Abstract
The International Compression Club (ICC) is a partnership between academics, clinicians and industry focused upon understanding the role of compression in the management of different clinical conditions. The ICC meet regularly and from these meetings have produced a series of eight consensus publications upon topics ranging from evidence‐based compression to compression trials for arm lymphoedema. All of the current consensus documents can be accessed on the ICC website (http://www.icc‐compressionclub.com/index.php). In May 2011, the ICC met in Brussels during the European Wound Management Association (EWMA) annual conference. With almost 50 members in attendance, the day‐long ICC meeting challenged a series of dogmas and myths that exist when considering compression therapies. In preparation for a discussion on beliefs surrounding compression, a forum was established on the ICC website where presenters were able to display a summary of their thoughts upon each dogma to be discussed during the meeting. Members of the ICC could then provide comments on each topic thereby widening the discussion to the entire membership of the ICC rather than simply those who were attending the EWMA conference. This article presents an extended report of the issues that were discussed, with each dogma covered in a separate section. The ICC discussed 12 ‘dogmas’ with areas 1 through 7 dedicated to materials and application techniques used to apply compression with the remaining topics (8 through 12) related to the indications for using compression.
Keywords: Compression bandages; Compression stockings; Intermittent pneumatic compression; Leg ulcers; Lipoedema; Lymphoedema
Dogma: ‘Compression affects the superficial veins more than deep veins?’ (presented by G. Mosti, J. F. Uhl and H. Partsch)
This statement appears to be based more on ‘common sense’ than on experimental findings. With the introduction of new imaging capabilities including magnetic resonance imaging (MRI) used while subjects adopt different postures including standing, supplemented by three‐dimensional (3D) reconstruction and volume quantification, experimental data has now challenged this dogma 1, 2. For example, 12 individuals (CEAP C0 in 4 and C2‐C4 in 8) were studied using T2‐weighted MRI of the calf of both legs while in different postures (12 in supine, 3 in prone and 6 in upright) before and after the application of compression using a variety of stockings and bandages (2). Throughout the study interface pressure at the B1 location was measured using the Picopress® pressure sensor (Microlab, Milan, Italy). From the MRI scans 3D vector models were constructed from cross‐sectional segmentation of all relevant anatomical structures (skin, muscle and bone, deep and superficial veins). For each leg an interactive model of the calf allowed quantification of the effect of compression upon the local anatomy including changes in the volume of the veins. While supine a light compression of 20 mmHg reduced the volume of the deep calf veins by 60–80% with this observation noted for the posterior tibial, fibular and soleus veins but not for the anterior tibial vein. Similar reductions of the volume of the deep veins were also reported by Downie and co‐workers (3) while prone but not in a supine posture (4). In the standing position light compression reduced the volume of the deep, but not of the superficial system and the varices (Figure 1). As the compression applied to the leg increased, the greater volume reduction of the deep veins compared with the superficial system remained until external pressures of around 70 mmHg were applied at which point the superficial veins were reduced in volume during standing. This observation has practical significance in that it may require high local pressures to occlude a superficial vein perhaps using additional pads placed beneath compression systems to achieve a local ‘high pressure point'.
Figure 1.

3D reconstruction of the leg comparing the venous system in standing position before and after applying a compression stocking exerting a pressure of 22 mmHg. Under the stocking the cross‐section of the leg is reshaped into a more circular structure. V, varix; A, anterior tibial veins; P, posterior tibial veins; F, fibular veins; S, soleus veins.
Dogma: Compression devices applied to the leg must provide a pressure gradient with decreasing pressures from distal to proximal? (presented by G. Mosti)
The concept of a graduated or ‘degressive’ pressure is based on the assumption that under physiological conditions flow will always go from a distal point at high pressure to a proximal point at lower pressure and that creating a reverse pressure gradient by applying higher proximal compression would impede venous return. Experimental data have refuted this hypothesis at least for the mobile patient during walking (4). In 30 patients with severe chronic venous insufficiency, the ejection fraction of the calf pump was significantly increased by the use of compression stockings that applied an inverse pressure gradient rather than by conventional, graduated products. This observation may be explained through the external compression exerting higher pressures over the calf compared with the ankle; the higher calf pressures will force a greater volume of blood from the local veins during muscle systole. Couzan and co‐workers have advocated this concept for several years 5, 6, and beneficial effects of high applied calf pressures have been reported in sports medicine (7). Couzan and co‐workers 5, 6 put forward the idea that progressive compression, which is just the opposite of degressive compression, could be more efficient because calf veins contain much more blood and are more accessible to compression compared to ankle veins. This concept has been tested in one randomised trial in 130 patients suffering from mild venous insufficiency: disappearance or major improvement of leg heaviness was observed in 73% of patients wearing progressive elastic stockings and 62·5% of those wearing degressive elastic stockings, showing that progressive compression was at least as efficient as degressive compression, in this situation (6). It must be stressed that the enhanced venous flow that might be induced using reverse gradient stockings or bandages may be of relevance only for mobile patients; for the immobile, further investigation is required to rule out inadvertent adverse effects of applying high pressure at the calf and lower pressure at the ankle. Up to now no clinical outcome data concerning oedema reduction or ulcer healing have been reported.
Dogma: Is a pressure gradient the best way to improve haemodynamics? answers from a computer model. (presented by M. Chauveau, F. Cros and P. Gelade)
Clinical (8) and experimental data have shown that in bedridden patients graduated, degressive compression is better than non‐degressive compression in reducing venous stasis and preventing deep vein thrombosis. Also when standing at rest, a degressive compression is logical for counteracting hydrostatic pressure in order to prevent venous distension and occupational oedema.
Computer simulations have been performed based on a mathematical model of venous haemodynamics, ‘the venous return simulator’ (VRS). The VRS takes into account the architecture, dimensions and distensibility of the venous network, blood viscosity, valve function, subject's posture and the external pressures applied to the veins (for example, by muscular contraction and compression therapy). Using the laws of hydrodynamics, it provides the diameters, pressures and flows throughout the network, while at rest and also during exercise.
A validation study of the VRS has been performed by comparing the values of ambulatory venous pressure (AVP) computed by the VRS, with AVP values reported in literature. In normal subjects , as well as in three schematic cases of valve incompetence (great saphenous vein (GSV), deep veins and combined incompetence), the computed AVP was in agreement with values reported in patients with similar valve defects; computed AVP increasing as the extent of valve defects was increased (9).
In the actual study, AVP achieved during a 10 tiptoe exercise was computed for a virtual normal subject and for a virtual patient characterised by a 55% increase in the diameters of the GSV and the posterior accessory vein resulting in a functional reflux in these two veins. The computed AVP was 25 mmHg in the normal case and 42 mmHg in the virtual patient.
Two forms of ‘steady compression’ with an interface pressure kept constant during tiptoe exercise were applied to this virtual patient: a degressive compression (25 mmHg ankle and 12·5 mmHg calf) and a progressive compression (11·5 mmHg ankle and 23 mmHg calf), with both achieving a spatial mean pressure of 15 mmHg. Both compressions resulted in a 9 mmHg decrease in AVP. This simulation therefore did not reveal a difference between graduated and non‐graduated compression.
A third modality, ‘dynamic’ compression was tested, where the interface pressure on the calf varies parallel to muscular contraction. Two levels of systolic–diastolic amplitude corresponding to a walking pressure amplitude (WPA) were simulated: 15 and 30 mmHg, the time‐space integrated value of interface pressure being in both cases the same as with steady compression: 15 mmHg. AVP decreased to 29 mmHg with a WPA of 15 mmHg and to 23 mmHg with a WPA of 30 mmHg showing that higher pressure amplitudes corresponding to stiffer material resulted in a more effective reduction of ambulatory venous hypertension.
According to this model, in the case of superficial reflux resulting from venous dilatation, AVP improvement does not depend on the degressive versus progressive compression pattern; instead it is better with dynamic compression and increases as WPA increases. This is in agreement with the correlation between the stiffness of a compression bandage and the haemodynamic improvement showed by Mosti and co‐workers (10).
Up to now, dynamic compression could only be achieved by means of short stretch bandages, which are not easy to use, both for physicians and for patients. New stockings with high stiffness should be developed and clinically evaluated in the future.
Dogma: Inelastic material exerts low pressure at rest? (presented by H. Partsch)
Every compression device produces a resting pressure which increases when movement causes the calf muscle to contract (‘working pressure’). The difference between working and resting pressure characterises the stiffness of the compression product. Compression stockings of low compression class and round‐knitted bandages and many elastic bandages have a low stiffness. Inelastic bandages, stockings of high compression class or flat‐knitted, and also multicomponent bandages consisting of different elastic bandages provide higher stiffness 11, 12.
The concept that inelastic material always exerts low pressure at rest is mainly based on traditional bias endorsed by outdated classification schemes derived from laboratory testing. Pressure measurements on the human leg published during the last years have clearly challenged this statement (12).
In the British Standard 7505 document (British Standard 7505: elastic properties of extensible bandages) short stretch bandages like Comprilan® (BSN) were put into the category of ‘light support' (‘type 2’ of British Standard) and only elastic material was classified in the group of compression (‘type 3’) (13). This is in agreement with the previous concept to differentiate between ‘passive contention’ exerted by inelastic material and ‘active’ by elastic textiles.
In the meanwhile, this differentiation has been abandoned by regulatory organisations like the French Haute Autorité de Santé(14).
Comprilan® like any other inelastic bandage can be applied with very high force, the strength used by the bandager being the deciding criterion for the final sub‐bandage pressure. The bandager, and not the bandage, creates the pressure. Mainly due to a reduction of oedema there is a pressure drop starting immediately after application of an inelastic bandage, so that after wearing such bandages for some time the statement of a ‘low resting and a high working pressure’ may be justified. However, it could be shown that after wearing inelastic bandages for 1 week their beneficial haemodynamic effects were maintained in spite of a pressure loss of more than 50% (15). In a previous consensus document ‘strong compression’ of a bandage was defined as a range between 40 and 60 mmHg (12). When strong compression is indicated this pressure loss together with the anticipated wearing time should be taken into consideration and inelastic bandages should be applied with an initial pressure of 60 mmHg. For elastic material showing less pressure drop 40 mmHg may be sufficient to produce a ‘strong compression bandage’.
Dogma: Immobile patients need elastic compression? (presented by H. Partsch)
Accepting the traditional view that only elastic material can produce sufficiently ‘high resting pressure’ due to the strength of the elastic fibres, while inelastic compression would provide high pressure only during movement then inelastic bandages would be ineffective when used in the care of immobile patients who by definition do not move. Pressure measurements on patients' legs show that this is a misconception (see discussion above) (12). A patient with swollen legs sitting in a wheelchair can tolerate very well compression pressures of up to 60 mmHg, applied by inelastic bandages or by intermittent pressure pumps. In addition, smallest active or passive movements even performed only with the toes will increase the pressure under inelastic material (‘massaging effect') much more than under elastic, yielding material (Figure 2). Unfortunately, there is still some discrepancy between the high incidence of chronic oedema and few recommendations concerning an optimal management of immobile patients.
Figure 2.
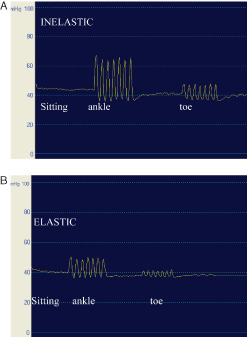
Sub‐bandage pressure measurements on the distal medial leg in the sitting position with dorsiflexions and toe movements under inelastic (A) and elastic bandaging (B). The same curves are obtained by active and passive movement. Starting from a pressure of around 40 mmHg the pressure amplitudes during movement are much higher with inelastic than with elastic material (‘stronger massaging effect').
Such immobile patients may also benefit from multicomponent bandages which may be better tolerated during night time because of their low resting pressure/high stiffness as stated above.
Dogma: Effects on skin‐changes: static or dynamic compression? (presented by M. Flour)
Trial data are lacking on the benefits of compression stockings in the management of venous eczema, atrophie blanche or lipodermatosclerosis (LDS). Since chronic ambulatory venous hypertension is the primary underlying cause in venous disorders, compression treatment should be an effective conservative treatment option. The exerted pressure induces increased subcutaneous pressures in patients with CVI, with and without clinical oedema, and this is expected to counteract capillary leakage.
The effect of compression is more than purely haemodynamic or mechanical, there is most probably an effect at the cellular/matrix level. In bioengineering, experimental evidence shows that cells (e.g. fibroblasts) behave differently when their 3D scaffold is stressed (static or dynamic) compared with a lack of mechanical stimulation (16).
Intermittent pneumatic compression (IPC) is an excellent model for ‘dynamic compression’ and under this modality, release of vasodilating and anti‐inflammatory mediators from the endothelial cells has been shown (17). Similar effects may be expected by using stiff compression material exerting high pressure peaks during walking.
The Clinical Knowledge Summaries (NHS, UK) in their latest review of the subject in 2008 (18) did not identify any national guidelines on the assessment of venous eczema or LDS.
There are several clinical parameters to consider, like inflammation, oedema, induration, pain, scarred atrophic areas in atrophie blanche and skin pigmentation. Experimental data supports the effectiveness of distinct levels of compression upon different aspects of LDS: oedema reduction has been shown both with bandages or IPC using pressures of 10–20 mmHg and 20–30 mmHg and improvement of microcirculation was achieved with bandages or IPC at 20–30 mmHg (19). Treatment with 35–45 mmHg compression stockings for 6 months has been shown to reduce the area of LDS in patients with healed venous ulcers (20). Objective assessment of outcome parameters may be difficult, expensive, cumbersome or not validated.
Inflammation and pain may be severe in the acute phase, rendering compression treatment painful and so less likely to be tolerated. Several publications mention that compression needs to be adapted to from the start, and supplementary treatment such as anti‐inflammatory agents (topical or systemic), or ‘fibrinolytic’ medication must be prescribed (Stanazolol 2 mg bid) (21). Clinical experience of many is that stiff bandages (including wadding/padding) are well tolerated and very effective at reducing pain and inflammation. No studies could be identified on this subject.
Dogma: Leg compression needs always to include foot and ankle? (presented by D. Bender)
A general misconception exists that the compression level applied to the foot must be equal to or greater than the compression level at the ankle otherwise a swollen foot will result. For the most part, this misconception has developed from a combination of several factors. First, the logic that compressing only part of a limb will result in a constriction point (tourniquet) and fluid distal to this point will be prevented from flowing up the leg resulting in swelling. Second, we are taught that compression must be applied in a gradient fashion to promote fluid flow from distal to proximal and that stockings are designed to be graduated. And third we have a lack of knowledge of the specific design parameters of ‘gradient compression stockings' due to a limited description of the product from the manufacturers.
In fact, we find that there are no specifications nor published criteria regarding the actual compression requirements for the feet.
Interestingly and contrary to what most believe, the compression level in the foot of traditional gradient elastic stocking is less than that at the ankle. A review of designs from the major compression stocking manufacturers around the world revealed that the compression level currently provided in the foot portion of a stocking is lower than the ankle pressure. While the manufacturers would not provide specific compression design parameters for the foot, across the board the design objective is to provide the same general level of compression as is provided in the calf, which is 50–80% of the ankle pressure.
Additionally pressure measurements under a RAL class II elastic stocking revealed that the pressures at the dorsal and ventral measuring points of the foot (X position in Figure 3) are indeed in the same range as the calf measurements. Also of note is that the lateral and medial measurements are significantly higher, an effect of the smaller radius associated with the side of the foot. This does draw our attention to the potential for too high a compression level in these areas of the foot and possible detrimental consequences.
Figure 3.
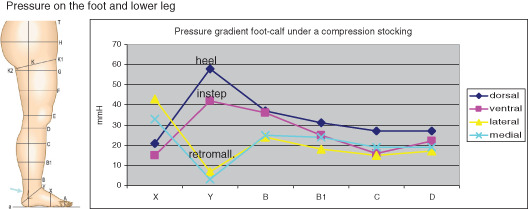
Pressure measurements under a compression stocking performed on the dorsal, ventral, medial and lateral aspect of the foot (X), the ankle (Y) and the lower leg (B–D) using a circular pressure probe of 45 mm diameter (Picopress®) (HP). On the foot (segment X) the highest pressure is measured over the lateral and medial edge of the foot and the lowest over the flat parts (dorsum and foot sole) according to the law of Laplace.
Thus, what we find in a traditional gradient elastic stocking is a compression profile similar to that shown in Figure 4 where the foot compression level is less than either the ankle or B1 landmarks.
Figure 4.
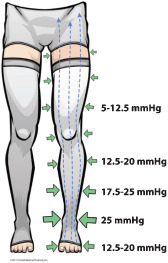
Compression profile of a traditional gradient elastic stocking: compression level at the foot is less than either the ankle or B1 measuring points.
The discussion then led to a series of questions that need to be considered in the future design and applications of compression devices. (i) Is compression of the foot necessary from a haemodynamic perspective? (ii) When should we compress the feet? Only when oedema is present? (iii) What about the higher pressures medial and lateral on the foot, is that detrimental? (iv) How much compression of the foot is enough? For what indications? (v) When a venous ulcer is present below the malleolus does the ulcer itself require direct compression over the wound? And if so, at what pressure? (iv) Is there ever a time to consider not compressing the foot at all?
For example, we know that bulky compression material applied in many layers over the ankle joint will impede mobility and thus calf muscle pump function and act in a counterproductive manner (22). If we could compress the leg without compressing the foot would we achieve an improved outcome or better patient compliance?
All these aspects should be investigated by future studies.
The discussion in total brought attention to an area of compression therapy that most of us simply overlook in regards to its importance and impact in the outcomes of our patients.
Dogma: Compression bandages need to contain padding? (presented by A. Andriessen and J. Schuren)
During the discussion of this contentious issue it emerged that the recommendation to use padding as routine is more a safety concern than a feature of better performance. In attempts to avoid skin damage, different foams or orthopaedic wool are applied first before the compression layers are applied (23). Padding changes both pressure and stiffness of multicomponent bandages in a way that is difficult to predict (24). Andriessen presented a meta‐analysis encompassing 110 trials with a total of 184 effect sizes, which was conducted as part of another project and presented at the SAWC 2003 meeting (abstract printed in the proceedings issue of this meeting). The most common outcome measure was healing rate at 12, 16 or 24 weeks of treatment. The commonly used compression systems were four‐layer or a two‐layer short stretch system. Padding (orthopedic wool or foams) was shown to help prevent iatrogenic damage and to ensure even distribution of pressure, especially when compression is applied in nursing homes or in a community setting where delivery of compression is often poor in chronic elderly and frail patients, and where there often is a lack of training and skills (25).
A pilot study in a community care setting employing 10 patients as their own control compared two different padding materials applied under short stretch adhesive compression bandages for venous leg ulcer (VLU) treatment. A foam padding layer was easier to apply than orthopaedic wool and slightly more comfortable, with less slipping (26). Randomised controlled trials comparing padding versus no padding are lacking.
Schuren presented arguments against the routine use of padding, showing that padding used to reshape or fill the leg, as is often seen in clinical practice and recommendations may have a detrimental effect. For many reasons, compression therapy for chronic venous ulceration can be compared to cast treatment for fractures. Maintaining or improving circulation and functional activities are common objectives. While older texts upon casting and compression therapy do not recommend the use of padding with good arguments, it is interesting that currently padding materials are routinely used underneath casts and compression bandages. The reason for this shift in clinical practice is probably the trend for more safety and comfort. Schuren noted that the use of padding materials can have detrimental effects on the stabilisation of fractures within a cast. It was also showed that the forces exerted inside a cast during functional activities are absorbed by the padding where ideally the cast should support the function of the muscles during their contraction and consequent venous return, this absorption of force within the padding layer would have a similar effect under compression bandages. Schuren presented three laboratory studies related to compression studies that clearly showed that the use of padding materials should be limited. The main conclusions from these studies are as follows:
Padding materials have an effect on the even distribution of sub‐bandage pressures, especially if they are used to ‘flatten’ or ‘fill’ irregularly shaped legs.
Padding materials have an effect on the stiffness of applied compression systems.
Padding materials have an effect on the calculation of sub‐bandage pressure measurements with the modified Laplace equation (27).
There are occasions where padding should be used, mainly to protect bony prominences such as the tibia crest or the malleoli.
Dogma: Arterial occlusive disease is an absolute contraindication for using compression? (presented by A. Cornu‐Thénard, G. Mosti and E. Arkans)
The basis for this dogma is the observation that in patients with reduced arterial flow an external compression could further reduce the arterial inflow and cause major tissue damage. Several consensus papers and guidelines define an ankle brachial pressure index (ABPI) of lower than 0·5 or a toe pressure < 30 mmHg as ‘critical ischemia’ which is a clear contraindication for sustained compression 28, 29. Most ulcer healing studies have excluded patients with an ABPI lower than 0·8. However, special forms of intermittent pump compression may be beneficial even in severe stages of arterial occlusive disease (see below). In the presence of oedema or mixed ulceration, modified compression exerting a reduced pressure of 30–40 mmHg has been shown to be safe and effective in patients with an ABPI over 0·5 (30).
During discussion it was agreed that the distal systolic pressure (DSP) (ankle or toe pressure measured in mmHg) is more relevant than the quotient between ankle and arm pressure (ABPI) 30, 31. Patients with a systolic ankle pressure between 60 and 100 mmHg in the lying position may be bandaged safely using non‐elastic, stiff material exerting an initial pressure of less than 40 mm Hg (30). This material will produce pressure peaks during walking which may have a massaging effect on the swollen leg and will lead to an increase of venous return thereby increasing the arterio‐venous pressure gradient.
If compression stockings are used, care should be taken to adjust the chosen compression class to the DSP. By knowing the DSP in mmHg and knowing the exerted pressure of the MCS in mmHg ready‐made MCS can be prescribed for patients with AOD, provided the systolic ankle pressure is higher than 60 mmHg. In the upright position, the ankle pressure is about 80 mmHg higher than in supine. In some situations, the MCS superposition technique can be used in order to obtain an acceptable compression pressure (32).
Skin necrosis due to compression especially in cases of unrecognised arterial occlusive disease has been described (33). This may occur even after low pressure elastic stockings are used especially in immobile patients. In such cases and also in patients with peripheral neuropathy – who may not feel any pain due to a pressure induced skin defect – daily change of the compression device with careful inspection of the skin is recommended.
In contrast, IPC may be an excellent indication for treating ischaemic disease of the extremities.
Increased pedal blood flow in normal subjects was observed when experimenting with rapid foot compression during a venous study. A literature review showed several investigators, as far back as 1934, that measured acute effects of increased blood flow in ischaemic limbs and relief of symptoms. However, there was poor understanding of the physiological mechanisms and a great variety of compression schemes and devices were reported.
Physiological studies showed that the acute mechanisms are: increased arterial‐venous pressure gradient, reduced peripheral resistance and abolition of the veno‐arteriolar reflex. These studies also provided data to optimise pressure, timing and tissues to compress. On the basis of these data a device was optimised providing short and high peak pressures of 120 mm Hg followed by a long period without pressure (ArtAssist® device, ACI Medical, San Marcos, CA).
Three randomised controlled trials were performed on patients with intermittent claudication. Aside from doubling or tripling of walking distances, sustained improvements led to the question of what long‐term mechanisms might be at work. Additional studies showed that arteriogenesis, the opening of collaterals, was responsible for improved pulse volume recordings (the arterial pulsatility of a limb segment), ‘permanently’ improved walking distances in claudicants, increased limb salvage in patients not able to undergo revascularisation procedures and resolved rest pain 34, 35, 36.
IPC is a very powerful adjunctive treatment modality mostly used in combination with conventional wound care therapy. A wide variety of products are on the market providing different sequences of pressures which need to be validated concerning optimal performance in different clinical indications. This is especially important for pumps that were not specifically designed for the indication of arterial occlusive disease but rather for prophylaxis of deep vein thrombosis and for the treatment of lymphoedema.
Dogma: Low pressure is enough for ulcer healing? (presented by D. Milic)
Although many studies have proven the efficacy of compression therapy in the treatment of venous ulcer patients, there is still no agreement on what type of compression and sub‐bandage pressure values should be used in order to achieve the best possible healing results. Theoretically, an ideal compression device should have a relatively low pressure in a resting position, especially during bed rest, which is well tolerated by the patient. A higher pressure is required when patients stand up in order to compensate for the increase in the hydrostatic load. Clinical studies have shown that the effect of compression therapy in chronic venous insufficiency depends mainly on two factors: (i) the interface pressure of the fabric on the affected leg and (ii) on the elastic property (stiffness) of the material that determines the performance of the product during standing and walking.
There are some data favouring high compression pressure for better healing of VLUs and for keeping them healed 37, 38. However, a recent meta‐analysis has questioned this concept (39) by stating that ‘leg compression with stockings is clearly better than compression with bandages, has a positive impact on pain, and is easier to use’. This conclusion is in many ways doubtful because of several reasons: some studies were incorrectly analysed, and good compression stockings have been compared with inadequate bandages. Also, the vast majority of patients included in the reported studies had small ulcers (usually up to 5 cm2 and no larger than 10 cm2) and were of short duration. The natural history of VLUs is that they tend to close and open for many years. Perhaps a more convincing way to compare the efficacy of compression systems (and sub‐bandage pressure values) is to include long lasting and large venous ulcers in randomised controlled trials (RCTs), excluding ulcers which have risk factors for non‐healing such as fixed ankle joint and a small calf to ankle ratio indicating patients with impaired calf muscle pump. Therefore, it cannot be generalised that low pressure compression (stockings) could provide the same or even better results compared with systems that provide high sub‐bandage pressure in the treatment of VLUs. In a recent RCT (40), three different compression systems with three different sub‐bandage pressure values were compared: compression stockings alone versus the stockings with one and with two bandages. This study showed that the healing rate after 26 weeks was correlated with the sub‐bandage pressure: the highest healing rate (74%) was seen in group C (resting pressure 74 mmHg), while the lowest (25%) was seen in group A (resting pressure 36 mmHg) (Figure 5)
Figure 5.
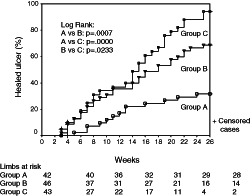
Cumulative healing rate of venous leg ulcers (VLUs) in three different compression systems with three different sub‐bandage pressure values (40). Group A: patients treated with Tubulcus stockings (36 mmHg); group B: patients treated with Tubulcus stockings + one elastic bandage (54 mmHg); group C: patients treated with Tubulcus stockings + two elastic bandages (74 mmHg).
From clinical experience and validated research (41), it is well known that patients with larger calf circumference (CC) need a much higher sub‐bandage pressure in order to achieve healing of a venous ulcer compared to patients with smaller CC. Therefore, it is prudent to determine compression system for the treatment of venous ulcers according to individual characteristics of the leg (CC) and venous ulcer itself (surface and duration). However, there are no data regarding this matter.
A simple formula was proposed that could be used as a starting point for establishing optimal sub‐bandage resting pressure (at B1 measuring point) of compression systems used for ulcer healing: sub‐bandage pressure value = CC + CC/2. The formula is suggested as a starting point in determining a compression system and sub‐bandage pressure value for the treatment of VLU that will have the best balance between the high pressure needed for VLU healing and non‐concordance due to bandage discomfort. The formula correlates well to the results obtained in the above mentioned study (40). One practical consequence would be that in future studies reporting the pressure of a compression device the circumference of the corresponding leg segment should be given as well.
Nevertheless, more RCT are needed in order to determine the optimal sub‐bandage pressure values and best treatment modalities in the treatment of VLUs.
Dogma: Venous ulcer healing is due to stiffness or pressure? (presented by J. P. Benigni)
Stiffness is defined as the increase of compression pressure induced by the increase of CC during muscle activity. This important parameter (‘stretch’) is measured by the producers of compression products in textile laboratories but often not declared. For bandages stiffness of the end‐product on the bandaged limb will vary depending on the amount of layers. Concerning compression stockings, only the product names are specified with the class of pressure. Interface pressures and stiffness in vivo are never specified.
Rather high pressure levels are recommended for healing venous ulcers 37, 40 and higher pressure seems also to be preferable for avoiding recurrence after ulcers are healed (38). Experimental data have shown that venous ejection fraction correlates significantly with compression pressure in the upright position, but also with the stiffness of the compression products used (10).
We do not know if it is the pressure or the stiffness of compression that plays a major role on the healing of the venous ulcers. Could stiff compression with a lower level of resting pressure be even superior by resulting in a better compliance?
Futures studies should clarify this question. Some specific points which should be considered:
Interface pressures and stiffness in vivo of tested compression devices should be specified in healthy subjects before starting clinical trials in patients with venous ulcers.
Clinical trials on healing of venous ulcers comparing a 30–40 mmHg compression with a low stiffness (stockings for instance) to a compression with the same interface pressure and a high stiffness.
Clinical trials on healing of venous ulcers comparing a 30–40 mmHg compression with a low stiffness (stockings for instance) to a compression with a low interface pressure (20–25 mmHg) and a high stiffness.
Dogma: Compression and lymphoedema: the higher pressure the better? (presented by R. Damstra and H. Partsch)
Up to now only a few studies have analysed a dose response relationship between compression pressure and oedema reduction (41). New trials measuring oedema reduction in relationship to the exerted pressure of compression products have indicated that there is obviously an upper pressure limit beyond which further increase of pressure seems contra productive. This upper limit is around 30–40 mm Hg of initial pressure exerted by inelastic bandages on the arm and around 50–60 mmHg on the lower extremity. This statement was based on the outcomes of two randomised controlled trials.
In one RCT on 36 patients with postmastectomy arm lymphoedema it was shown that classical inelastic, multicomponent bandages applied with a lower pressure (20–30 mmHg) were better tolerated and achieved the same amount of arm volume reduction as bandages applied with higher pressure (44–58 mmHg) in the first 24 h (42). In another RCT that studied 42 patients with chronic oedema of the leg, compression stockings (23–32 mmHg) were compared with inelastic bandages (pressure 53–88 mmHg). While stockings in the range between 20 and 40 mmHg showed a good correlation between exerted pressure and volume reduction, bandages applied with a resting pressure of more than 60 mm Hg resulted in a decreasing volume reduction (43).
The explanation for these findings may be based on a different threshold of efficacy of compression on the two main mechanisms of oedema reduction, which are the reduction of capillary filtration and an improvement of the lymphatic drainage. In clinical practice, comfort and ease of application, which have deciding importance for the compliance, will also favour lower compression pressures.
Dogma: Lipoedema cannot be improved by compression? (presented by G. Szolnoky)
According to a general view, fat tissue cannot be compressed and therefore its volume is barely reducible. Two studies seem to disagree with this concept 44, 45. Both studies found significant volume decrease due to compression supplemented with physiotherapy, but the results are insufficient to have a considerable impact on limb shape, cosmetic appearance and mobility.
Two major hallmarks of lipoedema are capillary fragility and spontaneous or minor injury provoked pain. Complex decongestive physiotherapy [manual lymph drainage, IPC (46), multicomponent and multilayered inelastic compression, skin care and walking exercise] is capable of reducing pain intensity (47) and capillary fragility (48).
Another dogma is that lipoedematous legs do not tolerate high compression pressure.
Some anecdotal reports argue that high compression pressure does not work in lipoedema because patients can only tolerate low pressure. To explore this controversy a retrospective cohort study was conducted to compare the compliance of wearing pantyhose with 23–32 mmHg (lower) and 34–46 mmHg (higher) pressures. Seventeen patients wearing lower pressure pantyhose and 22 patients wearing higher pressure pantyhose were included. Interface pressure measurement was performed at B1 in each limb and subjective perceptions were assessed with chronic venous insufficiency questionnaire (CIVIQ) and a visual analogue scale (VAS) (47). Compliance was assessed based on the patients' answer in the questionnaire: ‘How many days a week do you wear your panty in average?’. The resulting values did not discriminate between the two study groups.
There was a significant difference between mean interface pressure values of the two groups. None of the median scores of the identical CIVIQ items and mean VAS scores showed a difference between lower and higher pressure stocking groups. Unlike the dogma, this clinical trial supports the experience that lipoedema patients can tolerate both lower and higher pressures.
In the ensuing discussion Schingale pointed out that compression, but not manual lymph drainage, is able to reduce lipoedema. In general it may be stated that lymphoedema and lipoedema are domains where clinical studies are urgently needed to put widely performed traditional treatment strategies on a more scientific basis. Proposals concerning compression therapy trials in lymphoedema of the upper extremities and chronic oedema of the legs have been formulated in previous International Compression Club consensus meetings 49, 50, 51.
In conclusion, several unproven dogmas are conveyed in compression therapy, which need to be clarified in future examinations. These should not only include randomised controlled trials concentrating on clinical outcomes but should also consider experimental study designs comparing conventional compression devices with new innovative modalities.
Acknowledgements
The meeting was financially supported by companies manufacturing compression materials whose delegates were present at the meeting and contributed to presentations and discussions according to the constitutions of the ICC. We wish to thank the following companies for their presence at the meeting and their support: 3M, ACI Medical, Activa Healthcare, Arjo Huntleigh, Bauerfeind, BSN, Circaid, Cizeta Medica, Innothera, Karl Otto Braun, Lohmann‐Rauscher, Medi, Pierre Fabre, Sigvaris, Thuasne, Urgo, Uriel PG and Varitex.
References
- 1. Uhl JF. 3D multislice CT to demonstrate the effects of compression therapy. Int Angiol 2010;29:411–5. [PubMed] [Google Scholar]
- 2. Partsch H, Mosti G, Mosti F. Narrowing of leg veins under compression demonstrated by magnetic resonance imaging (MRI). Int Angiol 2010;29:408–10. [PubMed] [Google Scholar]
- 3. Downie SP, Firmin DN, Wood NB, Thom SA, Hughes AD, Wolfe JN, Xu XY. Role of MRI in investigating the effects of elastic compression stockings on the deformation of the superficial and deep veins in the lower legs. J Magn Reson Imaging 2007;26:80–5. [DOI] [PubMed] [Google Scholar]
- 4. Mosti G, Partsch H. Compression stockings with a negative pressure gradient have a more pronounced effect on venous pumping function than graduated elastic compression stockings. Eur J Vasc Endovasc Surg 2011;42:261–6. [DOI] [PubMed] [Google Scholar]
- 5. Couzan S, Prufer M, Ferret JM, Mismetti P, Pouget JF. A new concept of support‐compression: application of colour echo‐doppler, with venous pressure measurements and MRI. Phlébologie 2002;55:159–70. [Google Scholar]
- 6. Couzan S, Assante C, Laporte S, Mismetti P, Pouget JF. Booster study: comparative evaluation of a new concept of elastic stockings in mild venous insufficiency. Presse Med 2009;38:355–61. [DOI] [PubMed] [Google Scholar]
- 7. Garreau C, Pibourdin JM, Nguyen Le C, Boisseau MR. Elastic compression in golf competition. J Mal Vasc 2008;33:250–1. [DOI] [PubMed] [Google Scholar]
- 8. Sigel B, Edelstein AL, Savitch L, Hasty JH, Felix WR Jr. Type of compression for reducing venous stasis: a study of lower extremities during inactive recumbency. Arch Surg 1975;110:171–5. [DOI] [PubMed] [Google Scholar]
- 9. Chauveau M, Gelade P, Cros F. The venous return simulator: comparison of simulated with measured ambulatory venous pressure in normal subjects and in venous valve incompetence. Vasa 2011;40:205–17. [DOI] [PubMed] [Google Scholar]
- 10. Mosti G, Mattaliano V, Partsch H. Inelastic compression increases venous ejection fraction more than elastic bandages in patients with superficial venous reflux. Phlebology 2008;23:287–94. [DOI] [PubMed] [Google Scholar]
- 11. Partsch H, Clark M, Bassez S, Benigni JP, Becker F, Blazek V, Caprini J, Cornu‐Thénard A, Hafner J, Flour M, Jünger M, Moffatt C, Neumann M. Measurement of lower leg compression in vivo: recommendations for the performance of measurements of interface pressure and stiffness. Dermatol Surg 2006;32:224–33. [DOI] [PubMed] [Google Scholar]
- 12. Partsch H, Clark M, Mosti G, Steinlechner E, Schuren J, Abel M, Benigni JP, Coleridge‐Smith P, Cornu‐Thénard A, Flour M, Hutchinson J, Gamble J, Issberner K, Juenger M, Moffatt C, Neumann HA, Rabe E, Uhl JF Zimmet S. Classification of compression bandages: practical aspects. Dermatol Surg 2008;34:600–9. [DOI] [PubMed] [Google Scholar]
- 13. Thomas S. Compression bandaging in the treatment of venous leg ulcers. URL http://www.worldwidewounds.com/1997/september/Thomas‐Bandaging/bandage‐paper.html.http://www.worldwidewounds.com/ [accessed 29 March 2001].
- 14. Haute Autorité de Santé. Recommandations pour la pratique clinique. Managing venous leg ulcers (excluding dressings) June 2006 (Prise en charge de l'ulcère de jambe à prédominance veineuse hors pansement. Recommandations. juin 2006). URL www.has‐sante.fr [accessed on June 2006].
- 15. Mosti G, Partsch H. Inelastic bandages maintain their hemodynamic effectiveness over time despite significant pressure loss. J Vasc Surg 2010;52:925–31. [DOI] [PubMed] [Google Scholar]
- 16. Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three‐dimensional substrates. J Cell Physiol 1998;175:323–32. [DOI] [PubMed] [Google Scholar]
- 17. Chen AH, Frangos SG, Kilaru S, Sumpio BE. Intermittent pneumatic compression devices: physiological mechanisms of action. Eur J Vasc Endovasc Surg 2001;21:383–92. [DOI] [PubMed] [Google Scholar]
- 18. NHS Clinical Knowledge Summaries (CSK). Venous eczema and lipodermatosclerosis: management. URL http://www.cks.nhs.uk/venous_eczema_and_lipodermatosclerosis‐341190. [accessed on October 2008]
- 19. Partsch H, Flour M, Coleridge Smith P, Benigni JP, Cornu‐Thénard A, Delis K, Gniadecka M, Mariani F, Mosti G, Neumann HAM, Rabe E, Uhl F, Benhamou AC, Brandjes D, Cavezzi A, Clark M, Schuren J. Indications for compression therapy in venous and lymphatic disease: consensus based on experimental data and scientific evidence. Int Angiol 2008;27:193–219. [PubMed] [Google Scholar]
- 20. Vandongen YK, Stacey MC. Graduated compression elastic stockings reduce lipodermatosclerosis and ulcer recurrence. Phlebology 2000;15:33–7. [Google Scholar]
- 21. Burnand K, Clemenson G, Morland M, Jarrett PEM, Browse NL. Venous lipodermatosclerosis: treatment by fibrinolytic enhancement and elastic compression. BMJ 1980;280:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lentner A, Späth F, Wienert V. Modification of the flexibility of the upper and lower ankle joint by medical compression stockings. Vasa 1996;25:60–4. [PubMed] [Google Scholar]
- 23. Wilson M. How to use wool padding with compression bandaging. Wound Essentials 2008;3:61–5. [Google Scholar]
- 24. Mosti G, Mattaliano V, Partsch H. Influence of different materials in multicomponent bandages on pressure and stiffness of the final bandage. Dermatol Surg 2008;34:631–9. [DOI] [PubMed] [Google Scholar]
- 25. Wong IKY. Assessing the value of a leg ulcer education programme in Hong Kong. J Wound Care 2003;12:17–9. [DOI] [PubMed] [Google Scholar]
- 26. Moers N, Kraan van der A, Kuijper Y. Comparative pilot study evaluating two types of under padding applied with short stretch compression bandages. Brussels: Poster EWMA, 2011. [Google Scholar]
- 27. Schuren J. Compression unravelled. Germany: Margreff Druck GmbH, Essen, 2011. [Google Scholar]
- 28. Hopf HW, Ueno C, Aslam R, Burnand K, Fife C, Grant L, Holloway A, Iafrati MD, Mani R, Misare B, Rosen N, Shapshak D, Benjamin Slade J Jr. West J, Barbul A. Guidelines for the treatment of arterial insufficiency ulcers. Wound Repair Regen 2006;14: 693–710. [DOI] [PubMed] [Google Scholar]
- 29. World Union of Wound Healing Societies (WUWHS). Principles of best practice: compression in venous leg ulcers: a consensus document. London: MEP Ltd, 2008. [Google Scholar]
- 30. Mosti G, Iabicella ML, Partsch H. Compression therapy in mixed ulcers increases venous output and arterial perfusion. Vasc Surg 2011;55:122–8. [DOI] [PubMed] [Google Scholar]
- 31. Schuren J, Vos A, Allen JO. Venous leg ulcer patients with low ABPIs: how much pressure is safe and tolerable? EWMA J 2010;10:29–34. [Google Scholar]
- 32. Cornu‐Thenard A, Boivin P, Carpentier PH, Courtet F, Ngo P. Superimposed elastic stockings: pressure measurements. Dermatol Surg 2007;33:269–75. [DOI] [PubMed] [Google Scholar]
- 33. Callam MJ, Ruckley CV, Dale JJ, Harper DR. Hazards of compression treatment of the leg: an estimate from Scottish surgeons. BMJ 1987;295:1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delis KT, Nicolaides AN, Cheshire NJW, Wolfe JHN. Improvement in walking ability, ankle pressure indices and quality of life in vascular claudication using intermittent pneumatic foot and calf compression: a randomized controlled trial. Br J Surg 2002;88:605–6. [Google Scholar]
- 35. Van Bemmelen P, Char D, Giron F, Ricotta JJ. Angiographic improvement after rapid intermittent compression treatment (ArtAssist®) for small vessel obstruction. Ann Vasc Surg 2003;17: 224–8. [DOI] [PubMed] [Google Scholar]
- 36. Sultan S, Hamada N, Soylu E, Fahy A, Hynes N, Tawfick W. Sequential compression biomechanical device in patients with critical limb ischemia and non‐reconstructable peripheral vascular disease. J Vasc Surg 2011;54:440–7. [DOI] [PubMed] [Google Scholar]
- 37. O'Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev 2009; Issue 1. Art. No.: CD000265. DOI: 10.1002/14651858.CD000265.pub2.. [DOI] [PubMed] [Google Scholar]
- 38. Nelson EA, Bell‐Syer SE, Cullum NA. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2000;4:CD002303. [DOI] [PubMed] [Google Scholar]
- 39. Amsler F, Willenberg T, Blättler W. In search of optimal compression therapy for venous leg ulcers: a meta‐analysis of studies comparing diverse bandages with specifically designed stockings. J Vasc Surg 2009;50:668–74. [DOI] [PubMed] [Google Scholar]
- 40. Milic DJ, Zivic SS, Bogdanovic DC, Jovanovic MM, Jankovic RJ, Milosevic ZD, Stamenkovic DM, Trenkic MS. The influence of different sub‐bandage pressure values on venous leg ulcers healing when treated with compression therapy. J Vasc Surg 2010;51:655–61. [DOI] [PubMed] [Google Scholar]
- 41. Margolis D, Berlin J, Strom B. Which venoous leg ulcers will heal with limb compression bandages? Am J Med 2000;109:15–9. [DOI] [PubMed] [Google Scholar]
- 42. Vanscheidt W, Ukat A, Partsch H. Dose‐response of compression therapy for chronic venous edema: higher pressures are associated with greater volume reduction: two randomized clinical studies. J Vasc Surg 2009;49:395–402. [DOI] [PubMed] [Google Scholar]
- 43. Damstra RJ, Partsch H. Compression therapy in breast cancer related lymphoedema: a randomized controlled comparative study of relation between volume and interface pressure changes. J Vasc Surg 2009;49:1256–63. [DOI] [PubMed] [Google Scholar]
- 44. Mosti G, Picerni P, Partsch H. Compression stockings with moderate pressure are able to reduce chronic leg oedema. Phlebology 2011. [Epub ahead of print 16 November 2011]. [DOI] [PubMed] [Google Scholar]
- 45. Deri G, Weissleder H. Vergleichende prä‐ und posttherapeutische Volumenmessungen in Beinsegmenten beim Lipödem. Lymph Forsch 1997;1:35–7. [Google Scholar]
- 46. Szolnoky G, Borsos B, Barsony K, Balogh M, Kemeny L. Complete decongestive physiotherapy of lipedema with or without pneumatic compression: a pilot study. Lymphology 2008;41:50–2. [PubMed] [Google Scholar]
- 47. Szolnoky G, Varga E, Varga M, Dósa‐Rácz É, Kemény L. Complex decongestive physiotherapy decreases pain intensity in lipedema. Lymphology 2011;44:178–82. [PubMed] [Google Scholar]
- 48. Szolnoky G, Nagy N, Kovacs RK, Dósa‐Rácz É, Szabó A, Bársony K, Balogh M, Kemény L. Complex decongestive physiotherapy decreases capillary fragility in lipedema. Lymphology 2008;41:54–6. [PubMed] [Google Scholar]
- 49. Partsch H, Stout N, Forner‐Cordero I, Flour M, Moffatt C, Szuba A, Milic D, Szolnoky G, Brorson H, Abel M, Schuren J, Schingale F, Vignes S, Piller N, Döller W. Clinical trials needed to evaluate compression therapy in breast cancer related lymphedema (BCRL): proposals from an expert group. Int Angiol 2010;29:442–53. [PubMed] [Google Scholar]
- 50. Rabe E, Partsch H, Jünger M, Abel M, Achhammer I, Becker F, Cornu‐Thenard A, Flour M, Hutchinson J, Issberner K, Moffatt Ch, Pannier F. Guidelines for clinical studies with compression devices in patients with venous disorders of the lower limb. Eur J Vasc Endovasc Surg 2008;35:494–500. [DOI] [PubMed] [Google Scholar]
- 51. Stout N, Partsch H, Szolnoky G, Forner‐Cordero I, Mosti G, Flour M, Damstra R, Piller N, Geyer MJ, Benigni JP, Moffat C, Cornu‐Thenard A, Schingale F, Clark M, Chaveau M. Chronic oedema of the lower extremities: international consensus recommendations for compression therapy clinical research trials. Int Angiol. In press. [PubMed] [Google Scholar]


