Abstract
Skin breakdown and infiltration of skin flora are key causative elements in poststernotomy wound infections. We hypothesised that surgical incision management (SIM) using negative pressure wound therapy over closed surgical incisions for 6–7 days would reduce wound infections in a comprehensive poststernotomy patient population. ‘All comers’ undergoing median sternotomy at our institution were analysed prospectively from 1 September to 15 October 2013 (study group, n = 237) and retrospectively from January 2008 to December 2009 (historical control group, n = 3508). The study group had SIM (Prevena™ Therapy) placed immediately after skin suturing and applied at −125 mmHg for 6–7 days, whereas control group received conventional sterile wound tape dressings. Primary endpoint was wound infection within 30 days. Study group had a significantly lower infection rate than control group: 1·3% (3 patients) versus 3·4% (119 patients), respectively (P < 0·05; odds ratio 2·74). In the study group, when the foam dressing was removed after 6–7 days, the incision was primarily closed in 234 of 237 patients (98·7%). SIM over clean, closed incisions for the first 6‐7 postoperative days significantly reduced the incidence of wound infection after median sternotomy. Based on these data SIM may be cost‐effective in patients undergoing cardiac surgery.
Keywords: Mediastinitis, Poststernotomy infection, Prevena Therapy, Surgical incision management, Surgical site infection
Introduction
The problem
With regard to the incidence of wound infections after median sternotomy, it is assumed that in Germany alone, 4000–6000 patients per year undergo surgical revision with application of negative pressure wound therapy (NPWT; V.A.C.® Therapy, KCI, San Antonio, TX) to the open surgical wound and often additional plastic surgery 1, 2. For a particular patient this means repeated interventions under general anaesthesia and a prolonged hospital stay of 3 weeks on average or, sometimes, even a fatal outcome 1, 2, 3. From the economics point of view, excess costs arise primarily owing to the prolonged hospital stay and the need for repeated surgical procedures, amounting to about 9000€ per case for the hospital 3.
The cause
Suture breakdown, which allows infiltration of bacteria into the wound, is the primary cause of poststernotomy infection, and Gram‐positive bacteria are identified in ≥80% of cases 1, 4, 5. Median sternotomy as the standard approach in cardiac surgery shares a critical factor with most orthopaedic interventions: bone with injured periosteum and osteosynthesis material susceptible to bacterial infection directly beneath the skin incision. In addition, after sternotomy the skin incision is subject to traction forces that pull apart skin edges, especially in obese patients; in female patients in supine position the skin edges are pulled apart by the breasts (Figure 1) and in sitting position the inframammary fold is bent (Figure 2).
Figure 1.
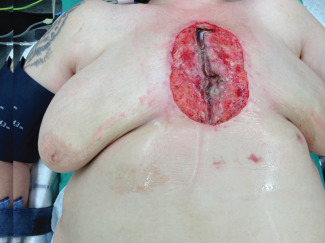
Patient's open wound was debrided and treated with negative pressure wound therapy (NPWT) following deep wound infection after sternotomy. Skin edges were pulled apart by breasts in supine position (traction forces on skin incision).
Figure 2.
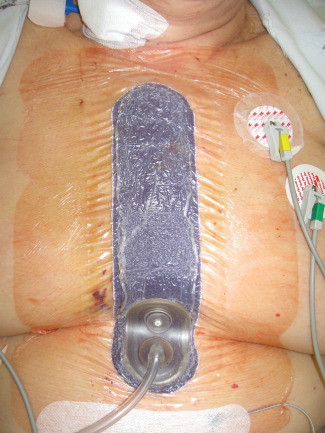
Patient in sitting position, with surgical incision management (SIM) foam dressing: The incision is bent in the inframammary fold provoking traction forces that would pull apart skin edges. However, SIM dressing conforms to body contours and helps to hold incision edges together.
The concept
Surgical incision management (SIM; Prevena™ Incision Management System, KCI, San Antonio, TX, USA) is an NPWT system designed specifically for use over surgical incisions. Both SIM and incisional NPWT have been evaluated experimentally as well as tested successfully after orthopaedic surgery 6, 7, 8. Furthermore, we have evaluated SIM in a high‐risk group of obese patients undergoing cardiac surgery via median sternotomy 9. The SIM dressing conforms to body contours, helping to hold incision edges together at least to a certain degree (Figure 2), and provides a clean, protected wound environment until dressing removal. Furthermore, there is experimental evidence that SIM and NPWT support wound healing by improving wound secretion drainage 6, 8.
Study purpose and hypothesis
In addition to our study in high‐risk patients mentioned above, we performed this study to compare SIM and conventional wound dressings in an ‘all comers’ population with special regard to wound complications and infections. We hypothesised that SIM applied immediately after skin closure and continued for 6–7 days would reduce the number of poststernotomy wound infections requiring surgical revision.
Methods
From 1 September to 15 October 2013 we performed a prospective ‘all comers’ cohort study in patients undergoing cardiac surgery via median sternotomy at our institution. A SIM dressing was placed immediately after skin suturing and negative pressure of −125 mmHg was applied for 6–7 days (SIM study group; n = 237). A historical cohort of ‘all comers’ undergoing cardiac surgery via median sternotomy between January 2008 and December 2009 served as a control group (n = 3508) [in 2010 we started SIM at our institution in high‐risk patients 9]. The control group received conventional wound tape dressings, which were changed for the first time on the first or second postoperative day and every 1–2 days thereafter. Study group wounds were inspected for the first time after SIM removal and every 1–2 days thereafter. Primary endpoint was wound infection within 30 days requiring surgical revision and application of NPWT to the open surgical wound in most cases.
Results
SIM was well tolerated in all patients, and all patients were followed up for at least 30 days. In the study group, a total of 258 patients were enrolled, but 21 (8·1%) patients were excluded from further analyses owing to insufficient therapy (i.e. dressing air leaks) or death <30 days. Of the remaining 237 patients, 3 (1·3%) suffered from wound infections. One patient suffered from an infected left ventricular assist device and a second patient suffered from mediastinitis owing to sternum mobility after postoperative delirium. A third patient underwent superficial wound infection without sternal involvement. For 98·7% (234/237) of study group patients without infection, the incision was primarily closed at removal of the SIM foam dressing. In the control group (n = 3508), the incidence of sternal wound infection requiring surgical revision (n = 119) was 3·4% (P < 0·05; odds ratio 2·74).
Discussion
Poststernotomy wound infections
Gårdlund et al. identified three categories of postoperative mediastinitis based on a retrospective study of 126 cases 4. Type 1 mediastinitis involved coagulase‐negative staphylococci, which accessed the wound through sternal dehiscence often associated with patient obesity or sometimes chronic obstructive pulmonary disease. Staphylococcus aureus from perioperative contamination of the mediastinal space characterised Type 2 mediastinitis and Type 3 resulted from aerobic Gram‐negative rods from concomitant infections (primarily pneumonia) in the immediate postoperative period 4. Sternal dehiscence was seen in 68% (86/126) of the mediastinitis patients 4, which highlights the importance of skin breakdown prevention as a strategy for reducing the risk of mediastinitis.
Concept of SIM
Atkins et al. recently demonstrated that incisional NPWT over clean, closed poststernotomy incisions decreased wound complications. In their retrospective study of 57 patients treated with incisional NPWT, patient risk factors such as obesity and diabetes warranted anticipation of three poststernotomy wound infections; however, no wound complications developed 10. Application of NPWT over closed incisions in various wound types has been shown to provide protection from external contamination and to facilitate drainage of wound secretions 6, 7, 8, 11.
Impact of SIM on wound infection
In our study, 119 of 3508 (3·4%) patients in the control group contracted poststernotomy wound infection, whereas only 3 of 237 (1·3%) patients in the SIM group experienced infection (P < 0·05; odds ratio 2·74). The fact that no wound infections occurred after removal of the foam dressing may be owing to the extent of wound healing during the 6–7 days of SIM application. Sufficient closure during that period appeared to provide a barrier against later skin breakdown and consequent infiltration of skin flora into the wound (the causative factor in poststernotomy wound infections).
Economic aspects of SIM
According to a recent study by Graf et al. on economic aspects of deep sternal wound infections, the median overall costs of treating a case with deep sternal wound infection (i.e. requiring surgical revision) were 36 261€ compared with 13 356€ per patient without infection; additional overall costs of 22 905€ per case arose for society 3. The median reimbursement from health care insurance companies was 27 107€ per case, which means a financial loss of 9154€ per patient for the hospital and of 13 751€ for the health care insurance company 3.
Assuming a reduction of wound infections requiring surgical revision by 62% (3·4% vs. 1·3%) based on this study and a number of affected patients of 4000–6000 per year in Germany, the avoidable economic loss per year in Germany amounts to about 60 000 000€–90 000 000€, which under current conditions of reimbursement have to be paid in two fifths by the hospitals (24 000 000€–36 000 000€) and in three fifths by the health care insurance companies (36 000 000€–54 000 000€).
Based on device costs of ∼350€ per SIM unit, comprehensive use of this therapy in cardiac surgery in Germany (90 000 cases per year) would cost about 30 000 000€ per year, which should be seen in relation to the annual cost of 60 000 000€–90 000 000€ calculated above. Therefore, use of SIM may be cost‐effective for hospitals and for health care insurance companies not only in high‐risk patient groups but also in the general cardiac surgery patient population.
Conclusions and considerations
We conclude that applying SIM over clean, closed incisions for the first 6–7 postoperative days reduced the likelihood of postoperative wound infection after median sternotomy not only in high‐risk patients but also in a comprehensive patient population.
This may be achieved by the mechanical stabilisation of incision edges and the clean, protected environment provided for 6–7 days. In addition, wound healing facilitated by improved wound secretion drainage establishes an adequate microbacterial barrier 1 week postoperation, which in turn may contribute substantially to the clinically significant reduction rate of wound infections.
Based on our data, the potential economic benefit of comprehensive use of SIM in Germany may amount to a saving of between 60 000 000€ and 90 000 000€ per year, which could be realised in two fifths by the hospitals (24 000 000€–36 000 000 €) and in three fifths by the health care insurance companies (36 000 000€–54 000 000€).
Conflicts of Interest
Dr OG presented as a faculty member during the 2013 International Surgical Wound Forum (ISWF), an annual educational event sponsored by Kinetic Concepts, Inc. (KCI). His article is part of a KCI‐funded educational supplement based on 2013 ISWF faculty presentations about wound care strategies using negative pressure wound therapy (V.A.C.® Therapy and Prevena™ Incision Management System) over closed surgical incisions and negative pressure therapy (V.A.C.® Abdominal Dressing System and ABThera™ Open Abdomen Negative Pressure Therapy) to treat the open abdomen. We thank Anne Gale and KCI for providing editorial assistance with the manuscript. PM and Drs AN, BT, FH, MH and RH state no conflict of interests or financial relationship with KCI.
References
- 1. El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61:1030–6. [DOI] [PubMed] [Google Scholar]
- 2. Gelape CL. Surgical wound infection following heart surgery. Arq Bras Cardiol 2007;89:e3–9. [DOI] [PubMed] [Google Scholar]
- 3. Graf K, Ott E, Vonberg RP, Kuehn C, Haverich A, Chaberny IF. Economic aspects of deep sternal wound infections. Eur J Cardiothorac Surg 2010;37:893–6. [DOI] [PubMed] [Google Scholar]
- 4. Gårdlund B, Bitkover CY, Vaage J. Postoperative mediastinitis in cardiac surgery – microbiology and pathogenesis. Eur J Cardiothorac Surg 2002;21:825–30. [DOI] [PubMed] [Google Scholar]
- 5. Durham SJ, Gold JP. Late complications of cardiac surgery. In: Cohn LH, editor. Cardiac surgery in the adult. New York: McGraw‐Hill, 2008:535–48. [Google Scholar]
- 6. Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz‐Drost S, Schlechtweg P, Pauser J, Gelse K, Brem MH. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop 2012;36:719–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stannard JP, Atkins BZ, O'Malley D, Singh H, Bernstein B, Fahey M, Masden D, Attinger CE. Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage 2009;55:58–66. [PubMed] [Google Scholar]
- 8. Stannard JP, Robinson JT, Anderson ER, McGwin G, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. J Trauma 2006;60:1301–6. [DOI] [PubMed] [Google Scholar]
- 9. Grauhan O, Navasardyan A, Hofmann M, Muller P, Stein J, Hetzer R. Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg 2013;145:1387–92. [DOI] [PubMed] [Google Scholar]
- 10. Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov 2009;16:140–6. [DOI] [PubMed] [Google Scholar]
- 11. Doss M, Martens S, Wood JP, Wolff JD, Baier C, Moritz A. Vacuum‐assisted suction drainage versus conventional treatment in the management of poststernotomy osteomyelitis. Eur J Cardiothorac Surg 2002;22:934–8. [DOI] [PubMed] [Google Scholar]


