Abstract
Topical hyaluronic acid (HA) is routinely used in the local treatment of chronic wounds, but few data have been reported to date. A 60‐day double‐blind, randomised, controlled superiority trial was designed to investigate the efficacy and safety of a gauze pad containing HA in local treatment of venous leg ulcers, compared with its neutral vehicle. The primary endpoint was the percentage of wound size reduction after 45 days. Totally 89 patients were included. At day 45, the percentage of ulcer surface reduction was significantly greater in the HA group (73 ± 4·6%) versus neutral vehicle group (46 ± 9·6%) (P = 0·011). The number of healed ulcers was significantly higher in the HA group at day 45 (31·1% versus 9·3% respectively) and day 60 (37·8% versus 16·3% respectively; P < 0·05). At day 30, pain intensity based on visual analogue scale was significantly lower in the HA group (12·4 mm ± 2·6 versus 22·8 mm ± 3·8; P = 0·026). Tolerance of both treatments was comparable in the two groups. HA gauze pad, in local treatment of venous leg ulcers, was significantly more effective than the neutral vehicle on wound size reduction, healed ulcers rate and pain management with a good safety profile.
Keywords: Double‐blind, randomised, controlled trial; Gauze pad; Hyaluronic acid; Leg ulcer; Reduction of wound
INTRODUCTION
Venous leg ulcer is a chronic, recurring condition affecting about 0·10–0·80% of the general population. It is even more frequent in elderly patients (65 years and over) with a prevalence rate of 1·69 (95% confidence interval: 1·65–1·74) (1).
This condition is a significant cause of morbidity, pain and decreased quality of life in elderly patients. Medical needs in this indication remain partially unmet as wounds take time to heal and are characterized by a significant rate of recurrence 2, 3, 4. Even though the underlying venous and/or arterial disorders are adequately handled, local treatment remains an essential part of the management of these lesions 4, 5. In France, different types of compressions are recommended by the French Health Authorities (HAS) based on literature review: low elasticity bandages with short, less than 20% stretch, elastic bandages with long more than 20% stretch, multilayered bandages and elastic compression stockings. The usual local treatment, recognised as the gold standard in the treatment of venous leg ulcers, is mainly based on multilayer compression of the wound wrapping primary contact dressings applied directly on the wound 6, 7. Different ranges of dressings are used with different kind of properties but none of them has clearly showed any significant advantage on wound healing in a controlled clinical trial 8, 9.
Hyaluronic acid (HA) is a major component of the extracellular matrix. It discloses hygroscopic and viscoelastic properties that plays a pivotal role in wound healing process (10). Beyond its structuring function, it is actively involved in all stages of wound healing including inflammation, granulation, remodelling and re‐epithelialisation. As a non immunogenic molecule, when highly purified, HA is currently used in routine in local treatment of chronic wound to promote tissue healing. Some products deliver HA under the form of cream or gel but also through impregnated gauze pad. Whatever the mode of delivery, few clinical data with optimal methodology have been reported to date as regards the HA specific efficacy in venous leg ulcer 11, 12, 13, 14. Therefore, a double‐blind, randomised, comparative controlled trial was designed to accurately investigate the performance and safety of a local application of HA impregnated gauze compared with a neutral vehicle in the treatment of leg ulcers of venous or mixed origin.
MATERIALS AND METHODS
Trial design
A prospective, multicentre, comparative, parallel‐group, randomised, double‐blind clinical trial was conducted in inpatients or outpatients with one or several leg ulcers of venous or mixed arterial/venous origin. For each patients included in the study, a target ulcer was selected by the investigator and randomly assigned to be locally treated once daily either by a 0·05% HA impregnated cotton gauze pad (ialuset® gauze pad manufactured by Laboratoires Genévrier, Sophia‐Antipolis, France) or by a neutral vehicle (same formulation as ialuset® gauze pad but without HA) for a maximal duration of treatment of 60 days or until complete healing. The ulcer was cleaned with physiological serum, and the assigned dressing was then applied by a nurse at the patient's home (for outpatients), or in various care facilities (for inpatients) except during evaluation visits when the dressing was applied by the investigator. The gauze pad was applied to the wound, covered with sterile gauze and then covered with an appropriate bandage. Surgical wound excision procedures were authorised if necessary with or without previous local anaesthesia. Systemic antibiotics could be used in case of clinically relevant infection. Systemic analgesics were authorised, provided they were interrupted at least 10 hours before each visit to allow a proper evaluation of wound‐related pain. The use of high‐dosage systemic corticosteroids, of cytostatic and immunosuppressive drugs and local use of proteolytic enzymes for wound debridement were not permitted during the time of the study.
Trial population
Male or female inpatients or outpatients aged 18 years or over, with one or several leg ulcers of venous or mixed arterial/venous origin present for >2 months and <4 years were primarily considered for inclusion. The inclusion criteria were the following: surface of the selected target ulcer comprised between 5 and 40 cm2 with no necrotic tissue; wound consistent with the use of an appropriate compression device; documented past history of deep venous thrombosis of the lower limbs and/or clinical evidence of post‐thrombotic syndrome with chronic oedema and lipodermatosclerosis and/or available data of an arterial‐venous Doppler examination performed within the previous 6 months and showing post‐phlebitic sequels (residual thrombosis), and/or a superficial or profound reflux on the venous system; no local use of HA within the 3 months before inclusion; albuminaemia ≥25 g/l; ankle/brachial Doppler systolic pressure index ≥0·8; daily use of efficient compression devices for ambulatory patients as recommended by French health authorities that is elastic bandage with long stretching properties or multilayer bandage; patients covered by a health insurance system. Women of childbearing age had to use a reliable contraceptive method for at least 3 months before and during the study.
Patients with an ulcer of non vascular origin or because of a general cause, diabetic patients, patients with significant arterial insufficiency (ankle/brachial Doppler systolic pressure index <0·8), patients with hepatic or renal failure, with a recent history of venous thrombosis (<3 months), pregnant or breastfeeding women or women planning to be pregnant were not allowed to participate in the study. Patients allergic to local anaesthetics or to investigational treatments components or under treatment delaying the healing process were also excluded, as were patients having participated in a clinical investigation within the 2 months preceding the inclusion visit (Table 1).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Male or female in‐ or outpatients | Pregnant or breast feeding women or women planning to be pregnant in the course of the study |
| Patient aged ≥18 years | Patient with an ulcer of non vascular origin (phagedenic pyodermatitis,…) |
| Patient with albuminaemia ≥25 g/l | Patient with clinical evidence of significant arterial insufficiency (claudication, pain at decubitus) |
| Patient with a diagnosis of leg ulcer for more than 2 months and less than 4 years | Patient with an ulcer due to a general cause (haematological cause,…) |
| Patient with one or several leg ulcers of varicose or post‐thrombotic origin, or of mixed venous arterial origin | Patient with any type of diabetes |
| Patient having undergone an arterial‐venous Doppler examination for <6 months showing post‐phlebitic sequels (residual thrombosis) AND/OR a superficial or profound reflux on the venous system AND/OR a well‐documented past history of deep venous thrombosis of the lower limbs AND/OR clinical evidence of post‐thrombotic syndrome with chronic oedema and lipodermatosclerosis | Patient suffering from hepatic disorders (ALAT/ASAT ≥2.5 ULN) |
| Patient with ankle/brachial Doppler systolic pressure index ≥0.8 | Patient suffering from renal disorders (creatinine clearance <30 ml/min) |
| Patient with a surface area of the targeted ulcer comprised between 5 and 40 cm2 included | Patient with known allergy to local anaesthetics such as to XylocaÏne®, LidocaÏne® or PrilocaÏne® |
| Patient without necrotic tissue | Patient with a clinical suspicion of general infection (erysipelas, phlegmon,…) |
| Patient with no local HA treatment within the three previous months before inclusion | Presence of at least one of the following symptoms, reminiscent of the local and/or general infection: peri‐ulcerous inflammation, odorous and purulent flow, adenopathy, lymphangitis, fever, unexpected healing interruption |
| Patient with an adapted compression treatment which was worn during all the study | Presence of a recent venous thrombosis (<3 months) |
| Patient having given his/her written informed consent for their participation in the clinical trial | Known allergy to one of the component of the investigational medical devices |
| Patient able to adhere to the protocol and to respect it at the interpretation of the Investigator | Patient under treatments delaying the healing process: systemic corticosteroids, cytostatic drugs, immunosuppressive agents |
| Patient covered by an insurance policy | Participation in any type of clinical investigation concurrently or within the 2 months preceding the inclusion visit |
| Patient having a satisfactory general condition at the interpretation of the investigator (life expectancy longer than the duration of the study) | |
| Woman of childbearing age using a reliable contraceptive method for at least 3 months before the study start and during the study or woman being in a menopause phase for at least 1 year (amenorrhoea). |
Ethical considerations
The study was conducted in accordance with the principles of the Declaration of Helsinki and its modifications (Washington, Tokyo and Seoul included), the relevant 2006 ordinance of the French Ministry of Health on Good Clinical Practice, ISO 2009 requirements and European Directive 93/42/CEE on medical devices, MEDDEV guidelines and rules of Good Clinical Practice. Study protocol was approved by Independent Ethics Committees: ‘CPP Est I’ of Dijon for France, Biomedical Research Ethics Committee of ‘Mohammed V Souissi’ University of Rabat for Morocco, and Bioethics Committee at Regional Medical Chamber of Lodz for Poland.
Data collected
A total of five visits were carried out by the same investigator for each patient: an inclusion visit (day 0), three intermediary evaluation visits (day 15 ± 2, day 30 ± 3, day 45 ± 3) and a final evaluation visit (day 60 ± 3). At inclusion visit (day 0), an informed consent form was signed by all patients, before undergoing a general clinical examination and study‐related procedures, as hereinafter described. The shape of the wound was drawn by the investigator using a sterile tracing paper at each evaluation visit for subsequent measure of wound size. During all evaluation visits, the investigator assessed the aspect of the wound by reporting the percentage of necrotic, fibrinous or granulation tissue; the aspect of the peri‐ulcerous skin (oedema, purpura, erythema, maceration, oozing and horny edges) was reported using a semi‐quantitative four‐point scale (nil = 0, slight = 1, moderate = 2, important = 3), and the intensity of pain during dressing renewal procedures was assessed according to a 0–100 mm visual analogue scale (VAS) completed by the patient. Compliance to study treatment was evaluated by counting the number of applications performed by the investigator or nurse reported by patient in a diary. It was considered excellent (no day of missed application), good (<3 days of missed application), fair (from 3 to 7 days of missed application) or poor ≥7 days of missed application between each visit. A general clinical examination was also performed at the final evaluation visit.
Primary and secondary endpoints
The primary endpoint of the study was the percentage of wound size reduction at D45. Two independent readers, equally blind to treatment, measured the wound size based on the drawings on sterile tracing papers, in a centralised fashion and using a digital planimetrics system, Visitrak®. The percentage of reduction of the wound area at visits 2, 3, 4 and 5 was calculated using the following formula: Mean (sum([(size of the wound at D0) − ( size of the wound at D45)]/(size of the wound at D0)).
Secondary efficacy parameters included the percentage of wound size reduction at D15, D30 and D60, pain intensity, burden of pain estimated by the area under the curve of daily patient pain and aspect of peri‐ulcerous skin, these latter parameters assessed at all five visits. Percentage of patients with healed ulcer, the pattern of the wound and compliance to treatment were also assessed during all evaluation visits. No change was made in the trial conduct or outcomes after the trial started.
Safety endpoints
Adverse events (AEs) as well as the drop‐outs for AE were recorded at all evaluation visits including nature, severity, time of onset, duration, degree of relationship to the study treatment and a description of any action and/or pharmacological treatment undertaken to handle the event. In case of clinical symptoms suggesting the onset of a local infection of the target ulcer, the patient was not withdrawn from the study and a bacteriological swabbing was performed to identify the responsible bacteria.
Statistics
Power calculation
Sample size was determined under the hypothesis of a 20% difference between the percentages of wound size reduction with the HA‐containing gauze pad compared with the neutral vehicle at D45, as a primary endpoint. A standard deviation of 40% healing was evaluated based on a previous study (13) with values α = 5% and 1 −β = 90% respectively for the level of significance and power of the trial (15). According to these data, the theoretical sample size was 140 patients overall, taking into account 10% of drop‐outs. Interim analysis was planned on the statistical plan in a way to have enough patients to conclude on the superiority in terms of efficacy of one of the study treatment. According to the Good Clinical Practice, when the superiority of one arm is statistically showed in an interim analysis, it is not necessary to expose more patients in the study. The possibility of an early interruption of the trial was included in the protocol, based on the results of an interim analysis of the primary efficacy criteria on the first 80 subjects having completed the study and on previously defined stopping rules.
Randomisation
The randomisation list was prepared by Data Management & Statistics Unit of IBSA Institut Biochimique SA, Switzerland using a validated software from SAS Institute Inc, Cary, NC in accordance with international standards. The HA treatment gauze pad and neutral vehicle were allocated according to a randomisation list balanced per blocks of 4. The patients received one of the investigational medical devices based on the sequential order at each site.
Statistical analysis
The study was designed based on the hypothesis of the HA gauze pad clinical efficacy superiority compared with the neutral vehicle. The clinical efficacy primary endpoint, percentage of wound size reduction at D45, was considered as a valid surrogate endpoint for leg ulcer healing 16, 17. The primary analysis was conducted in intention to treat (ITT) on all randomised patients having received at least once one of the two compared treatments. A secondary analysis was performed on the per‐protocol (PP) population defined as all subjects having completed the 45‐day treatment period without any major deviation from the protocol. Variance analysis (ANOVA) was used to compare primary and secondary endpoints between the two arms. Qualitative variables were compared with a Chi‐squared test (or a Fisher's exact test if the theoretical sample size was inferior to 5). Tests were two‐sided and considered significant at an alpha (α) level of 5%. Last Observation Carried Forward (LOCF) was used in case of missing values in the ITT analysis for all primary and secondary endpoints. The same technique was applied to PP analysis for patients who dropped out because of complete wound healing or inefficacy/worsening. Variables concerned by the LOCF were wound size, ulcer healing, proportion of necrotic, fibrinous or granulation tissue, aspect of peri‐ulcerous skin and pain intensity.
RESULTS
Patient disposition
Thirty‐one investigators from 29 centres participated in the study: 18 centres in France, 3 in Morocco and 8 in Poland. As planned by the protocol, an interim analysis was performed upon 80 subjects completing the study. Accordingly, 89 subjects were finally included in the analysis (ITT population), instead of the 140 patients previously calculated. These 89 patients were included from 8 November 2007 to 24 November 2009 (Figure 1): 45 in the HA group and 44 in the neutral vehicle group and constituted the ITT population. All of the 89 patients received at least one study treatment application. Overall, 28 patients did not complete the study (n = 18 for HA, n = 10 for neutral vehicle).
Figure 1.
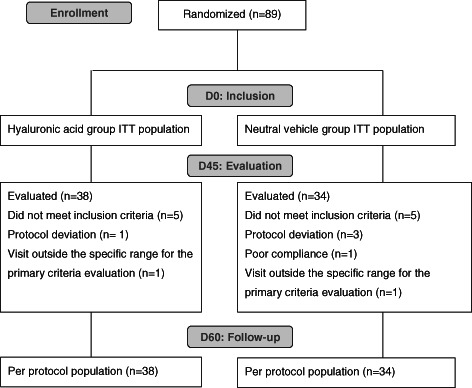
Flow chart. ITT, intention to treat.
The PP population (72 patients) was defined as ITT subjects who completed the 45 days treatment period without any major deviation from the protocol. Major protocol violations were reported for 17 patients during the study course. In the PP group, 38 patients received the HA‐containing gauze pad and 34 received the neutral vehicle (Figure 1).
Demographics
Patients were not significantly different between the two arms as regards gender, age and body mass index, frequency of medical and/or surgical background, clinical examination, total numbers of ulcers, localisation and duration of target ulcer, proportion of fibrinous or granulation tissue (Table 2).
Table 2.
Patient and ulcer demographics
| Treatment | ||||
|---|---|---|---|---|
| HA gauze pad (n = 45) | Neutral vehicle (n = 44) | |||
| Demographic characteristics | ||||
| Female gender: n, % | 20 | 44.4% | 24 | 54.5% |
| Age: mean, SEM | 59.4 | 2.5 | 64.1 | 2.7 |
| Body mass index: mean, SEM | 30.3 | 0.7 | 29.3 | 1.1 |
| Presence of medical or surgical history: n, % | 40 | 88.9% | 35 | 81.4% |
| Total number of reported ulcers | ||||
| One ulcer: n, % | 28 | 62.2% | 25 | 56.8% |
| Two ulcers or more: n, % | 17 | 37.8% | 19 | 43.2% |
| Localisation of the target ulcer | ||||
| Supra‐malleolar internal side | 23 | 69.7% | 17 | 58.6% |
| Supra‐malleolar external side | 4 | 12.1% | 6 | 20.7% |
| Malleolar | 2 | 6.1% | 0 | 0% |
| Sub‐malleolar | 2 | 6.1% | 1 | 3.4% |
| Other | 2 | 6.1% | 5 | 17.2% |
| Leg of the target ulcer | ||||
| Left | 25 | 55.6% | 25 | 56.8% |
| Right | 20 | 44.4% | 19 | 43.2% |
| Age of ulcer (months) | ||||
| Mean, SD | 12.4 | 12.3 | 12.8 | 12.2 |
| Missing values | 8 | 2 | ||
| Characteristics of the target ulcer (%) | ||||
| Proportion of fibrinous tissue: mean, SEM | 32.4 | 4.4 | 40.4 | 4.9 |
| Proportion of granulation tissue: mean, SEM | 66.9 | 4.4 | 62.9 | 4.7 |
| Missing values | 1 | 2 | ||
| Peri‐ulcerous skin with (n, %) | ||||
| Oedema | 26 | 57.8 | 32 | 72.7 |
| Purpura | 18 | 40.1 | 24 | 54.5 |
| Erythema | 28 | 62.2 | 33 | 74.9 |
| Oozing | 36 | 80.0 | 35 | 79.5 |
| Maceration | 16 | 35.6 | 15 | 34.1 |
| Surface area of the target ulcer (cm 2) | ||||
| Mean, SEM | 13.8 | 1.3 | 12.9 | 1.3 |
| Missing values | 0 | 1 | ||
| Mean, SEM | 33.2 | 3.7 | 33.4 | 4.0 |
HA, hyaluronic acid; SD, standard deviation; SEM standard error of mean; VAS, visual analogue scale.
Efficacy
After 45 days of treatment, average percentage of wound size reduction (primary endpoint) was greater in the HA treatment arm (73 ± 4·6%) than in the neutral vehicle arm (46 ± 9·6%) showing a statistically significant difference (P = 0·011) (Figure 2A).
Figure 2.
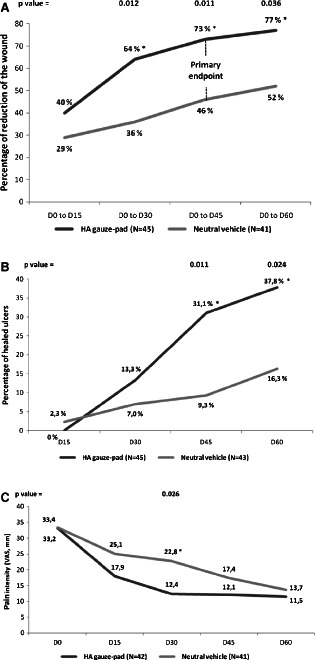
Percentage of reduction in wound size (A), percentage of healed ulcers (B) and pain intensity (C), according to treatment (ITT population). Between‐group comparisons were made using ANOVA. Statistical significance was set as P < 0·05. Abbreviations: ANOVA, analysis of variance, ITT, intention to treat.
Regarding the secondary endpoints, the percentage of size reduction of the ulcer was also significantly greater at days 30 and 60 in the HA group. Proportion of healed ulcers was significantly higher in the HA gauze pad group than in the neutral vehicle group at D45 (31·1% versus 9·3% respectively; P = 0·011) and D60 (37·8% versus 16·3% respectively; P = 0·024) (Figure 2B).
Between D0 and D60, the percentage of granulation tissue decreased by 8·5% (±7·6) in the HA group, while it increased by 9·0% (±8·0) in the neutral vehicle group. This evolution was significantly different between groups at visit D30 (P = 0·047) and D45 (P = 0·004). The evolution of the necrotic and fibrinous tissue remained comparable between groups during the study.
From D0 to D60, pain intensity (based on VAS) decreased with a favourable trend towards the HA group: decrease from 33·2 ± 3·7 mm to 11·5 ± 2·8 mm in average in the HA group and from 33·4 ± 4·0 mm to 13·7 ± 2·9 mm in average in the neutral vehicle group. The maximum difference between groups was observed at D30, pain intensity was then significantly lower in the HA gauze‐pad group (12·4 ± 2·6) than in the neutral vehicle group (22·8 ± 3·8) (P = 0·026) (Figure 2C).
Evolution of peri‐ulcerous skin was comparable between treatment groups except for oozing (P = 0·014; at D60), where HA gauze pad group patients showed reduced signs.
Other performance secondary endpoints (time to complete ulcer healing and global performance) were comparable between treatment groups, at any visit. Results from the PP analysis globally confirmed these findings; as a representative result, the reduction of size of ulcer between D0 and D45 was 72% versus 45% in the HA treatment and the neutral vehicle arm, respectively (P = 0·029).
The type of compression applied during the study was a type 2 compression with long stretching elastic bandage for a majority of patients (>96% of patients) and was not significantly different between groups.
The efficacy data of this study were all the more robust as the compliance to study treatment was highly satisfying for >87% of patients who did not miss any daily application of the allocated treatment; this excellent compliance was not significantly different between the two arms of the study at any visit.
Based on the significant difference observed for the primary performance parameter in this interim analysis, the study was stopped because according to the Good Clinical Practice, it is not necessary to expose more patients to the inferior treatment, the neutral vehicle.
Safety
A total of 48 AEs were reported in 27 patients during the course of the study. Most of them were not local AE (Table 3). Four patients dropped out from study because of an AE (two in the HA treatment group and two in the neutral vehicle group), but none of them was treatment related.
Table 3.
Adverse events (AEs)
| Hyaluronic acid gauze pad (n = 45) | Neutral vehicle (n = 44) | |||
|---|---|---|---|---|
| Local AEs | n | % * | n | % |
| Wound complication | 1 | 5.9 | 4 | 12.9 |
| Wound secretion | 0 | 0 | 3 | 9.7 |
| Application site eczema | 1 | 5.9 | 0 | 0 |
| Erysipelas | 1 | 5.9 | 1 | 3.2 |
| Wound haemorrhage | 1 | 5.9 | 1 | 3.2 |
| Wound infection | 1 | 5.9 | 0 | 0 |
| Application site erythema | 0 | 0 | 1 | 3.2 |
| Application site pruritus | 0 | 0 | 1 | 3.2 |
| Wound inflammation | 0 | 0 | 1 | 3.2 |
| Phlebitis | 0 | 0 | 1 | 3.2 |
| Non local AEs | 12 | 70.5 | 18 | 56.6 |
| Total | 17 | 100 | 31 | 100 |
*Percentages calculated for total number of AE.
Neither the overall incidence of AE nor the incidence of treatment‐related AE was statistically different between the two groups; more specifically, 22·2% of patients experienced at least one AE in the HA group versus 38·6% in the neutral vehicle group for overall AE (P = 0·233), whereas 6·7% of patients experienced at least one treatment‐related AE in the HA group versus 18·2% in the neutral vehicle group (P = 0·099).
AEs were mainly mild or moderate (75% of all AEs). Only 12 AEs (25%) were rated as severe: 8 in the HA group versus 4 in the neutral vehicle group. This difference between the two treatment groups was significant (P = 0·036). However, the severe AEs in the HA group were mostly reported by one patient (6/8 AEs) and only one AE was treatment‐related (Pain).
Only one serious adverse event (SAE) was reported during the study: suspicion of heart attack in the HA group. The SAE resulted in death of the patient but was not related to the treatment according to the investigator.
DISCUSSION
Application of exogenous HA has been shown to be of interest and currently used as local treatment option under dressing, cream or gel formulation in the treatment of chronic wounds such venous leg ulcer 11, 12, 14.
In this indication, gauze pads dressings represent a widely used treatment option, although they failed to show clear evidence of any increased benefit towards the multilayer compression bandaging recognised as the gold standard in the treatment of venous leg ulcers 6, 7.
The interesting option of combining HA and traditional gauze pad to improve healing process leads to the development of an HA impregnated gauze pad. This dressing is nowadays currently used in the local treatment of chronic wounds and has been evaluated in some preliminary studies 11, 13.
Although these preliminary and exploratory studies outlined the interest of HA‐based gauze pad in chronic wounds, they were not run under optimal methodology according to all the gold standards of proper clinical trial design.
Therefore, the present design of this study met the MEDDEV guidelines (18), recommendations of recent reviews and expert consensus on chronic wound dressings 8, 9, 19, which notably emphasised the need for double‐blind assessment and good methodology practice for clinical research on chronic wound dressing.
This multicentre, randomised, double‐blind study was designed to compare the efficacy and safety of an HA‐containing gauze pad and of its neutral vehicle administered once daily for 60 days in patient with leg ulcer of venous or mixed origin. At baseline, the demographic characteristics of patients and the characteristics of the ulcer were similar between the two treatment groups. Compliance was globally excellent and comparable between treatment groups throughout the study. Regarding the primary endpoint, the analysis based on surface measurement performed by two independent readers on tracing papers using planimetrics system unambiguously confirmed for the first time that a significantly greater reduction of wound size was obtained after 45 days of treatment with the HA‐containing gauze pad compared with the neutral vehicle (73 ± 4·6% versus 46 ± 9·6% respectively; P = 0·011). Wound surface reduction was also significantly more important at days 30 and 60 in the HA group.
The percentage of wound size reduction obtained at 45 days is widely used in such clinical short‐term studies, more consistent with real‐life conditions, as a valid surrogate endpoint as it can reliably predict subsequent ulcer healing 16, 17, which usually heal over a 12‐ to 24‐week period.
Interestingly, even on this relative study short time period, the proportion of healed ulcer was significantly higher in the HA gauze pad group than in the neutral vehicle group: 31·1% versus 9·3% at D45 (P = 0·011) and 37·8% versus 16·3% at D60 (P = 0·024). Showing the ability of HA gauze pad to show important and early efficacy on effective wound healing criteria.
Other endpoints observations supported these results: between D0 and D60, the percentage of granulation tissue decreased by 8·5% (±7·6) in the HA group, while it increased by 9·0% (±8·0) in the neutral vehicle group and this evolution was significantly different between groups at visit D30 (P = 0·047) and D45 (P = 0·004). This trend difference of evolution of the granulation tissue between groups does not reflect a less favourable evolution in the HA group, but is more suggesting a faster healing process in this group, as granulation tissue firstly increased (between D0 and D15) and tended to disappear when healing is close to end. The evolution of the necrotic and fibrinous tissue remained comparable between groups during the study.
Pain management in this indication is also an important challenge in the daily care especially during dressing application and removal. In this study, pain‐related secondary endpoints such as pain intensity compared with initial level during the whole study also favoured the HA arm. Although dressings were changed everyday, these results suggest that HA might have a favourable impact on wound‐related pain and on patients' quality of life.
Tolerance of the HA gauze pad was overall satisfying. The rate of patients experiencing at least one treatment‐related AE was not statistically different between the two groups, and no treatment‐related SAE was reported during the study. AEs were mainly mild or moderate. These results are supported by previous clinical trials using HA, as they also reported good tolerance and low AE rates 11, 12, 13, 14.
The good efficacy results obtained in the neutral vehicle group support the already recognised efficacy of multilayer compression along with any dressing to overall satisfy the healing process management in chronic wounds 6, 7. But the significantly stronger and faster effect of HA impregnated gauze pad supports the hypothesis that HA delivered in a gauze pad formulation significantly contributes to the restoration of optimal local physiologic conditions that are necessary to promote ulcer healing, resulting in a favourable final outcome 10, 20. Although neutral vehicle composition was comparable to other cotton gauze pad used on the marketplace, further studies using marketed reference dressings with good standards of care in chronic wounds as comparator, could be necessary to confirm these results.
The intimate mechanisms of venous leg ulcer healing are still a matter of investigations, but the effects of exogenous HA observed herein are consistent with the current knowledge of endogenous HA role in wound repair process. Indeed, endogenous HA appears to be both a major structural component of the extracellular matrix and a molecular actor of primary importance in wound healing deeply involved in all stages of this complex process.
CONCLUSIONS
For the first time, to the best of our knowledge such a well‐conducted clinical study clearly shows that the local application of HA using an impregnated gauze pad on venous leg ulcers is significantly more effective than neutral vehicle gauze pad regarding a widely accepted quantitative endpoint (i.e. reduction of wound size at D45) and effective wound closure rate with a good safety profile.
ACKNOWLEDGEMENTS
Project management and monitoring of the study were carried out by the Sponsor, Laboratoires Genévrier, with the support of local contract research organisations in France, Morocco and Poland.
References
- 1. Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46:381–6. [DOI] [PubMed] [Google Scholar]
- 2. Van Gent WB, Wilschut ED, Wittens C. Management of venous ulcer disease. BMJ 2010;341:c6045. [DOI] [PubMed] [Google Scholar]
- 3. Grey JE, Harding KG, Enoch S. Venous and arterial leg ulcers. BMJ 2006;332:347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzalez‐Consuegra RV, Verdu J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs 2011;7:926–44. [DOI] [PubMed] [Google Scholar]
- 5. Korber A, Klode J, Al‐Benna S, Wax C, Schadendorf D, Steinstraesser L, Dissemond J. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J Dtsch Dermatol Ges 2011;9:116–21. [DOI] [PubMed] [Google Scholar]
- 6. Nelson EA. Compression therapy, dressings and topical agents for venous ulcer healing. Phlebology 2010;25(Suppl 1):28–34. [DOI] [PubMed] [Google Scholar]
- 7. O'Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev 2009:CD000265. [DOI] [PubMed] [Google Scholar]
- 8. Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D. Systematic reviews of wound care management: (2). Dressings and topical agents used in the healing of chronic wounds. Health Technol Assess 1999;3(17 Pt 2):1–35. [PubMed] [Google Scholar]
- 9. Palfreyman S, Nelson EA, Michaels JA. Dressings for venous leg ulcers: systematic review and meta‐analysis. BMJ 2007;335:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen 1999;7:79–89. [DOI] [PubMed] [Google Scholar]
- 11. Barrois B, Carles M, Rumeau M, Tell L, Toussaint JF, Bonnefoy M, de Vathaire F. Efficacy and tolerability of hyaluronan (ialuset) in the treatment of pressure ulcers: a multicentre, non‐randomised, pilot study. Drugs R D 2007;8:267–73. [DOI] [PubMed] [Google Scholar]
- 12. Meaume S, Ourabah Z, Romanelli M, Manopulo R, De Vathaire F, Salomon D, Saurat JH. Efficacy and tolerance of a hydrocolloid dressing containing hyaluronic acid for the treatment of leg ulcers of venous or mixed origin. Curr Med Res Opin 2008;24:2729–39. [DOI] [PubMed] [Google Scholar]
- 13. Ortonne JP. A controlled study of the activity of hyaluronic acid in the treatment of venous leg ulcers. J Dermatol Treat 1996;7:75–81. [Google Scholar]
- 14. Abbruzzese L, Rizzo L, Fanelli G, Tedeschi A, Scatena A, Goretti C, Macchiarini S, Piaggesi A. Effectiveness and safety of a novel gel dressing in the management of neuropathic leg ulcers in diabetic patients: a prospective double‐blind randomized trial. Int J Low Extrem Wounds 2009;8:134–40. [DOI] [PubMed] [Google Scholar]
- 15. Kirby A, Gebski V, Keech AC. Determining the sample size in a clinical trial. Med J Aust 2002; 177:256–7. [DOI] [PubMed] [Google Scholar]
- 16. Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420–5. [DOI] [PubMed] [Google Scholar]
- 17. Papazoglou ES, Zubkov L, Mao X, Neidrauer M, Rannou N, Weingarten MS. Image analysis of chronic wounds for determining the surface area. Wound Repair Regen 2010;18:349–58. [DOI] [PubMed] [Google Scholar]
- 18. European Commission; Entreprise and Industry Directorate General. Guidelines on medical devices. Clinical evaluation: a guide for manufacturers and notified bodies. Brussels: European Commission; Entreprise and Industry Directorate General, 2009. [Google Scholar]
- 19. Chaby G. Management of leg ulcers. Rev Prat 2010; 60:970–8. [PubMed] [Google Scholar]
- 20. Brown JA. The role of hyaluronic acid in wound healing's proliferative phase. J Wound Care 2004; 13:48–51. [DOI] [PubMed] [Google Scholar]


