Endocrinol Metab 2009; 297:E525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorwitz RJ. A review of community‐associated methicillin‐resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J 2008;27:1–7. [DOI] [PubMed] [Google Scholar]
- 5. Anstead GM, Quinones‐Nazario G, Lewis JS. Treatment of infections caused by resistant Staphylococcus aureus. Methods Mol Biol 2007;391:227–58. [DOI] [PubMed] [Google Scholar]
- 6. Nordmann P, Naas T, Fortineau N, Poirel L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol 2007;10:436–40. [DOI] [PubMed] [Google Scholar]
- 7. Linares J. The VISA/GISA problem: therapeutic implications. Clin Microbiol Infect 2001;7 Suppl 4:8–15. [DOI] [PubMed] [Google Scholar]
- 8. Stadelmann WK, Digenis AG, Tobin GR. Impediments to wound healing. Am J Surg 1998;176: 39S–47S. [DOI] [PubMed] [Google Scholar]
- 9. Akbari CM, Saouaf R, Barnhill DF, Newman PA, LoGerfo FW, Veves A. Endothelium‐dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J Vasc Surg 1998;28:687–94. [DOI] [PubMed] [Google Scholar]
- 10. Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, DeGirolami U, LoGerfo FW, Freeman R. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 1998;47:457–63. [DOI] [PubMed] [Google Scholar]
- 11. Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev 2009;25:604–14. [DOI] [PubMed] [Google Scholar]
- 12. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–22. [DOI] [PubMed] [Google Scholar]
- 13. Isenberg JS, Ridnour LA, Espey MG, Wink DA, Roberts DD. Nitric oxide in wound‐healing. Microsurgery 2005;25:442–51. [DOI] [PubMed] [Google Scholar]
- 14. Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide 2002;7:1–10. [DOI] [PubMed] [Google Scholar]
- 15. Cals‐Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide 2004;10:179–93. [DOI] [PubMed] [Google Scholar]
- 16. Hegesh E, Gruener N, Cohen S, Bochkovsky R, Shuval HI. A sensitive micromethod for the determination of methemoglobin in blood. Clin Chim Acta 1970;30:679–82. [DOI] [PubMed] [Google Scholar]
- 17. Schwentker A, Billiar TR. Nitric oxide and wound repair. Surg Clin North Am 2003;83:521–30. [DOI] [PubMed] [Google Scholar]
- 18. Stenzler A, Miller C. Device and method for treatment of wounds with nitric oxide 2006;Patent WO/2006/1957:1–21.
- 19. Shabani M, Pulfer SK, Bulgrin JP, Smith DJ. Enhancement of wound repair with a topically applied nitric oxide‐releasing polymer. Wound Repair Regen 1996;4:353–62. [DOI] [PubMed] [Google Scholar]
- 20. Wheatley PS, Butler AR, Crane MS, Fox S, Xiao B, Rossi AG, Megson IL, Morris RE. NO‐releasing zeolites and their antithrombotic properties. J Am Chem Soc 2006;128:502–9. [DOI] [PubMed] [Google Scholar]
- 21. Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, Schoenfisch MH. Bactericidal efficacy of nitric oxide‐releasing silica nanoparticles. ACS Nano 2008;2: 235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti‐biofilm efficacy of nitric oxide‐releasing silica nanoparticles. Biomaterials 2009;30:2782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Lee PI. Controlled nitric oxide delivery platform based on s‐nitrosothiol conjugated interpolymer complexes for diabetic wound healing. Mol Pharm 2010;7:254–66. [DOI] [PubMed] [Google Scholar]
- 24. Chien S. Ischemic rabbit ear model created by minimally invasive surgery. Wound Repair Regen 2007;15:928–35. [DOI] [PubMed] [Google Scholar]
- 25. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 2004;37:395–400. [DOI] [PubMed] [Google Scholar]
- 27. Schulz G, Stechmiller J. Wound healing and nitric oxide production: too little or too much may impair healing and cause chronic wounds. Int J Low Extrem Wounds 2006;5:6–8. [DOI] [PubMed] [Google Scholar]
- 28. Frank S, Kampfer H, Wetzler C, Pfeilschifter J. Nitric oxide drives skin repair: novel functions of an established mediator. Kidney Int 2002;61:882–8. [DOI] [PubMed] [Google Scholar]
- 29. Noiri E, Peresleni T, Srivastava N, Weber P, Bahou WF, Peunova N, Goligorsky MS. Nitric oxide is necessary for a switch from stationary to locomoting phenotype in epithelial cells. Am J Physiol 1996;270:C794–802. [DOI] [PubMed] [Google Scholar]
- 30. Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor‐induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A 2001;98:2604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pollock JS, Webb W, Callaway D, Sathyanarayana, O’Brien W, Howdieshell TR. Nitric oxide synthase isoform expression in a porcine model of granulation tissue formation. Surgery 2001;129: 341–50. [DOI] [PubMed] [Google Scholar]
- 32. Bhide M. Nitric oxide delivery from polymeric wound dressings. Saarbrücken: VDM Verlag, 2006.
- 33. Ghaffari A, Jalili R, Ghaffari M, Miller C, Ghahary A. Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen 2007;15:368–77. [DOI] [PubMed] [Google Scholar]
- 34. Weinberger B, Laskin D, Heck D, Laskin J. The toxicology of inhaled nitric oxide. Toxicol Sci 2001;59:5–16. [DOI] [PubMed] [Google Scholar]
- 35. Guidance for Industry Chronic Cutaneous Ulcer and Burn Wounds – Developing Products for Treatment, FDA Document 2006:1–18. [DOI] [PubMed]
- 36. Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide‐related antimicrobial activity. J Clin Invest 1997;99: 2818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones ML, Ganopolsky JG, Labbe A, Prakash S. A novel nitric oxide producing probiotic patch and its antimicrobial efficacy: preparation and in vitro analysis. Appl Microbiol Biotechnol 2010;87:509–16. [DOI] [PubMed] [Google Scholar]


