Abstract
Small intestine submucosa (SIS), a bioactive extracellular matrix (ECM) containing critical components of the ECM including collagens, proteoglycans, and glycosaminoglycans, has been widely used for wound healing. The purpose of this study was to investigate the interaction between SIS and matrix metalloproteinases (MMPs). MMP‐1, MMP‐2, and MMP‐9 displayed different binding affinities, indicated by a loss in activity in solution upon incubation with SIS at 53·8%, 85·9%, and 36·9% over 24 hours, respectively. A cell migration study was conducted to evaluate the effects of MMPs and SIS on keratinocytes. The results indicated that MMPs inhibit keratinocyte migration in vitro, and that the inhibition can be significantly reduced by pre‐incubating the MMP solution with SIS. To evaluate activity in vivo a diabetic mouse wound healing study was conducted. Biopsy samples were collected on different days for analysis of MMP levels by gelatin zymography. MMP activity was found to be attenuated by SIS treatment on day 3 after wounding. On day 7, the attenuation became less significant indicating that the MMP binding ability of SIS had become saturated. SIS was able to reduce MMP activity immediately, and may reduce the inhibitory effects of MMPs on keratinocyte migration.
Keywords: MMP‐1, MMP‐2, MMP‐9, Small intestine submucosa, Wound matrix
INTRODUCTION
Matrix metalloproteinases (MMPs) are a family of zinc endopeptidases that are responsible for the degradation of a wide range of extracellular matrix (ECM) components including collagen and proteoglycans. There are currently more than 20 recognised members of this protease family. MMPs are believed to play an important role in wound healing. Under normal circumstances, the production and activation of MMPs in fibroblasts is closely regulated by numerous factors including mechanical strain, growth factors, and interaction of the cells with the ECM. This balance of activity allows normal wounds to progress through the healing stages. However, MMP activity may also impede healing by cleavage of growth factor receptors from the cell surface, shedding of cell adhesion molecules, and activation of other MMPs 1, 2. Various studies have reported the presence of high MMP levels and activity in chronic wound fluid (CWF) 3, 4. Unfortunately, the techniques used in many of these studies are not specific enough to clearly differentiate MMP species. This has led to controversy surrounding MMP levels in chronic wounds.
MMP family members that have been reported to display crucial activity in wounds include interstitial collagenases MMP‐1 (Collagenase 1), MMP‐8 (Collagenase 2), MMP‐13 (Collagenase 3); and gelatinases MMP‐2 (Gelatinase A) and MMP‐9 (Gelatinase B). In the collagenase subgroups, MMP‐1 and MMP‐8 have been found to be predominant in chronic wounds (5). MMP‐1 activity directly impacts keratinocyte migration and MMP‐8, which is secreted by more than 15 cell types, has the ability to cleave a broad range of substrates. MMP‐13 has been found to be expressed abundantly by fibroblasts deep in the chronic ulcer bed, but curiously is absent from acute wounds (6). The gelatinases (MMP‐2 and MMP‐9) play crucial roles in ECM remodeling and reepithelialisation of wounds. They further degrade the collagen‐1 cleavage products of MMP‐1 and directly cleave collagen‐IV, the primary collagen in basement membranes (7). Ladwig et al. reported that the elevation of MMP‐9 levels seen in CWF tended to decrease as wounds healed (8). In a study comparing CWF to serum and mastectomy fluid, Wysocki et al. (3) reported that CWF levels of MMP‐2 and MMP‐9 were increased 3‐ to 5‐fold and 10‐ to 20‐fold, respectively, over those found in mastectomy fluid, which contained markedly increased levels (5‐ to 10‐fold) of these MMPs compared with serum. Furthermore, zymograms of CWF displayed several products different from the proenzyme forms that were not observed in mastectomy fluid. It was postulated that these smaller forms most likely corresponded to the activated forms of the enzymes and thus indicated that not only were total MMP levels elevated but that levels of other active forms were also increased. These findings suggest that non‐healing ulcers develop an environment containing high levels of activated MMPs, which may result in chronic tissue turnover and failed wound closure. Over expression of MMPs observed in CWF is associated with tissue damage and a variety of elevated inflammatory states. MMPs have also been found to be able to degrade fibronectin, growth factors, and other proteins vital to wound healing (9). Therefore, it is logical to hypothesise that the excess levels of MMPs found in chronic wounds are a key contributor to wound chronicity and that reducing elevated levels of MMPs in chronic wounds should promote healing. Clinical evidence supporting this hypothesis was provided by Trengove and colleagues (4) who reported that levels of proteases decreased as chronic venous ulcers began to heal, and by Ladwig et al. (8) who reported that optimal healing of chronic pressure ulcers correlated with low values of the ratio of MMP‐9/tissue inhibitor of metalloproteinases‐1 (TIMP‐1). Thus, control of MMP levels may improve the poor healing conditions found in chronic wounds.
Small intestine submucosa (SIS) Wound Matrix (Cook Biotech, West Lafayette, IN) is a bioactive ECM for wound healing that contains critical components of ECM including collagens (10), proteoglycans (11), glycosaminoglycans (12), and growth factors (13). SIS derived from the porcine jejunum has been used as an acellular biologic scaffold in surgical applications 14, 15, 16. It has also been used to stimulate the closure of chronic non‐healing wounds (17). Results from many clinical studies indicate that SIS can be used to treat difficult‐to‐heal or chronic wounds 18, 19, 20, 21, including venous leg ulcers 17, 22 and diabetic foot ulcers (23). The proven bioactivity includes inducing growth factor secretion (24), promoting cell proliferation (25), supporting angiogenesis 25, 26, and promoting reepithelialisation 27, 28. To understand how SIS provides conditions for optimising healing, various studies at the molecular level have been conducted investigating interaction between SIS and different cytokines (29). As Shultz et al. (30) point out, dynamic interactions between growth factors/MMPs and ECM are integral to wound healing. The ECM can directly bind to and release certain growth factors, which may serve to sequester and protect growth factors from degradation, and/or enhance their activity. The purpose of this study was to investigate the interaction between SIS and human MMP‐1, MMP‐2, and MMP‐9 in vitro and in vivo. Two approaches were planned for studying the interaction between SIS and MMPs. First, the soluble bioactive substances were extracted from SIS. These extracts were then tested for inhibitory effects on MMP‐1, MMP‐2, and MMP‐9. Second, a solution depletion experiment was designed to study the physical binding of MMPs to SIS. In this case, SIS materials were incubated with MMP‐1, MMP‐2, and MMP‐9. At different time points, MMP activity in the solution phase was determined using a fluorogenic substrate. The time course of loss in MMP activity in the presence of SIS material was thus determined.
To examine the effect of SIS in vivo, a diabetic mouse wound healing study was conducted. Biopsy samples of both treated and untreated wounds were collected on different days for analysis of MMP levels by gelatin zymography. The effects of MMPs on cell migration in the presence of SIS extracts was also examined by a cell migration assay using human keratinocytes. The data from this study adds to the understanding of the healing properties of SIS, other than its scaffolding functions.
MATERIALS AND METHODS
All animals were treated humanely according to the guidelines provided in the Guide for the Care and Use of Laboratory Animals, published by the National Institute of Health. This study was approved by the Institutional Animal Care and Use Committee (IACUC) of University of North Texas Health Science Center, Fort Worth, TX.
Unless otherwise indicated, all chemicals and substrates were purchased from Sigma Aldrich Chemical Company (St. Louis, MO). FITC‐labeled collagen and rhodamine‐labeled elastin were obtained from Elastin Products Company (Owensville, MO). The fluorescence substrates used for MMP activity tests were purchased from MP Biomedicals, Inc. (Solon, OH). The buffer was 50 mM Tris with 100 mM NaCl and 10 mM CaCl2, and 0·05% Brij‐35 (pH 7·4).
Activation of MMPs
Human MMP‐1, MMP‐2, and MMP‐9 were purchased from Abcam (Cambridge, MA). MMP‐1 was already in active form, but MMP‐2 and MMP‐9 required activation. MMP‐1 was a recombinant fragment corresponding to amino acids 101–269 of human MMP‐1. MMP‐2 was a full‐length protein purified from human fibroblast cell culture media. MMP‐9 was Pro‐MMP‐9 from cell culture media of stimulated human fibroblasts.
MMP‐2 and MMP‐9 were activated using 4‐aminophenylmercuric acetate (APMA, from Sigma). APMA was made at a concentration of 3·5 mg/ml in DMSO. To activate, 200 µl buffer and 20 µl APMA was added to each vial containing 100 µl MMP solution. MMP‐2 was incubated at 37°C for 1 hour, and MMP‐9 at 37°C for 6 hours.
MMP activity assays
The enzymatic activity of MMP‐1 was determined using a fluorogenic peptide substrate 7‐MCA‐Pro‐Arg‐Pro‐Lys‐Pro‐Tyr‐Als‐Nval‐Met‐Lys(DNP)‐NH2 (31), whereas MMP‐2 and MMP‐9 used a different substrate, 7‐MCA‐Pro‐Leu‐Ala‐Nval‐DPA‐Ala‐Arg‐NH2 (31). A 10 mM peptide solution was prepared in dimethyl sulfoxide (DMSO), and the solution was further diluted 1:100 with the above Tris buffer. Activated MMP solutions were prepared in the same buffer. Ten microlitres (10 µl) of the testing MMP solution was mixed with 100 µl of the peptide substrate solution in the wells of a 96‐well plate. On a fluorescent plate reader (Safire II, Tecan, Germany), the fluorescent intensity (Ex/Em = 325 nm/393 nm) was measured using kinetics mode at 1‐minute intervals for 30 minutes at ambient temperature. The buffer was used as the blank for baseline correction. The slope of the kinetics curves in relative fluorescent units per minute (RFU/minute) was used to present the activity of MMPs.
SIS extracts effect on MMPs
A 5‐cm2 piece of SIS was immersed in 5 ml Tris buffer at 37°C for 24 hours. After the incubation, the SIS was removed from the solution. The extract solution was mixed 1:1 with each MMP solution at the following concentrations: MMP‐1 = 45 µg/ml, MMP‐2 = 22·7 µg/ml, MMP‐9 = 20 µg/ml. These concentrations were chosen based on the report activity in wounds fluid samples. Typical highest possible level of MMPs is around 10–20 µg/m range (8). Activated MMP‐2 and MMP‐9 were used in this experiment. Each mixture was incubated at 37°C for 30 minutes. The activity of MMP‐1, MMP‐2, and MMP‐9 was determined using the fluorogenic substrates as described in above section. The data of the same MMP solutions mixed with the Tris buffer, without SIS were the control to be subtracted from all test results and all activities were reported as RFU/minute.
The effect of SIS treatment on MMPs
The same MMP solutions as above were used in this experiment. A 1‐cm2 piece of SIS was combined with 0·5 ml of each MMP solution and incubated at 37°C for 24 hours. A 50‐µl aliquot was taken from each sample at T = 0, 0·5, 1, 3, 6, and 24 hours. The activity of each aliquot was determined using the same fluorogenic substrate as above.
Cell migration assay
An MMP solution containing MMP‐1, MMP‐2, and MMP‐9 was prepared in Tris buffer. The final concentrations were as follows: MMP‐1 = 45 µg/ml, MMP‐2 = 22·7 µg/ml, and MMP‐9 = 20 µg/ml. Human keratinocytes (Cascade, OR) at various passages were plated onto gold‐coated cover slips and treated with either epidermal growth factor (EGF, 1 µg/ml), untreated SIS (0·1% of incubation solution without MMP), MMP‐treated SIS solution (0·1% of incubation solution with MMPs), MMP solution (0·1% of the stock), or no treatment (NT). Migration was quantified by measuring the area of the tracks left in the gold coating after 20 hours of treatment.
Mouse impaired wound healing study
Four male obese diabetic Cg‐m+/+ Leprdb/J (BKS.Cg‐m+/+ Lepr db/J) mice between 6 and 8 weeks old were obtained from Jackson Laboratories and allowed to acclimate for 4 days prior to the beginning of the study. On day 0, animals were anaesthetised via CO2 inhalation, and the hair was removed from their backs via shaving followed by application of depilatory. Backs were then sterilised with povidone iodine and ethanol and two full‐thickness wounds were made through the panniculus carnosus using 6‐mm punch biopsies. Any remaining panniculus carnosus was removed with surgical scissors. Wounds were dressed and animals were returned to their cages for 24 hours. After 24 hours (day 1), animals were anaesthetised, dressings were removed and SIS matrix was applied to one wound followed by a hydrogel dressing. The corresponding wound received hydrogel alone. Both wounds were covered with a semi‐occlusive wound dressing followed by band‐aids and tubular dressings. At specific time points, animals were killed and wounds were excised and immediately flash frozen for further study. Animals were identified by letters A, B, C, and D. Mouse A and mouse B were sacrificed on day 3; mouse C and mouse D were sacrificed on day 7. A normal skin biopsy sample was included as a control.
Preparation of biopsy extracts
The tissue biopsy samples were frozen in liquid nitrogen then crushed using a mini pestle. The homogenised sample was transferred to a microcentrifuge tube. A 0·5 mL of the extraction buffer (20 mM Tris–HCl, 125 mM NaCl, 1% Triton‐X; pH 8·5) was added to each sample. All samples were placed on a rotary shaker for 2 hours at 4°C. The samples were then centrifuged at 20,800 g, and the supernatant was collected for future analyses.
Total protein analysis
The biopsy extract samples were analysed for total protein concentration using the Micro BCA assay (Pierce, Rockford, IL). The samples were diluted 1:1 in the extraction buffer (above). Twenty five microliters of each sample was added to 200 µl of working reagent (provided in the BCA kit), in a 96‐well plate. Samples were incubated at 37°C for 30 minutes, and then analysed at 562 nm using a plate reader (SpectraMax, Molecular Devices, Sunnyvale, CA). Bovine serum albumin was used as the reference standard. The total protein concentration was used to normalise the protein concentrations in all biopsy extract samples.
Gelatin zymogram
Gelatin zymography was performed to analyse the levels of active MMP gelatinases (MMP‐2 and MMP‐9). Ten percent acrylamide gels (mini protein gels) containing 10 mg gelatin in each were prepared. All normalised biopsy extract samples were diluted 1:1 in zymogram buffer before use. MMP‐2 and MMP‐9 proteins were used as the standards for each gel. Three microliters of all samples including the standards were loaded. The gels underwent a renaturation (2·5% v/v Triton‐X in H2O) and developing (50 mM Tris–HCl, 5 mM CaCl2, 200 mM NaCl, 0·02% w/v Brij‐35, pH 7·8) process. After staining with Coomassie Blue and destaining, gels were scanned and digitised. All bands were analysed using a gel densitometer (Bio‐Rad, Hercules, CA).
STATISTICS
Keratinocyte migration data for each treatment were averaged from a group of eight samples (n = 8). Multiple readings were also performed for each sample. Data between treated and no treatment were statistically compared using Mann–Whitney rank sum test. To determine statistical significance, P < 0·05 was the decision level. MMP activity data are presented as means ± SD. The mean values are derived from triplicates for the MMPs activity test. A t test (α = 0·05 with 4 degrees of freedom) was used to determined the statistical difference between the treated and no treatment. The decision level for statistical significance is P < 0·05.
RESULTS
Activity of MMP incubated with SIS extracts
Using the fluorogenic substrates, the enzymatic activity of MMP‐1, MMP‐2, and MMP‐9 was determined with and without incubation with SIS extracts. Figure 1 shows three graphs for each MMP's rate in RFU/minute. Incubation with SIS extracts significantly decreased MMP‐1 activity resulting in a 15% decrease in V max. The activity of MMP‐2 was reduced in the presence of SIS extracts but the result was not significant. MMP‐9 activity was increased in the presence of SIS extracts but the difference was again not statistically significant. The data suggest that the extracts from SIS may have a low inhibitory effect only to MMP‐1, with little to no impact on MMP‐2 and MMP‐9 activity. Therefore, interaction of MMPs with the soluble extracts appears to not be the primary reason for the loss of MMP activities.
Figure 1.

MMP‐1, MMP‐2, and MMP‐9 activity upon incubation with SIS extracts. MMP activity was measured in the presence and absence of SIS extracts using fluorogenic peptide substrates (* P < 0·05).
Binding of MMP to SIS
The above experiment did not let MMPs directly interact with SIS material. The results only explain whether the soluble extracts from SIS have any inhibiting/promoting effect on MMP activity. To evaluate the overall effect of SIS on MMP activity, MMPs were incubated with SIS individually. The activity of each MMP in solution was determined at various time points. Figure 2 shows the loss of activity of each MMP when incubated with SIS for different periods of time. As no dramatic inhibition was found in solution phases as indicated by the above experiments, the loss of activity can be attributed to binding of MMPs to SIS. The binding curves of the MMPs to SIS material were generated by plotting the percentage of activity loss versus time (Figure 2) using MMP solutions incubated under the same conditions as the baseline. All MMPs displayed a saturation curve. There was a dramatic loss of activity during the first 2 hours. MMP‐1, MMP‐2, and MMP‐9 displayed different binding affinities with 53·8%, 85·9%, and 36·9% bound to SIS during 24 hours, respectively. Thus, MMP‐2 showed the highest binding, whereas MMP‐9 was the lowest. Compared with MMP‐1, MMP‐2 and MMP‐9 displayed continuous increase in loss of activity after the first 2 hours. However, the changes were not as dramatic as observed within the first 2 hours. These data indicate that MMP‐1, MMP‐2, and MMP‐9 can lose activity when incubated with SIS simply by adsorption to SIS.
Figure 2.

MMP‐1,MMP‐2, and MMP‐9 activity loss in the presence of SIS at 37°C for 0, 1, 2, 3, 6, and 24 hours. SIS was incubated in each MMP solution at 37°C. The activity of each MMP at 1, 2, 3, 6, and 24 hours was compared with its original activity. The percentage of activity loss was calculated and plotted versus time.
MMP activity in wound biopsy
For band identification, MMP‐2 and MMP‐9 protein controls were analysed side by side with normal skin biopsy extracts. Both Pro‐MMP‐2 and active MMP‐2 bands were observed in the MMP‐2 protein control along with three higher molecular weight bands that could be MMP‐2 complexes. The MMP‐9 protein control was very active on the zymogram gel, showing intensive bands representing pro‐MMP‐9 and an MMP‐9 dimer. It also contained a lower molecular weight band, which could be an active fragment of MMP‐9.
Before running the biopsy extracts on zymogram gels, all samples were analysed for their total protein concentrations. The same total protein amount for each zymogram sample was loaded. The level of MMP‐1 in the biopsy samples was too low for analysis by gelatin zymography. Figure 3 shows zymographic band images of pro‐MMP‐2, active MMP‐2, pro‐MMP‐9, active MMP‐9, and MMP‐9 dimer bands in gelatin matrix for the biopsy samples on day 3 (SIS was placed on the wounds on day 1 after wounding) and day 7 after wounding. Gelatin zymograms of normal skin showed pro‐MMP‐2, but not active MMP‐2 and MMP‐9 bands. On day 3, elevated levels of pro‐MMP‐2, pro‐MMP‐9, and MMP‐9 dimer were found in the untreated wounds of both mouse A and mouse B. When the wounds on the same mice were treated with SIS, the levels of pro‐MMP‐9 and active MMP‐9 was substantially attenuated on both mice. The pro‐MMP 2 levels did not appear to be different when comparing untreated with treated. However, the active MMP‐2 levels were reduced (please see the faint bands under pro‐MMP‐2 bands shown on the untreated, not shown on the treated). On day 7, pro‐MMP‐9 and active MMP‐9 bands were also attenuated slightly in the treated wounds in mouse D. However, the differences were not as substantial as those observed in either mouse A or B on day 3. The levels of pro‐MMP‐2 and active MMP‐2 appeared mostly unchanged in mouse D. In mouse C, almost no difference could be found between treated and untreated wounds. The results show that the attenuation of MMP activity by SIS was high early after wounding and decreased later in the healing process.
Figure 3.
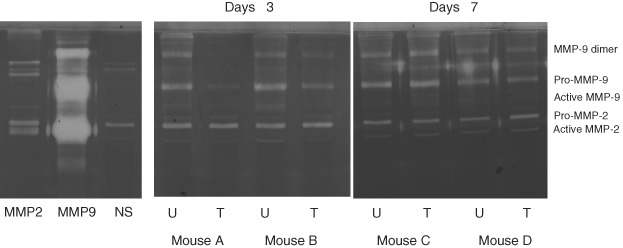
Gelatin zymographic analysis of biopsy samples from the mouse study. The left gel image depicts MMP‐2 and MMP‐9 proteins controls and protein extract from normal mouse skin on a gelatin zymography gel. This allowed for the identification of molecular weights and was used as a control. The centre and the right images show db/db mouse biopsy extracts from day 3 (A and B) and day 7 (C and D) run on gelatin zymography gels. NS = normal skin; U = untreated wounds; T = wounds treated with SIS.
Keratinocytes migration
Figure 4 plots the cell migration data with various treatments. Treatment with SIS extracts alone showed a significant increase in keratinocyte migration compared with no treatment (P < 0·05). Keratinocyte migration was inhibited following treatment with a solution containing MMP‐1, MMP‐2, and MMP‐9. When the MMP mixture was incubated with SIS, cells showed a significantly higher migration index compared with either cells treated with the MMP solution (P < 0·05) or untreated cells (P < 0·05). These results indicate that the MMP mixture had an inhibitory effect on keratinocyte migration and after incubating with SIS matrix, this inhibition was significantly reduced.
Figure 4.

Cell migration of human keratinocytes treated in the presence of SIS extracts, MMPs, MMP treated SIS extracts, and EGF. Human keratinocytes were plated on gold coated cover slips and treated with either epidermal growth factor (EGF), untreated SIS extract solution (0·1%), MMP treated SIS solution (0·1%), MMP solution (0·1%), or untreated (NT). Migration was quantified by measuring the area of the tracks left in the gold coating after 20 hours of treatment (* P < 0·05 comparing with no treatment; # P < 0·05 comparing with MMPs).
DISCUSSION
As stated earlier, MMPs are crucial to normal wound healing. However in the case of chronic wounds evidence suggests that the balance of ECM degradation and formation is disrupted and that elevated levels of certain MMPs hinder rather than help wound healing. The chronic wound state has been found to be associated with elevated levels of MMPs, especially the gelatinases MMP‐2 and MMP‐9 3, 5, 32, 33, 34, 35, 36, 37, 38, 39, 40. Liu et al. (35) reported recently that MMP‐9 levels rose in diabetic ulcers and suggested that high MMP‐9 levels and activity may be indicative of inflammation and poor wound healing. In pressure ulcers, elevated MMP levels may delay healing because of their excessive proteolytic and/or collagenolytic activities (36). Ladwig and Schultz (8) suggest using the ratio of MMP‐9/TIMP‐1 levels as a predictor of healing in pressure ulcers. Ulrich et al. (37) found MMP‐2 and MMP‐9 may also be involved in anti‐angiogenesis. Using CWF and MMP‐2/9 inhibitors, they verified in vitro that the reduced angiogenesis found in venous insufficiency ulcers was due at least in part to an anti‐angiogenic effect of MMP‐2 and MMP‐9. Inhibition of MMP‐2 and MMP‐9 resulted in a stimulation of angiogenesis.
MMPs have the unique ability to break down the highly collagenous ECM found in chronic wounds. This breakdown is essential for cell migration and wound healing. For example, it has been shown that MMP‐1 facilitates keratinocyte migration on Type I collagen in vitro. However if the matrices are damaged extensively, cell function and migration will be impaired, ultimately impeding healing. Keratinocytes have also been shown to express a more differentiated phenotype on damaged collagen matrix (38). Keratinocytes fail to spread and instead grow as dense colonies of isolated cells, as observed in a variety of conditions in which connective tissue damage occurs. In this study, we have shown in vitro that keratinocyte migration was significantly inhibited by treating the cells with a mixed MMP solution that contained MMP‐1, MMP‐2, and MMP‐9.
The most important collagen providing mechanical support is collagen Type I. The structure of collagen is based on the strength of the monomeric unit consisting of two α1(I) chains and one α2(I) chain inter‐wound into a triple helix. From this basic fibril higher order structures are formed eventually leading to mechanically strong fibrillar structures that are uniquely tailored to meet the needs of the tissue or organ in which they were created. These higher order fibrillar structures are also quite resistant to proteolytic cleavage.
Collagenolytic MMPs such as MMP‐1 and MMP‐13 contain a haemopexin C‐terminal domain, which is connected to the catalytic domain by a flexible proline‐rich linker 39, 40. In collagenases, the haemopexin C domain functions as a collagen‐binding domain (CBD) that is required for the cleavage of triple helical collagen 40, 41. Interestingly the haemopexin C‐terminal domain in the gelatinase MMP‐2 does not bind collagen. Gelatinases such as MMP‐2 and MMP‐9 bind to native collagen through a triple fibronectin type II repeat which acts as an alternative CBD. In fibronectin, the Type II modules are involved in binding denatured collagen. These modules are responsible for the ability of the gelatinases to bind to native Type I, V, and X collagen and denatured Type II, IV, and X collagens, gelatin, and laminin (42). These binding capabilities result in MMPs' potential to bind collagenous materials such as the SIS. SIS consists predominantly of fibrillar collagens (Types I, III, and V) (10) and it can act as a reservoir for various proteins possessing affinity to SIS matrix collagens. In this study, we have shown that MMP‐1, MMP‐2, and MMP‐9 bind to SIS quickly (most binding occurred within 3 hours), indicating the specific interaction between MMPs and SIS. However, as the adsorption study was performed with individual MMPs rather than combinations, the competitive binding between the MMPs is unknown. Clinically, multiple MMPs are present in wounds. The binding ability and specificity can vary greatly between the MMPs. For instance, in this study MMP‐9 displayed lower binding than MMP‐1 and MMP‐2 in vitro, when tested individually. However, the in vivo results suggest that SIS absorbs more MMP‐9 than MMP‐1 and MMP‐2. This is an indication that competitive binding may take place in the complex in vivo wound environment. When one considers the effect that molecular size has on protein absorption, it is not difficult to understand why MMP‐9 out‐competes MMP‐2. MMP‐9 is a more massive molecule than MMP‐2. At earlier time points, small proteins adsorb faster than larger proteins. However, at equilibrium larger proteins will out‐compete smaller ones. As observed in this study, the in vivo data (day 3) showed a hierarchy of binding with Pro‐MMP‐9 being absorbed to a greater degree than active MMP‐9 which in turn was absorbed more than Pro‐MMP‐2. The order matches the order of their molecular weights.
On day 7, the MMP levels of treated wounds were no different from the untreated. The wound environment is very different from that found in vitro. Over time, SIS is often digested and absorbed into the wound necessitating the application of additional layers. By day 7, the original SIS had been in place for 6 days and had been mostly incorporated into the wound matrix. Figure 5 depicts SIS immediately after placement (A) and 4 days after placement (B). Immediately after placement, SIS could be easily seen in the wound. The light yellow coloured material was the fresh SIS sheet. After 3 days in the wound, SIS was substantially degraded and incorporated into wound thus losing most of its binding ability. This explains why on day 7 we did not observe the same decrease as seen on day 3. Thus the absorptive capability of SIS decreases as it is incorporated into a wound. Continuous application of SIS may be needed depending upon the level of incorporation over time which will vary from wound to wound.
Figure 5.

Pictures of SIS treated and untreated wounds on db/db mice. The pictures show the wounds with and without SIS treatment on day 0 (Picture A) and day 4 (Picture B).
SIS contains other ECM molecules and growth factors that promote cell adhesion and migration. These molecules, including TGF‐β, FGF‐2, fibronectin, laminin, glycosaminoglycans, and proteoglycans (43), play important roles in cell proliferation and migration. In this study, we have found that SIS extracts displayed ability to control MMP activity, suggesting that they may have additional healing effects for chronic wounds. However, to fully understand the interaction between SIS and MMPs, a complete evaluation of the interaction between these molecules and MMPs and/or other wound proteases needs to be conducted.
In conclusion, the data presented in this article indicate that SIS has the potential to significantly inhibit MMP activity. MMP‐1, MMP‐2, and MMP‐9 displayed rapid losses in activity when interacting with SIS in vitro. This decrease in activity may explain how SIS attenuates MMP's inhibitory effect on keratinocytes migration. Furthermore, we show that SIS extracts have the ability to stimulate keratinocyte migration. The dynamic absorption of MMPs to SIS provides an explanation for the low MMP activity levels observed soon after its application in the animal study, indicating that SIS has the ability to decrease the activity of MMPs, especially MMP‐2 and MMP‐9 which are often elevated in chronic wounds. Overall, our data suggests that in addition to functioning as a scaffold at the wound site, SIS may also provide a supporting role in the promotion of wound healing.
ACKNOWLEDGEMENTS
The authors wish to thank Renée Carstens for medical writing contributions.
REFERENCES
- 1. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–74. [DOI] [PubMed] [Google Scholar]
- 2. Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 2004;16:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol 1993;101:64–8. [DOI] [PubMed] [Google Scholar]
- 4. Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 5. Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol 1996;106:1119–24. [DOI] [PubMed] [Google Scholar]
- 6. Vaalamo M, Weckroth M, Puolakkainen P, Kere J, Saarinen P, Lauharanta J, Saarialho‐Kere UK. Patterns of matrix metalloproteinase and TIMP‐1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 1996;135:52–9. [PubMed] [Google Scholar]
- 7. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–5. [DOI] [PubMed] [Google Scholar]
- 8. Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37. [DOI] [PubMed] [Google Scholar]
- 9. Telgenhoff D, Shroot B. Cellular senescence mechanisms in chronic wound healing. Cell Death Differ 2005;12:695–8. [DOI] [PubMed] [Google Scholar]
- 10. Voytik‐Harbin SL, Brightman AO, Waisner BZ, Robinson JP, Lamar CH. Small intestinal submucosa: a tissue‐derived extracellular matrix that promotes tissue‐specific growth and differentiation of cells in vitro. Tissue Eng 1998;4:157–74. [Google Scholar]
- 11. McPherson TB, Badylak SF. Characterization of fibronectin derived from porcine small intestinal submucosa. Tissue Eng 1998;4:75–83. [Google Scholar]
- 12. Hodde JP, Badylak SF, Brightman AO, Voytik‐Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng 1996;2:209–17. [DOI] [PubMed] [Google Scholar]
- 13. Voytik‐Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem 1997;67:478–91. [PubMed] [Google Scholar]
- 14. Rutner AB, Levine SR, Schmaelzle JF. Processed porcine small intestine submucosa as a graft material for pubovaginal slings: durability and results. Urology 2003;62:805–9. [DOI] [PubMed] [Google Scholar]
- 15. Pavcnik D, Uchida BT, Timmermans HA, Corless CL, O’Hara M, Toyota N, Moneta GL, Keller FS, Rosch J. Percutaneous bioprosthetic venous valve: a long‐term study in sheep. J Vasc Surg 2002;35:598–602. [DOI] [PubMed] [Google Scholar]
- 16. Musahl V, Abramowitch SD, Gilbert TW, Tsuda E, Wang JH, Badylak SF, Woo SL. The use of porcine small intestinal submucosa to enhance the healing of the medial collateral ligament – a functional tissue engineering study in rabbits. J Orthop Res 2004;22:214–20. [DOI] [PubMed] [Google Scholar]
- 17. Demling RH, Niezgoda JA, Haraway GD, Mostow EN. Small intestinal submucosa wound matrix and full‐thickness venous ulcers: preliminary results. Wounds 2004;16:18–22. [Google Scholar]
- 18. Barendse‐Hofmann MG, van Doorn LP, Oskam J, Steenvoorde P. Extracellular matrix prevents split‐skin grafting in selected cases. J Wound Care 2007;16:455–8. [DOI] [PubMed] [Google Scholar]
- 19. Romanelli M, Dini V, Bertone M, Barbanera S, Brilli C. OASIS wound matrix versus Hyaloskin in the treatment of difficult‐to‐heal wounds of mixed arterial/venous aetiology. Int Wound J 2007;4:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carson SN, Travis E, Overall K, Lee‐Jahshan S. Using becaplermin gel with collagen products to potentiate healing in chronic leg wounds. Wounds 2003;15:339–45. [Google Scholar]
- 21. Lown I, Kurt T, Tran H, Wayne M, Santiago C, Cioroiu M, Grossi R. Does bilayered extracellular matrix technology hasten wound healing in venous stasis ulcers? A retrospective study. Wounds 2005;17:27–31. [Google Scholar]
- 22. Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg 2005;41:837–43. [DOI] [PubMed] [Google Scholar]
- 23. Niezgoda JA, Van Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care 2005;18(5 Pt 1):258–66. [DOI] [PubMed] [Google Scholar]
- 24. Hodde J, Janis A, Hiles M. Effects of sterilization on an extracellular matrix scaffold. Part II. Bioactivity and matrix interaction. J Mater Sci Mater Med 2007;18:545–50. [DOI] [PubMed] [Google Scholar]
- 25. Nihsen ES, Johnson CE, Hiles MC. Bioactivity of small intestinal submucosa and oxidized regenerated cellulose/collagen. Adv Skin Wound Care 2008;21:479–86. [DOI] [PubMed] [Google Scholar]
- 26. Nihsen ES, Zopf DA, Ernst DM, Janis AD, Hiles MC, Johnson C. Absorption of bioactive molecules into OASIS wound matrix. Adv Skin Wound Care 2007;20:541–8. [DOI] [PubMed] [Google Scholar]
- 27. Gabriel A, Gollin G. Management of complicated gastroschisis with porcine small intestinal submucosa and negative pressure wound therapy. J Pediatr Surg 2006;41:1836–40. [DOI] [PubMed] [Google Scholar]
- 28. Dressel C, Furst A, Imhof A, Brehm W, Auer J. Clinical use of Small Intestine Submucosa in wound repair in 11 horses. Wiener Tierarztliche Monatsschrift (Vet Med Aust) 2004;91:142–50. [Google Scholar]
- 29. Nihsen ES, Zopf DA, Ernst DM, Janis AD, Hiles MC, Johnson C. Absorption of bioactive molecules into OASIS wound matrix. Adv Skin Wound Care 2007;20:541–8. [DOI] [PubMed] [Google Scholar]
- 30. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–62. [DOI] [PubMed] [Google Scholar]
- 31. Nagase H, Fields CG, Fields GB. Design and characterization of a fluorogenic substrate selectively hydrolyzed by stromelysin 1 (matrix metalloproteinase‐3). J Biol Chem 1994;269:20952–7. [PubMed] [Google Scholar]
- 32. Barone EJ, Yager DR, Pozez AL, Olutoye OO, Crossland MC, Diegelmann RF, Cohen IK. Interleukin‐1alpha and collagenase activity are elevated in chronic wounds. Plast Reconstr Surg 1998; 102:1023–7. [PubMed] [Google Scholar]
- 33. Bullen EC, Longaker MT, Updike DL, Benton R, Ladin D, Hou Z, Howard EW. Tissue inhibitor of metalloproteinases‐1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol 1995;104:236–40. [DOI] [PubMed] [Google Scholar]
- 34. Chen C, Schultz GS, Bloch M, Edwards PD, Tebes S, Mast BA. Molecular and mechanistic validation of delayed healing rat wounds as a model for human chronic wounds. Wound Repair Regen 1999;7:486–94. [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Min D, Bolton T, Nube V, Twigg SM, Yue DK, McLennan SV. Increased matrix metalloproteinase‐9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009;32:117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP‐8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res 1999;81:189–95. [DOI] [PubMed] [Google Scholar]
- 37. Ulrich D, Lichtenegger F, Unglaub F, Smeets R, Pallua N. Effect of chronic wound exudates and MMP‐2/‐9 inhibitor on angiogenesis in vitro. Plast Reconstr Surg 2005;116:539–45. [DOI] [PubMed] [Google Scholar]
- 38. Varani J, Perone P, Deming MO, Warner RL, Aslam MN, Bhagavathula N, Dame MK, Voorhees JJ. Impaired keratinocyte function on matrix metalloproteinase‐1 (MMP‐1) damaged collagen. Arch Dermatol Res 2009;301:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003;92:827–39. [DOI] [PubMed] [Google Scholar]
- 40. Overall CM. Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol 2002;22:51–86. [DOI] [PubMed] [Google Scholar]
- 41. Tam EM, Wu YI, Butler GS, Stack MS, Overall CM. Collagen binding properties of the membrane type‐1 matrix metalloproteinase (MT1‐MMP) hemopexin C domain. The ectodomain of the 44‐kDa autocatalytic product of MT1‐MMP inhibits cell invasion by disrupting native type I collagen cleavage. J Biol Chem 2002;277:39005–14. [DOI] [PubMed] [Google Scholar]
- 42. Nagase H. Matrix metalloproteinases. A mini‐review. Contrib Nephrol 1994;107:85–93. [PubMed] [Google Scholar]
- 43. Hodde J, Janis A, Ernst D, Zopf D, Sherman D, Johnson C. Effects of sterilization on an extracellular matrix scaffold. Part I. Composition and matrix architecture. J Mater Sci Mater Med 2007;18:537–43. [DOI] [PubMed] [Google Scholar]


