Abstract
The purpose of this prospective experimental and clinical study is to evaluate the effectiveness of the intralesional injection of platelet‐rich plasma (PRP), in the management of non‐healing chronic wounds. Skin defects were created in the ears of 20 white New Zealand rabbits. In the study group, autologous PRP was injected intralesionally. The control group was treated conservatively. Nineteen out of 20 cases of the study group healed within a mean time of 24·9 days. In the control group, seven defects healed within a mean period of 26·7 days, seven ulcers did not heal at day 28 and in six cases a full thickness ear defect was recorded. For a 3‐year period, 26 patients with chronic ulcers underwent surgical debridement and intralesional injection of PRP. A histological study was performed before and 7 days after PRP injection. Ten patients healed within a mean period of 7 weeks. In 16 cases, PRP prepared the wound bed for the final and simpler reconstructive procedure. Intralesional injection is a newly described method for application of PRP and represents an effective therapeutic option when dealing with non‐healing wounds.
Keywords: Chronic ulcer, Intralesional injection, Platelet‐rich plasma
Introduction
Chronic ulcers are wounds characterised by a delay of the normal healing process. Falanga suggested the term ‘impaired or weak healing process' to describe the non‐healing mechanism in chronic ulcers, instead of using the previous definition of a ‘healing failure’(1). Treatment of chronic ulcers may be conservative or surgical. The purpose of the conservative management is either the complete healing of the ulcer or the preparation of the wound bed for a successful final surgical intervention.
The goal in the treatment of chronic ulcers is to control local infection and improve tissue vascularity. Over the last years, new technologies allowed the manufacture and local application of autologous platelet components as accelerators of the healing cascade. Platelet‐rich plasma (PRP) plays a key role in the treatment of chronic ulcers and has already been applied in several clinical situations.
PRP is a plasma concentrate of the patient's blood that predominantly contains platelets. Various terms have been used in the literature to describe it, such as: ‘concentration of platelets', ‘platelet gel’ or ‘releasate of platelets' 2, 3. In the PRP, apart from a high concentration of platelets, an increase in all coagulation factors may also be recorded. Activated platelets release different growth factors that contribute to cell migration, proliferation, differentiation, angiogenesis, removal of tissue debris and regeneration of the appropriate type of tissues 4, 5, 6.
The α granules of the platelets contain a large number of proteins, also called secretion proteins, that influence wound healing. They belong to the group of growth factors, namely cytokines and chemokines and include platelet‐derived growth factors (PDGF‐aa, ‐bb and ‐ab isomeric), transforming growth factors‐b (TGF‐b, ‐b1 and ‐b2 isomeric), interleukin‐1, platelet‐derived angiogenesis factor, vascular endothelial growth factor, epidermal growth factor, platelet‐derived endothelial growth factor, epithelial cell growth factor, insulin‐like growth factor, fibronectin and others 5, 7, 8, 9, 10, 11, 12, 13.
Platelet activation leads to the binding of the α granules to the platelet membrane, which is followed by the activation of some secretion proteins (e.g. PDGF, TGF‐b) with the intervention of histones and carbohydrate chains 9, 12. The activated proteins are then secreted and linked to the membrane receptors of the target cells (e.g. mesenchymal stem cells, osteoblasts, fibroblasts, endothelial cells and epidermal cells), activating a specific intracellular protein, which, in turn, causes the gene sequence leading to cellular proliferation, extracellular matrix formation and collagen composition 4, 9.
It has been shown that the concentration of platelets is strongly related to the levels of secretion proteins and growth factors, as well as the proliferation rate of cells during the process of cicatrisation 3, 9, 14. However, many authors believe that PRP application may be effective only when the concentration of platelets in the plasma is increased by at least three to five times above the normal rate 3, 9, 14, 15, 16, 17.
Clinical applications of PRP have already been described in craniofacial and maxillofacial surgery, orthopaedics, spine surgery, as well as in wound healing, as described in previous studies 2, 3, 10, 11, 14, 15, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27. According to published data concerning the use of PRP in the management of chronic ulcers, PRP is usually applied on the surface of the wound, where the activated growth factors are expected to affect positively the progress of the healing process. Many authors propose the addition of 5000 IU of bovine thrombin and 10% calcium citrate in order to activate platelets within the concentrated PRP and release growth factors 11, 15, 21, 22, 23, 24, 25. Others advocate that the contact of the PRP with collagen and endothelial cells of the wound bed can equally lead to the activation of growth factors (28).
In this study, we propose a new PRP application technique. Performing an intralesional injection of the plasma at the bed and edges of the chronic wound, we believe that there is minimal risk of plasma loss, while the regular change of dressings and observation of the wound can be safely performed. The process can accelerate the healing phase and may control any signs of wound infection. Initially, we applied the technique in an experimental study and afterwards we performed a clinical study.
Experimental study
Materials and methods
An overall number of 20 white New Zealand rabbits (10 male and 10 female), weighing between 3 and 4·2 kg (average 3·5 kg) were used in our study. General anaesthesia with intramuscular injection of xylazine (5 mg/kg) and ketamine (35 mg/kg) was applied to each rabbit, ensuring instant induction and maintenance of the anaesthesia (29). We prepared the external auricular surface, by shaving any hair and applying alcohol solution as antiseptic and vasodilator. From the central artery of either the right or left ear, we drew 7 ml of blood/kg (average 24·5 ml blood per rabbit) using a 22G venflon (30). In ten cases, blood was drawn from the right auricle and in ten cases from the left. Using the Magellan‐Medtronic system, we centrifuged the blood for approximately 11 minutes, and obtained 3 ml of autologous PRP. For the quantitative measurement of the platelets concentration, we analysed 0·5 ml of the received PRP.
At the same time of centrifugation, we created skin defects over the external surface of the rabbits' pinna using a No. 15 blade, simultaneously to both ears and under the same conditions (Figure 1). Dimensions of the defects were 2 × 2 cm, involving the full thickness of soft tissues up to the level of the perichondrium. This was recorded as day 0 of the ulcer study.
Figure 1.
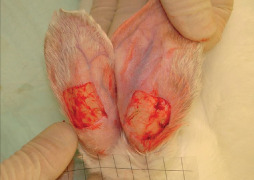
Skin defects up to the level of the perichondrium, measuring 2 × 2 cm at the external surface of the rabbits' pinna.
Ulcers of the rabbits' right ears constituted the ‘study group’ (n = 20), while the left ear ulcers (n = 20) constituted the ‘control group’.
Ulcers of the study group were treated using local treatment with PRP as following: we injected autologous PRP at the margins of the ulcer in equal distribution (Figure 2) and covered it with a transparent sterile self‐adhesive dressing of polyurethane (Tegaderm™, 3M, Marousi, Greece). The wound remained intact for 3 days; on day 4, the wound was gently washed out with normal saline and a Tegaderm™ dressing was applied again. Change of dressings continued regularly every other day for a period of 4 weeks. Ulcers of the control group were treated with regular change of dressings, washing of the wound with normal saline gauze and application of Tegaderm™ dressings, every other day for an equal period of the 4 weeks.
Figure 2.
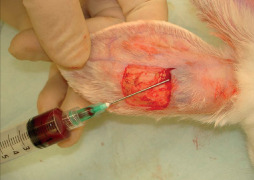
Injection of autologous platelet‐rich plasma at the margins of the ulcer.
The healing process was being recorded throughout this 4‐week period, up to day 28, which was considered the end of our macroscopic study. The morphometric characteristics of the defect area were again recorded and animals underwent a second operative procedure under general anaesthesia in order to get specimens for histological evaluation; tissue specimens were taken from the healed area or the edges of the remaining ulcer, if any. During histological examination we studied the presence of epidermis, ear's cartilage, fibroblasts and angiogenesis.
Results
In the 4‐week period, we had no rabbit loss, nor major complication occurred. Local infection of the ulcers was not recorded either.
Analysis of the PRP
The quantitative analysis of the platelet's concentration showed a significant increase (×7 −×25) in the number of platelets in the analysed PRPs. Specifically, although the initial concentrations of platelets in the blood varied from 155 000/ml to 519 000/ml (average 236 000/ml), the number of platelets in the PRP reached 1 730 000/ml to 6 909 000/ml (average 3 214 000/ml).
Macroscopical results
Four weeks after the intralesional injection of PRP, the macroscopic results in the healing phase of the ulcers of the ‘study group’ were the following: 19 of 20 ulcers were completely healed and new epithelium covered the surface of the ulcer. Completion of the healing procedure ranged from 21 to 28 days (average 24·9 days). In only one case, a delay of the healing process was documented, without, however, any signs of complication.
In the ‘control group’, we documented the following: seven ulcers had completely healed within the 4 weeks of the study; another seven were still under the healing process with no epithelialisation present over the wound bed, while in the remaining six ulcers a deterioration of the ulcer was noted. In those cases, the underlying cartilage and the soft tissue of the internal surface of the ear were affected, leading to a full thickness defect (Figure 3). The healing process for these wounds continued with contraction (Table 1).
Figure 3.
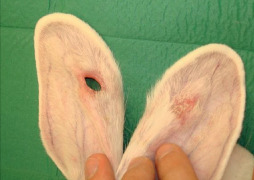
The ulcer at the right (study) ear of the rabbit is completely healed, while the ulcer at the left (control) ear led to a full thickness defect.
Table 1.
Experimental study: results of the healing status at day 28
| Number of rabbits | Sex | Weight (kg) | Study group Day of healing completion (post‐platelet‐rich plasma injection) | Control group Day of healing completion (after the creation of the wound) | |
|---|---|---|---|---|---|
| 1 | Female | 4 | Day 25 | Day 28 | |
| 2 | Female | 3·5 | Day 23 | Not healed at day 28 | |
| 3 | Female | 3·3 | Day 24 | Full thickness sequel (at day 28) | |
| 4 | Male | 3 | Day 21 | Full thickness sequel (at day 28) | |
| 5 | Male | 3·5 | Day 22 | Full thickness sequel (at day 28) | |
| 6 | Female | 3·6 | Day 23 | Full thickness sequel (at day 28) | |
| 7 | Male | 3·3 | Day 25 | Not healed at day 28 | |
| 8 | Male | 3·5 | Day 28 | Day 28 | |
| 9 | Female | 3·3 | Day 26 | Full thickness sequel (at day 28) | |
| 10 | Female | 3·8 | Day 25 | Full thickness sequel (at day 28) | |
| 11 | Male | 3 | Day 28 | Full thickness sequel (at day 28) | |
| 12 | Male | 4·1 | Day 27 | Day 27 | |
| 13 | Male | 4·2 | Day 26 | Not healed at day 28 | |
| 14 | Male | 3·8 | Day 26 | Day 26 | |
| 15 | Male | 3·7 | Not healed at day 28 | Day 27 | |
| 16 | Male | 3·9 | Day 26 | Day 25 | |
| 17 | Female | 3·5 | Day 24 | Not healed at day 28 | |
| 18 | Female | 3·6 | Day 25 | Not healed at day 28 | |
| 19 | Female | 3·4 | Day 24 | Not healed at day 28 | |
| 20 | Female | 3·5 | Day 26 | Day 26 |
Histological results
Epidermis
It was present in all 19 healed ulcers in the study group and only in one case epithelial coverage was not recorded. In the control group, epidermis was normal in four cases, and moderate or hyperplastic in three cases; in seven cases no epidermis was recorded over the wound and in six cases a full thickness defect had developed.
Cartilage
It was present with a normal appearance in 17 cases of the study group while hypoplasia was documented only in three cases. In the control group, cartilage absence was recorded in 12 cases while in 8 cases localised cartilage appearance was recorded (Figure 4).
Figure 4.
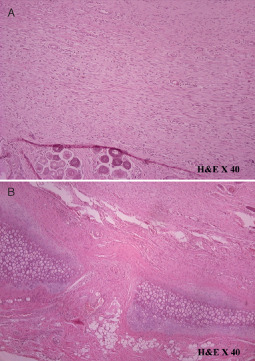
(A) Histological result of a ‘study’ specimen suggested organised interconnecting collagen fibres running parallel to each other. (B) Specimen of the ‘control’ group showed absence of cartilage.
Vessels
In the study group, small vessels were apparent in ten cases and moderate or large vessels in the remaining ten specimens. In the control group, only a few vessels were present and low or moderate angiogenesis was recorded in most (n = 14) ulcer areas.
Fibroblasts
In the study group, ten cases displayed organised interconnecting collagen fibres running parallel to each other and to the epidermis (Figure 4). In the remaining ten cases, a moderate collagen architecture or absence of the fibres orientation was recorded. In the control group, only three cases showed moderate fibre architecture. The remaining 11 specimens showed disorganised collagen fibres at various angles to each other and to the epidermis.
Clinical study
Patients
Our study included 26 patients (16 men and 10 women), who have been treated for chronic ulcers in our department over a period of 3 years. The mean age of our patients was 59 years (varying from 22 to 83 years). All patients presented a non‐healed ulcer for a time period varying between 1 and 13 months (average 4·6 months). Ulcers were located on the lower limb (15 patients), the trunk (9 patients), the upper limb (1 patient) and the head (1 patient). The size of the defects varied from 2 to 150 cm2. In 16 cases, exposure of the underlying tendons (n = 3) or bones (n = 13) was recorded. As far as aetiology is concerned, 13 ulcers resulted from a mechanical trauma, while 2 were related to thermal injury; five patients of our series presented a pressure sore and another six a postoperative dehiscence of a surgical wound.
The morphometric characteristics of the chronic ulcers were similar to those of a pressure sore and therefore we decided to use the staging system of the European Pressure Ulcer Advisory Panel (EPUAP) and Shea's classification (31) to stage the ulcers of our patients. According to the EPUAP and Shea's classification, 9 ulcers were classified as stage III and 17 ulcers as stage IV.
Thirteen of our patients were diabetic and ten suffered from an occlusive vascular disease that was already managed by vascular surgeons.
Although all patients had previously received some treatment for their ulcers, either conservative or surgical, complete healing had not been achieved; treatment modalities included conventional changes of dressings in 21 patients and surgical debridement followed by skin grafting in five cases. The majority of our patients (n = 20) were initially treated by general practitioners or general surgeons, who then referred them to our department.
At presentation and before starting any treatment, all wounds were evaluated clinically and examined both microbiologically and histologically in order to record the wound bed characteristics and exclude any neoplasia. In 19 of 26 cases, wound cultures confirmed the presence of some bacteria, that is Pseudomonas, Staphylococcus aureus or epidermis, and Escherichia coli, all having a bacterial burden greater than 105. In all cases, infection was limited to the wound area and no systemic symptom was noted.
Histological specimens were sectioned at 3‐µm thickness and stained for haematoxylin–eosin and masson trichrome. The results showed the presence of intense acute inflammation and irregular architecture of collagen fibres and fibroblasts; malignancy of the ulcer elements was excluded.
Method
Our patients were divided into two groups, according to the ulcer characteristics, that is size and stage of the lesion. Group A consisted of patients with stage III and any size of the ulcer and patients with stage IV ulcers sized up to 10 cm2 (n = 10). Group B consisted of patients presenting more severe lesions (stage IV ulcers), larger than 10 cm2 (n = 16). According to our protocol, we performed a conservative treatment with intralesional injection of autologous PRP within the ulcers of group A. For the group B ulcers, we planned a plastic surgical procedure with skin graft or flap, depending on the defect's characteristics. Prior to surgery of the group B patients, and in order to prepare the wound bed, we injected PRP at the edges and the wound bed of the chronic ulcer (Table 2).
Table 2.
Clinical study: patients demographics and results after the intralesional injection of platelet‐rich plasma
| Number | Sex | Age | Etiology | Ulcer location | Ulcer size (cm2) | Time period of unhealed ulcer (months) | Stage I–IV | Group | Treatment plan | Final procedure/healing | Follow‐up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 63 | Pressure sore | LL | 9 | 3 | IV | A | Conservative | Secondary intention | 12 |
| 2 | Female | 62 | Wound dehiscence | T | 5 | 9 | III | A | Conservative | Secondary intention | 18 |
| 3 | Female | 56 | Trauma | LL | 4 | 5 | IV | A | Conservative | Secondary intention | 20 |
| 4 | Male | 47 | Burn | LL | 4 | 2 | III | A | Conservative | Secondary intention | 20 |
| 5 | Female | 56 | Trauma | LL | 8 | 5 | III | A | Conservative | Secondary intention | 14 |
| 6 | Male | 57 | Trauma | LL | 4 | 2 | III | A | Conservative | Secondary intention | 24 |
| 7 | Female | 55 | Trauma | LL | 2 | 4 | III | A | Conservative | Secondary intention | 6 |
| 8 | Male | 63 | Trauma | LL | 50 | 4 | III | A | Conservative | Secondary intention | 16 |
| 9 | Female | 43 | Wound dehiscence | T | 4 | 3 | III | A | Conservative | Secondary intention | 6 |
| 10 | Female | 80 | Pressure sore | T | 38 | 6 | III | A | Conservative | Secondary intention | 4 |
| 11 | Male | 50 | Trauma | LL | 78 | 13 | IV | B | Flap | SSG | 20 |
| 12 | Male | 22 | Trauma | Upper limb | 24 | 3 | IV | B | Flap | SSG | 26 |
| 13 | Male | 62 | Wound dehiscence | T | 24 | 1 | IV | B | SG | SSG | 9 |
| 14 | Female | 83 | Wound dehiscence | T | 24 | 4 | IV | B | Flap | Full thickness skin graft | 22 |
| 15 | Female | 77 | Pressure sore | T | 150 | 7 | IV | B | Flap | Flap | 6 |
| 16 | Male | 61 | Burn | T | 37 | 1 | IV | B | Flap | SSG | 12 |
| 17 | Male | 54 | Trauma | LL | 16 | 6 | IV | B | Flap | Flap | 12 |
| 18 | Male | 65 | Trauma | LL | 19 | 3 | IV | B | SG | SSG | 6 |
| 19 | Male | 47 | Trauma | Head | 21 | 7 | IV | B | Flap | SSG | 18 |
| 20 | Male | 57 | Pressure sore | T | 82 | 6 | IV | B | Flap | Flap | 20 |
| 21 | Female | 72 | Trauma | T | 60 | 4 | IV | B | SG | SSG | 18 |
| 22 | Male | 58 | Wound dehiscence | LL | 39 | 3 | IV | B | SG | SSG | 14 |
| 23 | Male | 61 | Trauma | LL | 10 | 6 | IV | B | Flap | SSG | 24 |
| 24 | Female | 79 | Pressure sore | LL | 110 | 4 | IV | B | Flap | Flap | 1 |
| 25 | Male | 56 | Wound dehiscence | LL | 32 | 2 | IV | B | SG | SSG | 9 |
| 26 | Male | 51 | Trauma | LL | 22 | 3 | IV | B | Flap | Flap | 8 |
Secondary intention, healing with secondary intention; LL, lower limb; SG, skin graft; SSG, split skin graft; T, trunk.
For the following 2 weeks, all wounds underwent regular change of dressings and at the end of that period, a reevaluation of the chronic wound was performed. Specifically and according to our pretreatment records, we hypothesised that 10 of the 26 patients (group A) would be treated only with PRP, with no need of any further intervention. For the patients of group B, 5 patients would undergo skin graft and 11 patients local or distant flap coverage.
For ‘intralesional’ application of PRP, we drew 52 ml of blood from the peripheral vein of all patients, to which 8 ml of citreous ACD‐A of anticoagulant was added. Then, using the closed sterilised centrifuge circuit (Magellan‐Medtronic, Minneapolis, MN), we obtained 3 ml of concentrated platelets. A small quantity of this product was examined to determine the quantity of the elements. It was found that the number of platelets was increased by an average of 6·7 times, with a mean of 1 966 000/ml.
After meticulous wound debridement, PRP was injected in an equal distribution at the wound edges and bed of the chronic ulcers (Figure 5). The wound was then covered with Tegaderm™ dressing, which remained intact for 3 days; on day 4, we performed the first change of dressings, using gauze saline and application of a new Tegaderm™ dressing. The same method for change of dressings was repeated every day. On day 7 after the injection, tissue specimens were collected and microbiology and histology status were examined. The change of dressings was carried out in the same way, until the end of the second week, when a reevaluation of the ulcer was carried out. At that time, we determined any further – conservative or surgical – treatment modality for the management of the wound.
Figure 5.
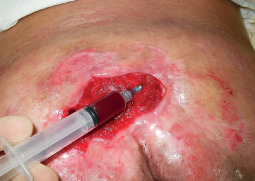
Intralesional injection of platelet‐rich plasma in an equal distribution at the wound edges and bed of the ulcer.
Results
On day 7 after the application of PRP, all 26 ulcer patients were found to be macroscopically improved with an intense presence of granulation tissue, reduction of the depth of the wound and epithelial deposition at the edges of the wounds (Figure 6).
Figure 6.
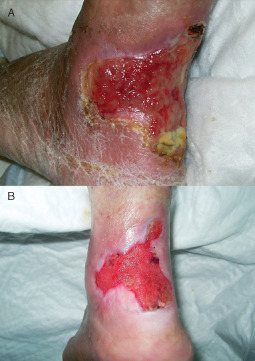
Chronic ulcer of the lower limb before (A) and 7 days after the platelet‐rich plasma injection (B) with a macroscopic improved lesion; intense presence of granulation tissue, reduction of the depth of the wound and epithelial deposition at the edges of the wounds.
The results of the histological examination that took place in the first week after PRP injection, confirmed the clinical findings showing decreased additions of elements of acute inflammation, an intense presence of fibroblasts in organised provision and a better orientation of collagen fibres (7, 8). Microbiology results after the application of the PRP also showed a reduction of the bacterial load. While, before the application of PRP, positive cultures (>105) were found in 19 patients, 1 week after treatment, the culture in 10 of 19 patients was negative or less than 105 without the use antibiotics.
Figure 7.
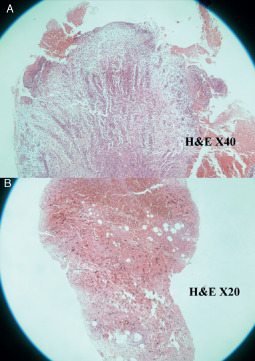
Ulcer of the epidermis before platelet‐rich plasma (PRP) treatment with a severe collagen orientation (A) [×40 haematoxylin and eosin (H & E)] and 7 days after PRP intralesional injection (B) (×20 H& E). A better orientation of collagen at the deep area is documented.
Figure 8.
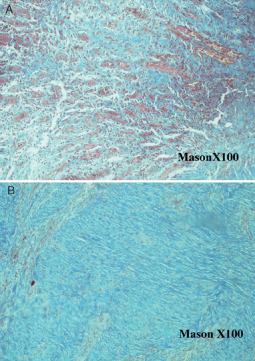
Tissue of the ulcer before platelet‐rich plasma (PRP) treatment with a severe collagen orientation (A) (×100 mason) and 7 days after PRP intralesional injection (B) (×100 mason). A better orientation of collagen is documented.
On the second week after the intralesional injection of PRP, reevaluation of the ulcers resulted in the following findings: all ten patients of group A, initially estimated to be candidates for conservative treatment, presented satisfactory clinical progress of the healing process and were offered continuation of regular change of dressings. The group B patients, where a surgical treatment was initially planned, presented a clinical improvement of the wound characteristics after PRP injection, that is while, before treatment, 5 patients had been considered candidates for skin graft and 11 for flap coverage, PRP injection led to a spectacular improvement of the local conditions of the ulcers, with growth of abundant granulation tissue and satisfactory coverage of the exposed bones and tendons. In these cases, flap reconstruction was not considered necessary; reconstruction with a skin graft was proved to be effective.
Finally, group A ulcers were successfully treated conservatively, with regular change of dressings after the initial intralesional PRP injection, and showed complete healing within a mean period of 7 weeks (Figure 9). In group B only 5 patients underwent a surgical intervention with local or distant flap, while a full or split‐thickness skin graft was placed to the remainder 11 (Table 2). In the cases where a skin graft was applied, a complete take of the graft was recorded (Figure 10). In five patients, in whom the deficit was covered with a flap, no major complications occurred.
Figure 9.
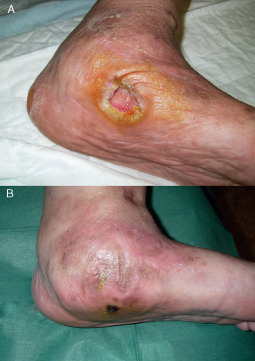
(A) An unhealed 9 months' ulcer of the lower limb of a 62 year old female patient. (B) Successful conservative treatment with intralesional platelet‐rich plasma injection. The wound remained intact 18 months after treatment.
Figure 10.
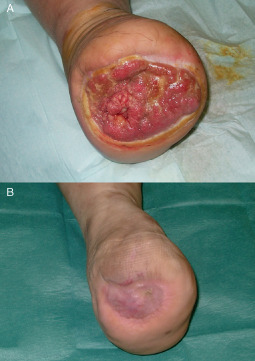
(A) Chronic ulcer of an amputated lower limb. (B) A complete take of the skin graft 3 months after surgery.
The mean follow‐up time was 14 months, varying between 1 and 26 months. During that period and after the completion of healing, only two diabetic patients presented an ulcer recurrence; the first patient had been managed conservatively, while the second had been treated with a split skin graft. These recurrences occurred on the first and fourth month, respectively, and were attributed to the insufficient wound care, the increase of the patients' body weight and the inadequate discipline of blood glucose control.
Discussion
During wound healing, the stages that follow and overlap are haemostasis and inflammation, proliferation and remodelling. In chronic ulcers, an impairment of the healing process leads to an alternation between the phases of inflammation and proliferation, which creates permanent conditions of chronic inflammation 4, 5, 6, 7, 13, 32. The general condition of the patient's health, the local conditions of the ulcer and the therapeutic actions that will take place can influence positively or negatively the healing phases.
The application of PRP seems to have a positive effect in the healing process of chronic ulcers, accelerating the rate of wound healing and decreasing local pain and the possibility of infection (33). Experimental studies usually present satisfactory results of PRP application; Carter et al. conducted an experiment in equine lower limb wounds by applying PRP onto ulcers. Histological results showed improvement of the wound healing, with a presence of organised fibroblasts and better orientation of collagen fibres (34). Yazawa used PRP in combination with fibre glue in rabbit ear ulcers and concluded that, from the seventh post‐treatment day, ulcers treated with PRP presented an increased progress in epithelialisation and limited appearance of granulation tissue, compared with the ulcers treated only with fibre glue 28, 35.
Clinical studies on the healing progress of chronic ulcers using PRP equally present encouraging results 2, 15, 26, 27. In some of them, an improvement or even a complete healing of previously non‐healed ulcers is described, such as ulcers in patients' diabetic legs, in percentages that ranged from 14% to 81% 2, 26. In an extended multicentre retrospective study, where healing rates of chronic ulcers in 3830 patients were compared, the results showed that patients treated with platelet concentration and systemic care showed considerably higher rates of healing and lower percentages of amputations compared with patients that were treated only with regular local care (27).
The regenerative ability of PRP depends on the activation of platelets 13, 15. In the technique where PRP is just applied over the surface of the ulcer, the process of activation should precede this application on the lesion. The usual method of activation is the addition of thrombin or CaCl2 in the concentrated platelets 3, 11, 15, 21, 36. Despite the fact that the creation of a clot with this method causes the activation of platelets, this does not necessarily mean that it is accompanied by a complete release of secretion proteins (37).
Another method of platelet activation is the contact of the plasma with elements of collagen fibres and endothelial cells, following which a clot of fibres is created and secretion proteins are released (28).
According to our method of PRP application, an ‘intralesional’ injection of platelets takes place in the wound bed and ulcer margins, offering the possibility of selective distribution of the PRP in some regions of the wound that require more aid in the healing process, while at the same time the loss of plasma away from the wound area is avoided. The technique we hereby propose is described for the first time in literature and constitutes a new way of application of PRP to the ulcer.
Given that the plasma is injected inside the wound, we believe that this method has more advantages compared with the classic method of simple placement of PRP on the ulcer, that is PRP intralesional injection allows the continuation of regular change of dressings without the risk of removing the growth factors from the wound; moreover, PRP application can be associated to any reconstructive procedure even in a single‐stage operation.
On the basis of our very encouraging results, we could suggest that stage III and small sized (<10 cm2) stage IV ulcers, according to EPUAP's classification, may be successfully treated only with PRP ‘intralesional’ injection. Larger lesions (>10 cm2) of stage IV ulcers may undergo a safe surgical procedure, which can occasionally be a simpler reconstructive method. In any case, surgical treatment that we followed led to the successful cover of the ulcer. Some of the patients of the second group could only be treated conservatively with local care and change of dressings; however, we decided to go for a surgical treatment modality, because the period for complete healing was estimated to be too long, thus increasing the possibilities of complications. In stage IV ulcers with particularly unfavourable local conditions because of poor vascularity, presence of infection or osteomyelitis, the beneficial action of treatment with PRP is doubtful.
According to our results, the clinical improvement of ulcers is associated with the proportional improvement of histological findings. After the addition of PRP, histological figures of the ulcers were altered, with an increased presence of fibroblasts and collagen fibres in a better orientation.
An additional finding that needs to be noted is the reduction of the bacterial load of the ulcers after this treatment. Even if the change of positive cultures into negative did not constitute the inquiring objective of this study, we speculate that the above finding could be attributed to the particularly careful local care of ulcers and/or the transport, with the PRP and concentrated line of white blood cells.
Despite the fact that white cells activation in the PRP has not been proved yet, we do believe that white cells may play a significant role in the reduction of the bacteria. The difference in pH of the PRP's pH from that of the blood is likely to be one of the factors that influence the whole procedure (9).
The mean follow‐up period of our patients after the healing of the ulcers was 14 months. The long follow‐up time confirms the effectiveness and the stability of this method.
Conclusion
The intralesional injection of platelet concentration constitutes a valuable alternative in the treatment of chronic ulcers. The method accelerates the healing process in the majority of non‐healing wounds and contributes to either the complete healing or the preparation of the wound bed for a final reconstructive procedure. Satisfactory improvement of the ulcer's morphology may lead to choose a minor reconstructive procedure which will possibly help to reduce the postoperative recovery time.
Acknowledgements
The authors want to thank the Haematology and Microbiology Departments of Papageorgiou Hospital, Thessaloniki, Greece, who performed the analysis of blood and specimen cultures for this study. We are grateful to the company Hospital Line SA for providing the centrifuge circuit Magellan‐Medtronic, Minneapolis, MN without any financial interest or claim. The authors declare no conflict of interest.
References
- 1. Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis 2004;32: 88–94. [DOI] [PubMed] [Google Scholar]
- 2. Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2001;24:483–8. [DOI] [PubMed] [Google Scholar]
- 3. Gonshor A. Technique for producing platelet‐rich plasma and platelet concentrate: background and process. Int J Periodontics Restorative Dent 2002;22:547–57. [PubMed] [Google Scholar]
- 4. Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg 2005;16:1043–54. [DOI] [PubMed] [Google Scholar]
- 5. Guyton AC. Human physiology, 3rd edn. Philadelphia: Saunders Company, 1982. [Google Scholar]
- 6. Giakoumetis A. Wound healing. Athens: Innovative, Johnson & Johnson, 2005. [Google Scholar]
- 7. Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg 2002;18:27–33. [DOI] [PubMed] [Google Scholar]
- 8. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 2004;91:4–15. [DOI] [PubMed] [Google Scholar]
- 9. Marx RE. Platelet‐rich plasma: evidence to support its use. J Oral Maxillofac Surg 2004;62: 489–96. [DOI] [PubMed] [Google Scholar]
- 10. Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet‐rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodontics Restorative Dent 2002;22:45–53. [PubMed] [Google Scholar]
- 11. Petrungaro PS. Using platelet‐rich plasma to accelerate soft tissue maturation in esthetic periodontal surgery. Compend Contin Educ Dent 2001;22:729–36. [PubMed] [Google Scholar]
- 12. Harrison P, Cramer EM. Platelet alpha‐granules. Blood Rev 1993;7:52–62. [DOI] [PubMed] [Google Scholar]
- 13. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet‐rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg 2002;30: 97–102. [DOI] [PubMed] [Google Scholar]
- 14. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet‐rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85:638–46. [DOI] [PubMed] [Google Scholar]
- 15. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet‐rich plasma: implications for wound healing. Plast Reconstr Surg 2004;114:1502–8. [DOI] [PubMed] [Google Scholar]
- 16. Kevy SV, Jacobson MS. Comparison of methods for point of care preparation of autologous platelet gel. J Extra Corpor Technol 2004;36:28–35. [PubMed] [Google Scholar]
- 17. Weibrich G, Kleis WK, Kunz‐Kostomanolakis M, Loos AH, Wagner W. Correlation of platelet concentration in platelet‐rich plasma to the extraction method, age, sex, and platelet count of the donor. Int J Oral Maxillofac Implants 2001;16:693–9. [PubMed] [Google Scholar]
- 18. Fennis JPM, Stoelinga PJW, Jansen JA. Mandibular reconstruction: a clinical and radiographic animal study on the use of autogenous scaffolds and platelet‐rich plasma. J Oral Maxillofac Surg 2002;31:281–6. [DOI] [PubMed] [Google Scholar]
- 19. Man D, Plosker H, Winland‐Brown JE. The use of autologous platelet‐rich plasma (platelet gel) and autologous platelet‐poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg 2001;107:238–9. [DOI] [PubMed] [Google Scholar]
- 20. Robiony M, Polini F, Costa F, Politi M. Osteogenesis distraction and platelet‐rich plasma for bone restoration of the severely atrophic mandible: preliminary results. J Oral Maxillofac Surg 2002;60:630–5. [DOI] [PubMed] [Google Scholar]
- 21. Shanaman R, Filstein MR, Danesh‐Meyer MJ. Localized ridge augmentation using GBR and platelet‐rich plasma: case reports. Int J Periodontics Restorative Dent 2001;21:345–55. [PubMed] [Google Scholar]
- 22. Tischler M. Platelet rich plasma. The use of autologous growth factors to enhance bone and soft tissue grafts. N Y State Dent J 2002;68:22–4. [PubMed] [Google Scholar]
- 23. Roseborough IE, Grevious MA, Lee RC. Prevention and treatment of excessive dermal scarring. J Natl Med Assoc 2004;96:108–16. [PMC free article] [PubMed] [Google Scholar]
- 24. Slater M, Patava J, Kingham K, Mason RS. Involvement of platelets in stimulating osteogenic activity. J Orthop Res 1995;13:655–63. [DOI] [PubMed] [Google Scholar]
- 25. Lozada JL, Caplanis N, Proussaefs P, Willardsen J, Kammeyer G. Platelet‐rich plasma application in sinus graft surgery: part I–background and processing techniques. J Oral Implantol 2001;27:38–42. [DOI] [PubMed] [Google Scholar]
- 26. Knighton DR, Ciresi K, Fiegel VD, Schumerth S, Butler E, Cerra F. Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet derived wound healing formula. Surg Gynecol Obstet 1990;170:56–60. [PubMed] [Google Scholar]
- 27. Glover JL, Weingarten MS, Buchbinder DS, Poucher RL, Deitrick GA, Fylling CP. A 4‐year outcome‐based retrospective study of wound healing and limb salvage in patients with chronic wounds. Adv Wound Care 1997;10:33–8. [PubMed] [Google Scholar]
- 28. Yazawa M. Platelet rich plasma for clinical application. In: Peterson BR, editor. Trends in blood transfusion research. Hauppauge, New York: Nova Science Publishers Inc, 2006:85–118. [Google Scholar]
- 29. Miskolczi L, Nemes B, Cesar L, Masanari O, Gounis MJ. Contrast injection via the central artery of the left ear in rabbits: a new technique to simplify follow‐up studies. AJNR Am J Neuroradiol 2005;26:1964–6. [PMC free article] [PubMed] [Google Scholar]
- 30. University of California San Diego Institutional Animal Care and use Committee IACUC. URL www.iacuc.ucsd.edu/policies/Policy5.03.pdf [accessed on 26 June 2011].
- 31. Shea JD. Pressure sores: classification and management. Clin Orthop Relat Res 1975;112:89–100. [PubMed] [Google Scholar]
- 32. Marlovits S, Mousavi M, Gabler C, Erdös J, Vécsei V. A new simplified technique for producing platelet‐rich plasma: a short technical note. Eur Spine J 2004;13 Suppl 1:S102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waters JH, Roberts KC. Database review of possible factors influencing point‐of‐care platelet gel manufacture. J Extra Corpor Technol 2004;36: 250–4. [PubMed] [Google Scholar]
- 34. Carter CA, Jolly DG, Worden CE, Hendren DG, Kane CJ. Platelet‐rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol 2003;74:244–55. [DOI] [PubMed] [Google Scholar]
- 35. Yazawa M, Ogata H, Kimura A, Nakajima T, Mori T, Watanabe N. Basic studies on the bone formation ability by platelet rich plasma in rabbits. J Craniofac Surg 2004;15:439–46. [DOI] [PubMed] [Google Scholar]
- 36. Roukis T, Zgonis T, Tiernan B. Autologous platelet‐rich plasma for wound and osseous healing: a review of the literature and commercially available products. Adv Ther 2006;23: 218–37. [DOI] [PubMed] [Google Scholar]
- 37. Zimmermann R, Arnold D, Strasser E, Ringwald J, Schlegel A, Wiltfang J, Eckstein R. Sample preparation technique and white cell content influence the detectable levels of growth factors in platelet concentrates. Vox Sang 2003;85:283–9. [DOI] [PubMed] [Google Scholar]


