Abstract
Impaired wound healing leading to skin ulceration is a serious complication of diabetes and may be caused by defective angiogenesis. Endothelial progenitor cells (EPCs) can augment neovascularisation in the ischaemic tissue. Experiments were performed to test the hypothesis that locally administered EPCs can promote wound healing in diabetes. Full‐thickness skin wounds were created on the dorsum of diabetic mice. EPCs were obtained from bone marrow mononuclear cells (BMMNCs) and applied topically to the wound immediately after surgery. Vehicle and non‐selective BMMNCs were used as controls. Wound size was measured on days 5, 10 and 14 after treatment, followed by resection, histological analysis and quantification of vascularity. Topical application of EPCs significantly promoted wound healing, as assessed by closure rate and wound vascularity. Immunostaining revealed that transplanted EPCs induced increased expression of vascular endothelial growth factor and basic fibroblast growth factor. Few EPCs were observed in the neovasculature based on in vivo staining of the functional vasculature. Ex vivo expanded EPCs promote wound healing in diabetic mice via mechanisms involving increased local cytokine expression and enhanced neovascularisation of the wound. This strategy exploiting the therapeutic capacity of autologously derived EPCs may be a novel approach to skin repair in diabetes.
Keywords: Basic fibroblast growth factor; Endothelial progenitor cells; Neovascularisation; Vascular endothelial growth factor; Wound healing
Introduction
Impaired wound healing is a major clinical problem in patients with diabetes (1). Poor healing of wounds is characterised by impaired angiogenesis and diminished formation of granulation tissue, with impairment of angiogenesis thought to play a pivotal role because this process is required for successful wound repair.
In the postnatal period, neovascularisation was originally thought to result from proliferation and migration of pre‐existing, fully differentiated endothelial cells (EC) residing within vessels; a process referred to as ‘angiogenesis’2, 3. Recently, however, endothelial progenitor cells (EPCs) have been isolated from peripheral blood cells and shown to undergo incorporation into new vessels in ischaemic regions 4, 5, 6, 7. This is a process consistent with ‘vasculogenesis’, a mechanism critical for establishing the primordial vascular network in the embryo (5). The therapeutic potential of EPC populations expanded ex vivo has recently been investigated in rodent models of ischaemic disorders that require neovascularisation, such as myocardial and hind‐limb ischaemia 8, 9, 10, 11, and EPC transplantation has been shown to promote liver regeneration and to improve survival after liver injury (12). These findings suggest that both angiogenesis and vasculogenesis may play major roles in the process of neovascularisation during adult wound healing.
The hypothesis that bone marrow (BM) is a reservoir of EPCs has prompted several groups to test the potential therapeutic effect of autologous cells of medullar origin in human ischaemic disease. BM mononuclear cells (BMMNCs) injected locally in patients with lower limb or cardiac ischaemia appear to contribute to regeneration of the vasculature 13, 14, 15. However, these studies constitute pioneering clinical trials and it is likely that a more purified cell therapy product will be used in the near future, thus limiting possible toxicity and side effects. In this context, autologous EPCs or macrophages purified from peripheral blood and expanded in vitro appear to be the best alternatives to crude BMMNCs.
Recent studies have shown that BM‐derived stem cells participate in cutaneous wound healing and skin regeneration, but have indicated little therapeutic advantage of these cells in excisional wound models, especially in healthy mice 6, 16. In contrast, human CD34+ peripheral blood mononuclear cells, which can function as endothelial progenitors, from healthy donors accelerate vascularisation and healing in full‐thickness skin wounds of hypoinsulinemic (streptozotocin‐treated) diabetic mice (17). A recent study showed that human peripheral blood‐derived EPCs transplanted into cutaneous wounds in nude mice accelerated dermal wound healing with increased recruitment of monocytes/macrophages and neovascularisation (18).
Humans with diabetes and diabetic animal models display poor wound healing and reductions in angiogenesis and granulation tissue formation 19, 20, 21. The circulating and wound levels of EPCs are also decreased in diabetes (22). The leptin receptor‐deficient diabetic (db/db) mouse is an established mouse model of type 2 diabetes and of deficient wound healing associated with diabetes (21). In this mouse model, topical application of vascular endothelial growth factor (VEGF) to cutaneous wounds has been shown to increase neovascularisation by mobilising and recruiting EPCs (23). We have also reported that dibutyryl cyclic adenosine monophosphate and topical sonic hedgehog gene therapy accelerate wound healing and promote angiogenesis by enhancing incorporation of EPCs into wounds in db/db mice 24, 25. In this study, we performed experiments using the db/db mouse as an autologous cell transplantation model of wound healing to test the hypothesis that topically applied EPCs can accelerate wound vascularisation and promote wound healing in diabetes.
Materials and methods
Animals
All protocols were approved by the Institutional Animal Care and Use Committee of Kyoto Prefectural University of Medicine and St. Elizabeth's Medical Center. Ten‐ to 12‐week‐old C57BL/6J‐m+/+ Leprdb homozygous mice (db/db mice) (Jackson Laboratory, Bar Harbor, ME) were used in the study.
Cell culture
Total BMMNCs were isolated from BM of diabetic mice by density gradient centrifugation with Histopaque‐1083 (Sigma, St Louis, MO) (13). Cells were plated on culture dishes coated with rat fibronectin (Sigma) and maintained in EC basal medium‐2 (EBM‐2) (Clonetics, San Diego, CA). The media was supplemented with EGM‐2 MV SingleQuots containing 5% fetal bovine serum (FBS), human VEGF‐1, human fibroblast growth factor‐2 (FGF‐2), human epidermal growth factor (EGF), insulin‐like growth factor‐1 (IGF‐1), and ascorbic acid. After 4 days in culture, non‐adherent cells were removed by washing with phosphate‐buffered saline (PBS), new media was applied, and the culture was maintained until day 7. Some cells were first incubated with dioctadecyl tetramethylindocarbocyanine (DiI)‐labelled acetylated low‐density lipoprotein (acLDL; Biomedical Technologies, Stoughton, MA) at 37°C for fluorescent labelling of EPCs before application, as described previously (26).
Wound model
The animals were housed in individual cages and wounds were created as described previously (19). In brief, mice were anaesthetised using an intraperitoneal injection of Avertin (16 mg/kg, Ben Venue Laboratories, Bedford, OH). The dorsal surface was shaved, and washed with povidone‐iodine solution and alcohol. Then a disposable 0·8‐cm diameter skin punch biopsy tool (Acuderm Inc., Fort Lauderdale, FL) was used to create a full‐thickness excisional wound down to the fascia. Tincture benzoin (Paddock Laboratories, Minneapolis, MN) was applied to the perimeter of the wound and allowed to dry. Immediately after surgery 5 × 104 EPCs in 10 µl PBS were placed into the wound bed; 5 × 104 BMMNCs in 10 µl PBS or 10 µl PBS vehicle were also applied topically as controls. A semipermeable transparent dressing (Tegaderm; 3M Health Care, St. Paul, MN) was placed over the wound and sealed at the edges with benzoin. All animals were given 1·0 ml of 0·9% saline intraperitoneally at the end of the surgical procedure and cages were placed on a heating pad until the mice fully recovered from anaesthesia. The dressing was maintained on the wound throughout the entire course of the experiments.
Wound‐closure analysis
Six mice in each group were examined. Wound closure was documented with a digital camera (Nikon Coolpix 995, Nikon, Japan) on days 0, 5, 10 and 14. Images were analysed using National Institute of Health (NIH) Image 1.60 software by tracing the wound margin with a fine resolution computer mouse and calculating the pixel area. Measurements were performed in duplicate and expressed as the percentage of closure from the original wound using the equation: %wound closure = 100 × (wound area on day 0 − wound area on day n)/wound area on day 0. The wound area data were compared using a paired Student t‐test.
Histological score
Wounds were harvested on days 5, 10 and 14 after injury, fixed in 10% neutral‐buffered formalin, and processed using normal histopathological approaches. Paraffin‐embedded tissue sections (5‐µm thick) were stained with haematoxylin and eosin and reviewed. Histological scoring was assigned in a blinded manner as described previously (19). Briefly, each specimen was given a score of 1–12: 1–3, none to minimal cell accumulation and granulation tissue or epithelial migration; 4 to 6, thin, immature granulation dominated by inflammatory cells, but with few fibroblasts, capillaries or collagen deposition and minimal epithelial migration; 7–9, moderately thick granulation tissue, ranging from domination by inflammatory cells to more fibroblasts and collagen deposition; and 10–12, thick vascular granulation tissue dominated by fibroblasts and extensive collagen deposition.
Evaluation of wound vascularity
For evaluation of neovascularisation during wound healing, the density of vascular structures was determined by immunostaining with anti‐PECAM‐1/CD31 IgG, followed by quantitative analysis. Wounds were harvested at intervals, fixed in 4% paraformaldehyde in PBS overnight at 4°C, quenched in PBS for 1 hour (4°C), and sedimented in 15% sucrose in PBS overnight at 4°C. The tissues were embedded in OCT (Electron Microscopy Sciences, Washington, PA) and frozen (−80°C). Cryostat sections (10 µm) were placed on poly‐lysine‐coated slides. The slides were washed with PBS and endogenous peroxidase activity was blocked with PBS containing 0·3% hydrogen peroxide for 30 minutes at room temperature. Samples were blocked using normal 10% goat serum in PBS for 30 minutes at room temperature. Sequential slides were incubated with a rat anti‐mouse PECAM (CD31) monoclonal antibody (1:2500 dilution, Pharmingen, San Diego, CA) with normal 5% goat serum for 1 hour at 37°C and then overnight at 4°C. Normal rat IgG was used as a negative control. Slides were rinsed with PBS and then incubated with biotinylated anti‐rat IgG (1:500, 1 hour at room temperature, Vector Laboratories, Burlingame, CA). The slides were washed with PBS and avidin–biotin complex (Vector Laboratories) was added. The slides were rinsed again in PBS, developed with chromagen 3,3′‐diaminobenzidine (Sigma‐Aldrich), and lightly stained with haematoxylin. For each slide, three images from different areas of the cross‐section were digitally captured and the vascular density was analysed using NIH Image software. The vessel density was calculated as: % vascularisation = (PECAM‐1‐positive area/total wound bed area) × 100.
In situ staining of the functional vasculature in the wounds
Before sacrifice, a subgroup of mice with transplanted DiI‐labelled EPCs was prepared for vascular labelling with FITC‐conjugated Bandeiraea simplicifolia lectin‐1I (BS‐1 lectin, Sigma). Ten minutes before sacrifice, 75 µl of BS‐1 lectin was injected into the left ventricle to visualise the functional vasculature in the healing wound. The lectin was allowed to perfuse for 10 minutes, after which the chest was entered, the left ventricle was cannulated, and the right ventricle was incised. The animal was perfused with PBS and fixed with 4% paraformaldehyde. The wounds were then harvested from the dorsum. Tissue sections were prepared as described above. Sections were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (1:5000) and mounted in aqueous mounting medium. Tissue sections were reviewed using a computer‐assisted Nikon fluorescence microscope with a digital camera (ECLIPS TE200, Nikon Inc).
Immunohistochemistry
Wounds from animals with transplanted DiI‐labelled EPCs were harvested on days 5, 10 and 14, and prepared for frozen sections as described above. Cryostat sections (6 µm) were washed with PBS and endogenous peroxidase activity was blocked with PBS containing 0·3% hydrogen peroxide for 30 minutes at room temperature. Samples were blocked using normal 10% goat serum in PBS for 30 minutes at room temperature. Sections were incubated overnight at 4°C with a rabbit polyclonal antibody against basic fibroblast growth factor (bFGF) (1:500) and VEGF (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA) diluted in 2% goat serum/PBS, followed by incubation for 1 hour with a secondary antibody, Cy2‐conjugated anti‐rabbit IgG (1:500) (Jackson ImmunoResearch). Normal rabbit IgG was used as a negative control. Nuclei were counterstained with DAPI. Tissue sections were reviewed using Nikon fluorescence microscopy.
Statistical analysis
All results are presented as mean ± SEM. Statistical comparisons between two groups were performed by Student t‐test. Analysis of variance was used for serial analyses. P < 0·05 was considered to indicate a significant difference in all analyses. All in vitro experiments were repeated at least three times.
Results
EPC accelerates healing and neovascularisation of wounds in diabetic mice
To investigate the effects of EPC transplantation on wound closure, we created full‐thickness skin wounds in the dorsal skin of db/db mice and applied EPCs, BMMNCs or PBS(pH 7·4) just after wounding. Wound areas on days 0 and 14 in PBS‐, BMMNC‐ and EPC‐treated diabetic mice are shown in Figure 1A. On day 14, EPC‐treated wounds showed about 65% epithelialisation, while <50% of the wound was epithelialised in the PBS group. EPC treatment produced a significantly smaller wound area after 5 days (Figure 1B). By day 14, this difference was maximal (% wound closure in the control versus BMMNC versus EPC groups: on day 5, 4·0 ± 4·85% versus 12·4 ± 5·90% versus 20·4 ± 6·23%, P < 0·0001; on day 10, 16·2 ± 4·92% versus 22·2 ± 3·56% versus 37·6 ± 6·62%, P < 0·0001; on day 14:41·6 ± 4·72% versus 52·8 ± 7·76% versus 64·4 ± 3·65%, P < 0·0001; Figure 1B).
Figure 1.
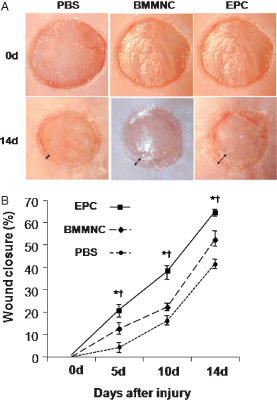
Effects of endothelial progenitor cell (EPC) transplantation on full‐thickness skin wound closure in genetically diabetic mice. EPCs, bone marrow mononuclear cells (BMMNCs) or phosphate‐buffered saline (PBS) were applied to the wound. (A) Macroscopic appearance of wounds after each treatment. (B) % wound closure. Topical application of ex vivo expanded EPCs significantly accelerated wound closure compared to controls (n = 5 per group, *P < 0·001 versus PBS, † P < 0·001 versus BMMNC).
Histological scoring
Haematoxylin and eosin staining showed thick granulation tissue and re‐epithelialisation in EPC‐treated wounds, whereas thin granulation tissue was found in the BMMNC and PBS groups (Figure 2A). Several mature blood vessels were present in granulation tissue of EPC‐treated wounds, whereas only a few vessels were found in granulation tissue in the other groups. Histological scores for EPC‐treated wounds were significantly higher than those for BMMNC‐ and PBS‐treated wounds (Figure 2B).
Figure 2.
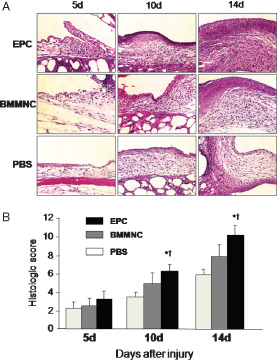
Topical application of endothelial progenitor cells (EPCs) accelerated formation of granulation tissue. (A) Sections from wounds of diabetic mice treated with EPCs, bone marrow mononuclear cells (BMMNCs) and phosphate‐buffered saline (PBS) were stained with H&E. Wound margins on days 5, 10 and 14 showed an improved wound appearance and increased granulation tissue with time for EPC‐treated wounds. In the PBS group, the wound bed shows a thin layer of granulation tissue over adipose tissue. In the EPC group, the wound bed shows a thick layer of granulation tissue covered with epithelium. (B) Histological score. The EPC group had a significantly higher histological score compared with the other two groups (n = 5 per group, *P < 0·001 versus PBS, † P < 0·001 versus BMMNC).
Wound vascularity
Since healing of skin wounds requires neovascularisation, we next evaluated the vascularity of wound granulation tissue. Wound angiogenesis was analysed by CD31 immunostaining of 10‐µm frozen sections to visualise neovascularisation. CD31 staining of wound granulation tissue on day 14 in PBS‐, BMMNC‐ and EPC‐treated diabetic mice is shown in Figure 3A. Tiny, narrow new vessels at the wound margin were present in the PBS and BMMNC groups, whereas larger and thicker vessels were growing widely in the EPC group on day 14. Topical EPC application significantly enhanced wound vascularity, as assessed by the % CD31 staining area (Figure 3B).
Figure 3.
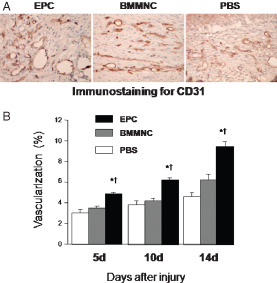
Effects of topical application of endothelial progenitor cells (EPCsI on wound vascularity. (A) The sections were stained with an antibody against CD31. On day 14, tiny and narrow new vessels at the wound margin were present in the bone marrow mononuclear cell (BMMNC) and phosphate‐buffered saline (PBS) groups, while larger and thicker vessels were growing widely in the centre of the wound in the EPC group. Original magnification ×100. (B) Topical application of EPCs significantly promoted wound vascularity (n = 5 per group, *P < 0·001 versus PBS, † P < 0·001 versus BMMNC).
Incorporation of EPCs into the vasculature
We also evaluated the functional vessels by injection of FITC‐conjugated BS1‐lectin. Neovascularisation at the margin of EPC‐treated wounds in diabetic mice on days 10 and 14 is shown in Figure 4A. Areas of red and green fluorescence indicate DiI‐labelled EPCs and pre‐existing or newly formed mature functional vessels, respectively. Therefore, yellow areas indicate EPCs incorporated into the functional neovasculature. These yellow areas were observed in EPC‐treated wounds, but relatively in rare instances only one or two yellow areas were found in 10 high power fields.
Figure 4.
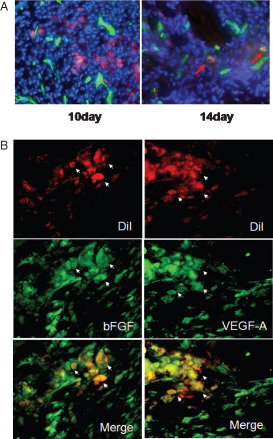
Endothelial progenitor cell (EPC) tracking. For EPC tracking, cells were marked with red fluorescent carbocyanine dioctadecyl tetramethylindocarbocyanine (DiI) dye‐labelled acetylated LDL. (A) Many DiI‐labelled EPCs were observed around the neovasculature by in vivo staining of the functional vasculature with FITC‐labelled Bandeiraea simplicifolia lectin I on day 10. In contrast, only a few DiI‐labelled EPCs were observed in the neovasculature on day 14 (red arrow). (B) Immunostaining revealed that DiI‐labelled EPCs expressed vascular endothelial growth factor‐A (VEGF‐A) and basic fibroblast growth factor (bFGF).
bFGF and VEGF expression of transplanted EPCs
Immunostaining performed to evaluate paracrine effects of EPCs revealed that transplanted EPCs induced increased expression of VEGF and bFGF, both of which act as angiogenic growth factors in wounds (Figure 4B).
Discussion
Recent studies suggest that BM‐derived progenitor cells contribute to skin wound healing in various organs through formation of endothelial cells, lymphatic endothelial cells, keratinocytes and myofibroblasts (27). On the basis of these findings, clinical evaluation has shown that topical application of BM cells improves impaired wound healing (28). In this study, we evaluated the efficacy and mechanisms of topical BM‐derived cells in wound healing, with a focus on neovascularisation. Topical application of BMMNCs to the wound resulted in marked promotion of wound closure with abundant neovascularisation and granulation tissue. However, topical application of ex vivo expanded EPCs showed greater effects on wound closure than BMMNCs. Neovascularisation was significantly increased in EPC‐treated wounds compared to wounds treated with BMMNCs. Total BMMNCs contain various types of cells, including progenitor cells and monocytes, which contribute to accelerating wound healing, and lymphocytes, which have little effect on wound healing. Of the progenitor cells, EPCs may be the most effective promoters of wound healing, since neovascularisation plays a pivotal role in this process.
The high level of CD31‐positive blood vessels indicated that EPCs increase neovascularisation. Examination of the mechanism of neovascularisation mediated by topically applied EPCs requires consideration of both angiogenesis and vasculogenesis, as mentioned above. The isolation of EPCs from peripheral blood cells has focused attention on vasculogenesis as a new mechanism of wound neovascularisation (29). A recent study showed that human peripheral blood‐derived EPCs injected into cutaneous wounds in nude mice were directly incorporated into newly formed capillaries, with about 7·5% of neovessels shown to contain the injected human EPCs (18). Contrary to these observations, we found that very few EPCs incorporated into the neovasculature in the PBS and BMMNC groups, and that incorporation of EPCs into the neovasculature only increased slightly in the EPC group. We speculate that the diabetic condition might be the major reason for the low rate of EPC incorporation, since previous studies have suggested impaired function of EPCs in diabetes (22). These results suggest that some of the topically applied EPCs may differentiate and incorporate into the neovasculature, but that the incorporated amount of cells is insufficient to accelerate wound healing. This indicates that vasculogenesis may not be the main mechanism of action of topically applied EPCs in a diabetic condition.
To evaluate the effects of EPCs on angiogenesis, we examined VEGF and bFGF expressions, since both these molecules are major angiogenic factors in EPCs. EPCs strongly expressed both VEGF and bFGF, and therefore these cells might act as a source of growth factors and promote local angiogenesis by paracrine effects. Therefore, we suggest that neovascularisation induced by topically applied EPCs is mainly mediated by promotion of angiogenesis, rather than vasculogenesis. In diabetes, the levels of growth factors essential for wound healing, such as VEGF‐A, are reduced (24). Impairment of EPC function has also been shown in patients with type 2 diabetes (30). Therefore, application of ex vivo expanded EPCs as a source of growth factors can compensate for changes induced by diabetes. In this study, EPCs were applied to wounds only once, but significant acceleration of wound healing was observed in the early stage of the healing process. Further studies are required to determine if repeated application of EPCs enhances the acceleration of healing.
In conclusion, our results demonstrate that topical application of EPCs accelerates cutaneous wound repair and angiogenesis in a murine model of diabetes, in part by providing a source of growth factors (VEGF and bFGF) that promote wound healing and angiogenesis. Clinically, topical application of BM cells to the wound is viable and may represent a new strategy for improvement of impaired wound healing in patients with diabetes, by exploiting the therapeutic capacity of autologously derived EPCs.
Acknowledgements
This work was supported by a grant from the Japanese Ministry of Education, Science, Sports and Culture (to JA).
The authors state no conflict of interest.
References
- 1. Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361: 1545–51. [DOI] [PubMed] [Google Scholar]
- 2. Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992;267:10931–4. [PubMed] [Google Scholar]
- 3. Risau W. Differentiation of endothelium. FASEB J 1995;9:926–33. [PubMed] [Google Scholar]
- 4. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–7. [DOI] [PubMed] [Google Scholar]
- 5. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999;85:221–8. [DOI] [PubMed] [Google Scholar]
- 6. Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 2003;196:245–50. [DOI] [PubMed] [Google Scholar]
- 7. Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow‐derived cells. Blood 2005;105:1068–77. [DOI] [PubMed] [Google Scholar]
- 8. Kalka C, Masuda H, Takahashi T, Kalka‐Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A 2000;97:3422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood‐derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest 2000;105:1527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001;103:634–7. [DOI] [PubMed] [Google Scholar]
- 11. Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 2003;107:461–8. [DOI] [PubMed] [Google Scholar]
- 12. Taniguchi E, Kin M, Torimura T, Nakamura T, Kumemura H, Hanada S, Hisamoto T, Yoshida T, Kawaguchi T, Baba S, Maeyama M, Koga H, Harada M, Kumashiro R, Ueno T, Mizuno S, Ikeda H, Imaizumi T, Murohara T, Sata M. Endothelial progenitor cell transplantation improves the survival following liver injury in mice. Gastroenterology 2006;130:521–31. [DOI] [PubMed] [Google Scholar]
- 13. Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kögler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106: 1913–8. [DOI] [PubMed] [Google Scholar]
- 14. Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 2001;103:897–903. [DOI] [PubMed] [Google Scholar]
- 15. Tateishi‐Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone‐marrow cells: a pilot study and a randomised controlled trial. Lancet 2002;360:427–35. [DOI] [PubMed] [Google Scholar]
- 16. Stepanovic V, Awad O, Jiao C, Dunnwald M, Schatteman GC. Leprdb diabetic mouse bone marrow cells inhibit skin wound vascularization but promote wound healing. Circ Res 2003;92:1247–53. [DOI] [PubMed] [Google Scholar]
- 17. Sivan‐Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res 2003;40:368–77. [DOI] [PubMed] [Google Scholar]
- 18. Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, Jang HS, Lee JS, Byun J, Choi JH, Jeon ES, Kim DK. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells 2005;23:1571–8. [DOI] [PubMed] [Google Scholar]
- 19. Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 1990;136:1235–46. [PMC free article] [PubMed] [Google Scholar]
- 20. Werner S, Breeden M, Hubner G, Greenhalgh DG, Longaker MT. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J Invest Dermatol 1994;103:469–73. [DOI] [PubMed] [Google Scholar]
- 21. Goodson WH 3rd, Hung TK. Studies of wound healing in experimental diabetes mellitus. J Surg Res 1977;22:221–7. [DOI] [PubMed] [Google Scholar]
- 22. Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal 2008;10: 1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow‐derived cells. Am J Pathol 2004;164:1935–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asai J, Takenaka H, Katoh N, Kishimoto S. Dibutyryl cAMP influences endothelial progenitor cell recruitment during wound neovascularization. J Invest Dermatol 2006;126:1159–67. [DOI] [PubMed] [Google Scholar]
- 25. Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, Eaton E, Iwakura A, Tsutsumi Y, Hamada H, Kishimoto S, Thorne T, Kishore R, Losordo DW. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell‐mediated microvascular remodeling. Circulation 2006;113:2413–24. [DOI] [PubMed] [Google Scholar]
- 26. Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch‐Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell‐derived factor‐1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003;107:1322–8. [DOI] [PubMed] [Google Scholar]
- 27. Falanga V. Bone marrow cells can manipulate healing. Blood 2009;113:982–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow‐derived cells. Arch Dermatol 2003;139:510–6. [DOI] [PubMed] [Google Scholar]
- 29. Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO‐mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF‐1 alpha. J Clin Invest 2007;117: 1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002;106:2781–6. [DOI] [PubMed] [Google Scholar]


