Abstract
The aim of this study was to assess the impact of an epidermal substitute, a lactocapromer terpolymer matrix, on microcirculation in wounds. Lactocapromer terpolymer matrices were placed into the dorsal skinfold chamber of mice (n = 10). Untreated chamber preparations served as controls (n = 10). The microcirculation in tissue adjacent to the implant was observed by intravital fluorescence microscopy. Alongside the stable microhaemodynamics, a strong induction of angiogenesis adjacent to the implants was observed. A progressive increase in the functional vessel density was detected throughout the observation time of 10 days. Additionally, a stable and increasing perfusion within the newly developed vascular network in the outer circumference of the matrix was noted. The lactocapromer terpolymer matrix showed no adverse effect on the microcirculation in the host tissue. In contrast, as detected by intravital microscopy, the biomaterial protected the microcirculation and induced angiogenesis.
Keywords: Angiogenesis, Burn, Suprathel, Vascularization, Wound dressing
INTRODUCTION
The impairment of tissue perfusion within the burn area can occur from shortly after the injury up to 48 hours post‐burn. Hindered microcirculation will result in the reduction of oxygen and nutrient supply to the affected tissue, while susceptibility to infection increases. Limitations of tissue perfusion as a consequence of both trauma and toxic wound treatment inversely affect wound healing 1, 2, 3.
Hence the use of wound dressings compromising the microcirculation should be avoided to obviate the risk of transforming an initially superficial burn wound into a deep one.
A variety of biological and synthetic wound dressings are currently in use in the treatment of burn wounds with varying success. A number of dressings appear to be beneficial in the management of superficial partial‐thickness burns. This benefit relates to wound healing time, the number of dressing changes and the level of pain. Associated costs and patient comfort should also be considered 4, 5, 6, 7.
The resorbable lactocapromer terpolymer matrix assessed in this study serves as a temporary epidermal substitute in clinical application for a number of years. The indication for the use of lactocapromer terpolymer matrix has been superficial partial‐thickness burns, which still retain a regenerative potential (8). Especially fresh, uninfected burns and childhood burns have been treated with excellent results avoiding complications associated with split skin grafting as well as their donor site morbidity (9). As shown by Uhlig and colleagues, this less painful treatment of burn wounds led to a decrease in scar formation and improved skin function. Besides its use in wound dressing for burns, lactocapromer terpolymer matrix has also been successfully applied on donor sites of split skin grafts (10). This study showed that the matrix supports wound reepithelialisation. The compact, but yet porous structure of lactocapromer terpolymer matrix is supposed to act as a temporary template, maintaining an optimal wound milieu and to enhance migration of the keratinocytes (11). The optimal time for the use of this matrix appears to be the first hours after burn injury. It should remain as a wound cover for at least 1 week, making painful dressing changes redundant 8, 9, 11
However, despite the reports about successful clinical application of this epidermal substitute no experimental studies were performed displaying the microcirculatory response to this material.
The aim of this study was to visualise and quantify the microcirculatory changes within wounds treated with lactocapromer terpolymer matrix, by means of intravital fluorescence microscopy in vivo.
MATERIALS AND METHODS
Animals
The experiment included 20 female balb/c mice (Charles River, Sulzfeld, Germany; body weight 18–22 g). The animals had access to standard laboratory chow and tap water ad libitum. The study was approved by the local governmental animal care committee and conducted in accordance with German legislation on protection of animals and the NIH Guidelines for the Care and Use of Laboratory Animals.
Preparation of the dorsal skinfold chamber
The implantation procedure of the dorsal skinfold chamber (Institute of Surgical Research, Ludwig‐Maximilian University of Munich, Germany) was performed as reported previously (12). In brief, the titanium chamber frames were implanted to sandwich an extended skinfold on the animal's back. The prepared skin layer containing the striated skin muscle was covered with the second titanium frame incorporating a removable coverslip.
Implants
The implants (diameter 2 mm and thickness 200 µm) were punched from gamma‐sterilised lactocapromer terpolymer matrix (Suprathel® , Institute of Textile and Process Engineering, Denkendorf, Germany).
The three‐dimensional matrix (Figure 1) possesses an interconnected pore structure (pore size 5–50 µm and porosity 70–80%) and consists of a synthetic copolymer mainly based on dl‐lactide. The components are polymerised using a melting procedure and dissolved in organic solvents in a second step. The material is subsequently processed by means of a modified phase inversion and freeze‐drying technique.
Figure 1.
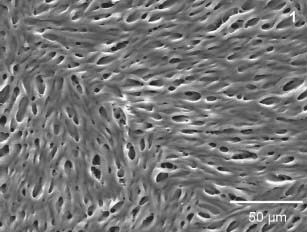
The scanning electron microscopy image shows the microporous structure of the lactocapromer terpolymer matrix (magnification 500‐fold).
Prior to implantation of the matrix, the animals were allowed to recover from anaesthesia and surgery for at least 48 hours to exclude alterations of the microcirculation as a result of surgical trauma. The implants were placed in the centre of each chamber with direct contact to the perfused striated skin muscle.
Intravital fluorescence microscopy
Intravital fluorescence microscopy was performed on days 1, 5 and 10 after implantation within the implant's border zones in awake animals. Six different regions of interest were selected at the implant's circumference (Figure 2) and within the centre of the chamber preparations in controls.
Figure 2.
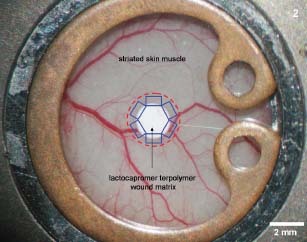
The intravital observations were performed in six different observation regions (blue boxes) within a defined border zone (red interrupted line) up to 500 µm from the circumference of the transplanted matrix. Matrices transplanted into the chamber directly contacted the perfused striated skin muscle layer of the wound.
Epi‐illumination was achieved using a 12 V, 100 W halogen lamp in conjunction with a Zeiss filter set (BP 450‐490, FT 510 and LP 520). For contrast enhancement, the fluorochromes were administered via the tail veins. FITC‐labelled dextran (1·0%, molecular weight 150 kDa) served as a plasma marker (Sigma Chemicals Co., St Louis, MO). A 40‐fold water immersion objective (Zeiss, Axiotech Vario 100 HD, Achroplan 40× 0·5 W; Carl Zeiss, Oberkochen, Germany) was used to observe the microvasculature.
Image processing
The images were captured using a charge‐coupled device video camera (AVT‐BC 71; AVT‐Horn, Aalen, Germany), digitised (Pinnacle Movie Box; Pinnacle Systems, Avid Technology, Munich, Germany) on hard disc for later off‐line analysis. The X−Y coordinates of the regions of interest were saved to ensure the exact relocation of the areas throughout the study (Axiovision 3.1. system; Carl Zeiss Imaging Solutions GmbH, Munich, Germany).
Microcirculatory measurements
Quantitative off‐line analysis of the digital images was performed using the image analysis system CapImage® (Zeintl, Heidelberg, Germany). The following parameters were assessed in capillaries as described previously in detail 12, 13:
-
1
Functional vessel density (cm/cm2) was defined as the length of red blood cell‐perfused microvessels per observation area reflecting nutritive tissue perfusion and oxygen delivery.
-
2
Microvascular diameters (µm) were measured at a 90° angle to the vessel wall.
-
3
Red blood cell velocity (mm/s) was analysed by a computer‐assisted image analysis system using the line‐shift method.
-
4
Macromolecular leakage (I e/I i) of the plasma marker FITC‐dextran into the extravascular space was assessed by a densitometric quantification of fluorescence intensity in the perivascular and intravascular areas.
Statistics
Mean and standard error of the mean (SEM) were calculated for each microcirculatory parameter. Differences between the groups at each time point were assessed using Mann–Whitney rank sum test. Differences between the time points (days of observation) within each group were assessed using Kruskal–Wallis one‐way analysis of variance on ranks followed by a pair‐wise multiple comparison procedure (Dunn's method) in the case of significant differences. Statistically significant differences are indicated in Table 1. Statistical significance was set at P < 0· 05. SigmaStat® (Version 15, SPSS GmbH, Munich, Germany) was used for statistical analysis of the data.
Table 1.
Quantitative analysis of microcirculatory parameters (mean ± SEM) in the border zones of the matrix and in controls
| Observation time | Day 1 | Day 5 | Day 10 | |||
|---|---|---|---|---|---|---|
| Microcirculatory parameters | Border | Control | Border | Control | Border | Control |
| Functional vessel density (cm/cm2) | 206·94 ± 7·7 | 209·82 ± 8·0 | 222·55 ± 9·3 [Link] , [Link] | 208·10 ± 8·9 | 243·73 ± 8·4 [Link] , [Link] | 210·64 ± 9·2 |
| Microvascular diameter (µm) | 4·2 ± 0·08 | 4·1 ± 0·09 | 4·7 ± 0·06 | 4·3 ± 0·07 | 5·2 ± 0·08 * | 4·1 ± 0·07 |
| Red blood cell velocity (mm/s) | 0·051 ± 0·005 | 0·052 ± 0·004 | 0·071 ± 0·005 [Link] , [Link] | 0·059 ± 0·004 | 0·085 ± 0·006 [Link] , [Link] | 0·056 ± 0·006 |
| Macromolecular leakage (I e/I i) | 0·855 ± 0·013 | 0·854 ± 0·012 | 0·899 ± 0·011 [Link] , [Link] | 0·855 ± 0·012 | 0·898 ± 0·013 * | 0·854 ± 0·011 |
The matrices induced a strong vascular response with an increasing microvessel density, accompanied by an increase in the blood flow as shown by quantification of microvascular diameters and red blood cell velocity. The macromolecular leakage was found to be significantly increased on days 5 and 10 compared with the control group. The functional vessel density in controls did not change significantly throughout the observation. Microvascular diameters, macromolecular leakage and red blood cell velocity in controls remained constant and were not altered significantly during the period of 10 days.
*Mann‐Whitney.
†ANOVA on ranks, P < 0· 05.
RESULTS
The quantitative analysis of the functional vessel density performed within the border zones of the implants showed a statistically significant increase from day 1 to day 10 of the experiment. Additionally, an increase in the microvessel diameter adjacent to the implants was noted. The vessel diameters increased exceeding those of the control tissue on days 5 and 10 (Table 1).
The red blood cell velocity showed a statistically significant increase on days 5 and 10 when compared with controls (Table 1). The macromolecular leakage as a parameter for vascular integrity was markedly elevated in the treated group on days 5 and 10, and remained above values in the control group starting from day 5 (Table 1).
Furthermore, the intravital observation of the microvasculature within the border zone of the implants showed a remarkable vascular remodelling. On day 5, the capillaries neighbouring the implants dilated and formed loop‐like formations presenting vessel sprouts at their flexures. On day 10, the outgrowing blood vessels extended throughout the implant's edge and interconnected to form perfused finger‐shaped formations at the implant's edge (Figure 3A–D). There was no breakdown of the microperfusion around the matrices.
Figure 3.
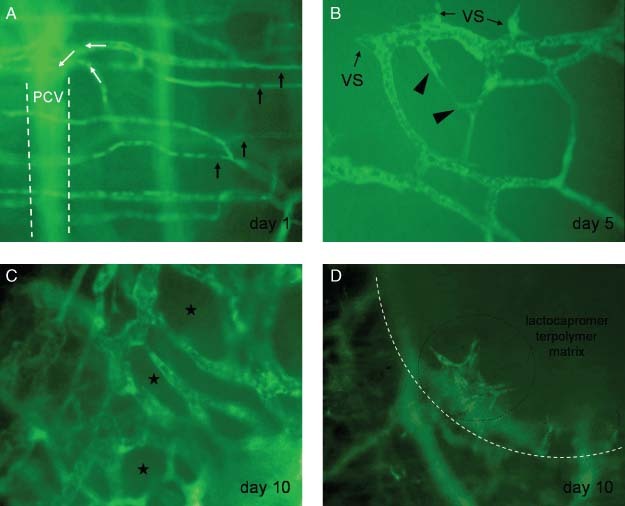
Intravital fluorescence microscopy images display the remodelling of the microvasculature noted within the border zone during the observation time of 10 days. Contrast enhancement was achieved by a macromolecular plasma marker FITC‐dextran (magnification 355‐fold). (A) The normal capillaries are parallely arranged within the muscle layer (black arrows) on day 1 after transplantation of the matrix. The blood flow (pointed by white arrows) within the capillaries is directed towards a postcapillary venule (PCV). The unsharp contour of the venule is signified by interrupted white lines. The venule is located underneath the observation plane. (B) Newly developed and outgrowing blood vessels were found on day 5. The dilated capillary loops bear at their vertex a few perfused vessels sprouts (VS). A newly developed connection between two vessel sprouts is presented (triangles). (C) Reduction in the intervascular space (asterisks) because of a rise in the development of new blood vessels was noted on day 10. The newly developed microvessels connected to form a perfused grid‐like perfused microvascular network. (D) The newly developed vessels interconnected to build finger‐shaped formations at the border zone (white line) of the lactocapromer terpolymer matrix (magnification 88‐fold). The vessel formations were detected to grow along the edge of the matrix and above the material surface (black encircled).
DISCUSSION
Burn wound progression is a complex process by which superficial partial‐thickness burns spontaneously advance into deep partial‐ or full‐thickness burns. This phenomenon is important because of the fact that burn wound depth may be a significant determinant of morbidity and treatment 1, 2. The impairment of the local tissue perfusion leads to tissue necrosis. The breakdown of skin microcirculation plays a key role in the extension of burns, especially in the zone of stasis 1, 2, 3, 13. However, a detailed knowledge of the pathogenic molecular and cellular mechanisms related to wound conversion is limited.
One of the major aspects to prevent burn wound progression is to preserve or improve microcirculation. A variety of dressings for the treatment of burn wounds to promote healing are designed 14, 15, 16, 17. The use of biosynthetic dressings is associated with a decrease in healing time and reduction in pain during dressing changes. Despite some potentially positive findings derived from experimental situation, however, the gain according to the clinical use is limited (5).
Numerous experimental settings were designed for analysing tissue perfusion. However, most of them were in vitro studies, where dynamic microcirculatory observations were not possible. In the animal model, the use of intravital fluorescent microscopy is still the gold standard for pathophysiological investigations, allowing long‐time evaluations of exactly the same vessel, enabling visualisation of angiogenesis 12, 13, 18.
In this study, the dorsal skinfold chamber instead of the ear model of hairless mice was used to increase the area of investigation. The renouncement of burn injury was chosen, because the angiogenesis and microcirculation during wound healing of burns and other wounds is up to a certain degree comparable and the quality of images of large burn areas declines significantly after burn infliction (13).
We observed a vascular remodelling of the host microvasculature progressing similar to what we have seen in previous studies using this model conducted to analyse angiogenesis and neovascularisation in scaffold biomaterials 12, 18.
The functional vessel density, which is an indicator of tissue perfusion (19), increased continuously during the observation period from day 1 to day 10. The quantification of the functional vessel density confirmed the observations of ongoing vessel development around the matrix. The functional vessel density remained significantly above the values in controls during all time. The increase in vessel diameters, subsequent to incorporation of matrices displayed the start of vessel sprouting, which could be visualised and was the main reason for the increase in vascular density. The red blood cell velocity in microvessels around the matrix showed significant elevation during the observation period. In combination with the increased diameter, it indicates a higher blood flow in tissue adjacent to implanted matrices.
The increase in macromolecular leakage underlined the temporary instability of the newly developed vasculature. The leakage of plasma marker into the perivascular tissue displayed a transitory integrity loss of the vessel wall as observed in ongoing angiogenesis.
In this study, the microcirculatory changes and angiogenic response induced by lactocapromer terpolymer matrix was assessed for the first time in an in vivo model. The results of our study reflect long‐term effects of the matrix on the wound microcirculation. With the technique used we were able to gain quantitative data in terms of microcirculatory changes under standardised conditions. The matrix did not disturb the microcirculation of the wound tissue. In contrast, the lactocapromer terpolymer matrix was found to induce angiogenesis.
We are aware that the model used allows only the investigations of the impact of lactocapromer terpolymer matrix in normal wounds. There are differences in the pathophysiology compared with burn wounds and the matrix should be investigated in further studies in established in vivo burn models as well. On the other hand, our findings suggest that lactocapromer terpolymer matrix does support and even induce angiogenesis, the most important factor for healing all wound types.
REFERENCES
- 1. Singh V, Devgan L, Bhat S, Milner SM. The pathogenesis of burn wound conversion. Ann Plast Surg 2007;59:109–15. [DOI] [PubMed] [Google Scholar]
- 2. Grunwald T, Garner W. Acute burns. Plast Reconstr Surg 2008;121:311e–9e. [DOI] [PubMed] [Google Scholar]
- 3. Goertz O, Ring A, Köhlinger A, Daigeler A, Andree C, Steinau HU, Langer S. Orthogonal polarization spectral imaging: a tool for assessing burn depths? Ann Plast Surg 2010;64:217–21. [DOI] [PubMed] [Google Scholar]
- 4. Shakespeare P. The role of skin substitutes in the treatment of burn injuries. Clin Dermatol 2005;23:413–8. [DOI] [PubMed] [Google Scholar]
- 5. Wassiak J, Cleland H, Campbell F. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev 2008;4:CD002106. [DOI] [PubMed] [Google Scholar]
- 6. Saffle J. Closure of the excised burn wound: temporary skin substitutes. Clin Plast Surg 2009;36:627–41. [DOI] [PubMed] [Google Scholar]
- 7. Goertz O, Abels C, Knie U, May T, Hirsch T, Daigeler A, Steinau HU, Langer S. Clinical safety and efficacy of a novel thermoreversible polyhexanide‐preserved wound covering gel. Eur Surg Res 2010;44:96–101. [DOI] [PubMed] [Google Scholar]
- 8. Uhlig C, Rapp M, Hartmann B, Hierlemann H, Planck H, Dittel KK. Suprathel‐an innovative, resorbable skin substitute for the treatment of burn victims. Burns 2007;33:221–9. [DOI] [PubMed] [Google Scholar]
- 9. Schwarze H, Küntscher M, Uhlig C, Hierlemann H, Prantl L, Ottomann C, Hartmann B. Suprathel, a new skin substitute, in the management of partial‐thickness burn wounds: results of a clinical study. Ann Plast Surg 2008;60:181–5. [DOI] [PubMed] [Google Scholar]
- 10. Schwarze H, Küntscher M, Uhlig C, Hierlemann H, Prantl L, Noack N, Hartmann B. Suprathel, a new skin substitute, in the management of donor sites of split‐thickness skin grafts: results of a clinical study. Burns 2007;33:850–4. [DOI] [PubMed] [Google Scholar]
- 11. Uhlig C, Rapp M, Dittel KK. [New strategies for the treatment of thermally injured hands with regard to the epithelial substitute Suprathel]. Handchir Mikrochir Plast Chir 2007;39:314–9. [DOI] [PubMed] [Google Scholar]
- 12. Ring A, Steinstraesser L, Muhr G, Steinau HU, Hauser J, Langer S. Improved neovascularization of PEGT/PBT copolymer matrices in response to surface modification by biomimetic coating. Eur Surg Res 2007;39:75–81. [DOI] [PubMed] [Google Scholar]
- 13. Goertz O, Vogelpohl J, Jettkant B, Daigeler A, Steinau HU, Steinstraesser L, Langer S. Burn model for in vivo investigations of microcirculatory changes. ePlasty 2009;9:e13. [PMC free article] [PubMed] [Google Scholar]
- 14. Morellini NM, Giles NL, Rea S, Adcroft KF, Falder S, King CE, Dunlop SA, Beazley LD, West AK, Wood FM, Fear MW. Exogenous metallothionein‐IIA promotes accelerated healing after a burn wound. Wound Repair Regen 2008;16:682–90. [DOI] [PubMed] [Google Scholar]
- 15. Bullock AJ, Pickavance P, Haddow DB, Rimmer S, MacNeil S. Development of a calcium‐chelating hydrogel for treatment of superficial burns and scalds. Regen Med 2010;5:55–64. [DOI] [PubMed] [Google Scholar]
- 16. Shakespeare P. Burn wound healing and skin substitutes. Burns 2001;27:517–22. [PubMed] [Google Scholar]
- 17. Vogt PM, Kolokythas P, Niederbichler A, Knobloch K, Reimers K, Choi CY. [Innovative wound therapy and skin substitutes for burns]. Chirurg 2007;78:335–42. [DOI] [PubMed] [Google Scholar]
- 18. Steinstraesser L, Ring A, Bals R, Steinau HU, Langer S. The human host defense peptide LL37/hCAP accelerates angiogenesis in PEGT/PBT biopolymers. Ann Plast Surg 2006;56:93–8. [DOI] [PubMed] [Google Scholar]
- 19. Nolte D, Zeintl H, Steinbauer M, Pickelmann S, Messmer K. Functional capillary density: an indicator of tissue perfusion? Int J Microcirc Clin Exp 1995;15:244–9. [DOI] [PubMed] [Google Scholar]


