Abstract
Biatain and Biatain‐Ag are two identical wound dressings except the fact that Biatain‐Ag releases silver. In the present multinational double‐blinded randomised controlled trial the effect of the two dressings were compared for treatment of venous leg ulcers. A total of 181 patients were treated for 6 weeks with either Biatain or Biatain‐Ag followed by 4 weeks treatment with Biatain. Biatain‐Ag showed superior performance in relative wound area reduction after 6 weeks treatment and the estimated treatment difference increased after 10 weeks indicating that the effect of silver continues at least for 4 weeks after treatment. A subgroup of the patients differed significantly from the others with respect to parameters associated with a poor healing prognosis; patients were older, had significant history of venous thrombosis, larger ulcers with longer duration and more often recurrent. For this subgroup of patients Biatain‐Ag showed significant (P < 0·05) better performance in terms of relative ulcer area reduction and healing rate. In conclusion, this study suggests the superior performance of Biatain‐Ag compared with the non silver‐releasing dressing Biatain in particular for patients having ulcers associated with a poor healing prognosis.
Keywords: Delayed healing, Randomised double‐blinded clinical trial, Silver foam dressing, Venous leg ulcers, Wound area regression
Introduction
Venous leg ulcers occur in 1–3% of the adult population and account for the majority of lower extremity ulcerations 1. Venous ulcers are characterised by a cyclic pattern of healing and recurrence. The large number of patients suffering from venous leg ulcers combined with the long duration causing high cost of care result in a large financial impact on society 2.
The current standard of care for chronic venous ulcers involves the use of compression bandages as a means to reduce ambulatory venous pressure, control oedema and improve venous return. Dressings are applied beneath the compression to maintain the wound in a moist environment. Modern dressings are occlusive or semi‐occlusive, classified according to their physical composition. They have been developed to reduce pain and healing time, to absorb blood and exudate and to be painless on application and removal 3. However, even with the best local care, around 50% to 60% of ulcers are not closed by week 20–24 4 and several studies have demonstrated that patients may suffer from leg ulcers for several years without any real improvement 5, 6. These potentially ‘non healing’ ulcers may not be easy to identify when seen for the first time 7, 8, 9. Nevertheless, a number of factors suggest a poor healing prognosis: a large ulcer size (>10 cm2), a long duration (open for 12 months or more) 10, reoccurrence and a deep vein involvement 11. Pathogenesis of non healing venous leg ulceration involves chronic inflammation and are often heavily colonised 12, 13. Ionised silver have both anti‐inflammatory and antibacterial properties, with a broad spectrum of action 14, 15, 16. In addition to controlling the progression of colonisation through reduction of bioburden, antiseptic dressings can reduce the risk of biofilm formation, aid in debridement, prepare the wound bed prior to healing and act in infection prevention and control 17. Several studies have investigated the safety and effect of silver in the treatment of venous leg ulcers 6, 18, 19, 20, 21, 22, 23, suggesting a beneficial effect on wound size reduction.
To confirm the causal relationship between silver and wound area regression, the present multinational randomised controlled study was designed to use the same basic treatment in both groups, where the only difference was that one dressing released silver.
Materials and Methods
Study design
The study was a comparative, multinational, randomised, double‐blinded controlled study with two parallel groups. Thirty‐eight centres in five countries participated in the study. The study was initiated by a 4‐week pre‐selection period to identify ulcers with delayed healing, for example ulcers with less than 20% ulcer area reduction despite optimal, etiological (adapted compression) and appropriate local treatment (no silver or antimicrobial containing dressings was allowed). Patients passing the pre‐selection criteria were centrally randomised (by computer system) and allocated to 6‐week treatment with either Biatain or Biatain‐Ag foam dressing using Interactive Voice Response Service (IVRS). The IVRS system was used in the blinding and packaging procedure of the dressings. The 6‐week treatment period was followed by a 4‐week open study only with Biatain treatment for all participants. Four visits were scheduled at day 0 (visit 1), day 28 (visit 2, 4 weeks), day 42 (visit 3, 6 weeks) and day 70 (visit 4, 10 weeks). Compression therapy according to the clinical practice of the centres was mandatory throughout the study period.
Patient selection
Patients of either gender and equal to or above age 18 years old were eligible for inclusion in the study if having (i) a venous or predominantly venous leg ulcer (Ankle/Brachial Index, ABI > 0·8) between 2 cm and 13 cm in all directions (C6 of the CEAP classification) 24, (ii) a moderately or severely exudating leg ulcer in the phase of debridement or formation of granulation tissue, (iii) a leg ulcer with size reduction less than 20% despite suitable and well‐conducted local treatment and use of appropriate compression in the 4 weeks prior to inclusion, (iv) patients available for monitoring for at least 10 weeks.
Patients excluded from the study were those having (i) clinically infected leg ulcers (including erysipelas and cellulitis of the skin around the ulcer) requiring systemic antibiotic treatment, (ii) undergone surgery on the saphenous trunk within the 2 months prior to inclusion, (iii) systemic use of antibiotics 2 weeks prior to inclusion or systemic use of corticoids or cytostatics within the 3 months prior to inclusion, (iv) unbalanced diabetes, (v) known allergy to one of the components in Biatain‐Ag or Biatain. Patients already taking part in another clinical study or were pregnant or breastfeeding were also excluded from the study.
Products/materials
The study was designed as double‐blinded. All products used in the study were produced with the same top film, packed in identical packing and blinded by an external company in the United Kingdom. Furthermore, all products had individual kit numbers and the code was not broken until the database was locked. No dressings could be compared by the subject or investigator in the knowledge that they were different products.
Biatain dressings (Coloplast A/S) are made of hydrophilic polyurethane hydrocellular and are covered by a plain polyurethane Biatain topfilm. The Biatain‐Ag dressings are similar containing evenly distributed silver ions (1 mg Ag/cm2) in the form of a complex. The two dressings used in the study were 15 cm × 15 cm and were supplied to the site in boxes.
Wound area measurement
The change in wound size and area was measured by planimetry using double‐sided tracing paper. After correct tracing the transparent tracing paper was repositioned on a white cardboard‐backed sheet with centimetre scales. A HP Scanjet 7400 c (Hewlett Packard, Palo Alto, CA) was used to scan the sheets and Image‐Pro Plus 4.1 (MediaCybernetics, Rockville, MD) was used for measurement. The planimetry records were read blind by a person who was not aware of the nature of the treatment.
Other assessments
At inclusion visit, the patient's baseline characteristics (gender, date of birth, weight, height, medical history, details of surgery and other illnesses and other treatments) were recorded. Baseline ulcer characteristics were also recorded (main site of ulcer, how long it had been there, aetiology, ankle/arm index, origin, recurrent nature of the wound, spontaneous pain around the ulcer between dressing changes, amount of exudate, odour, erythema around the wound, local oedema, other local treatment and venous compression). The patient was given a dressing change form to record all the dates on which dressings and compression therapy were changed between the scheduled study appointments and the reasons for doing so.
At visits 2, 3 and 4 the dressing change form was delivered to the investigator and the condition and size of the ulcer were determined as at inclusion visit. Furthermore, adverse events and concomitant medication were recorded.
Ethics
The study was carried out in accordance with the Declaration of Helsinki. Ethical approval was obtained according to the guidelines from local ethical committees. Patients were given verbal and written information about the study and consent form was signed before recruitment. Patients were also informed of their right to withdraw from the study at any time.
Data management and statistics
A priori sample size calculation was performed based on results obtained in the study by Humbert 25 investigating the effect of Biatain‐Ag versus Algosteril. Assuming a dropout rate of 20% it was estimated that a total of 180 participants were required to obtain a power of 80%. Data management was performed by Coloplast A/S (Humlebaek, Denmark) using a validated data management system. Data were entered twice and the first and second sets of entries were compared and discrepancies checked by the Data Manager. The planimetry records were read by a person blinded to the nature of the treatment used.
The main analysis was carried out on the intention to treat (ITT) population, defined as all patients who were assessed at least once while receiving treatment.
The statistical analyses were performed by an independent company using SAS version 9.2 (SAS Institute, Cary, NC). All statistical tests were two‐sided at the 5% level of significance and a two‐sided 95% confidence interval of the product difference was provided for all efficacy variables. Last observation carried forward was used for handling missing values for the area and perimeter of the ulcer. No baseline values were carried forward. All collected data were presented in the form of descriptive statistical tables. An ANCOVA model was used to estimate the primary endpoint and the treatment effects. The differences between these were estimated by least‐square mean (LSMeans) extracted from the model, including confidence intervals and P‐values for the treatment effect differences.
Results
Patient disposition and adverse reactions
A total of 182 subjects were randomised in the study; however, 1 patient was in systemic antibiotic treatment (shown after code breaking, Biatain‐Ag group) and was erroneously enrolled. Hence the ITT analysis set comprised 181 subjects of which 75 participants were from France, 52 from Germany, 4 from Belgium, 43 from Denmark and 7 from the United Kingdom. The participants were randomly selected for treatment with either Biatain (n = 94) or Biatain‐Ag (n = 88). The two treatment groups were comparable for inclusion characteristics (Table 1). The mean age was 73·5 ± 12·2 years for all participants and the mean ulcer area was 15 ± 13·7 cm2. Twenty‐nine subjects, 11 from the Biatain‐Ag group and 18 from the Biatain group withdrew for various reasons during the study (see Figure 1 flowchart). Four participants were lost to follow‐up, 12 experienced protocol deviations, 3 withdrew their consent, 1 subject died and 1 subject withdrew his consent because of no effect of treatment. Eight withdrew due to an adverse event. The occurrence and cause of adverse events were equally distributed between the study groups. Four device‐related adverse events were recorded for the Biatain group and six in the Biatain‐Ag group. The device‐related adverse events were primarily skin related: maceration, eczema, pain and burn.
Table 1.
Baseline characteristics
| Biatain (N = 94) | Biatain‐Ag (N = 87) | All (N = 181) | |
|---|---|---|---|
| Female/Male (%) | 47/53 | 61/39 | 54/46 |
| Age, mean (SD) | 72·1 (12·4) | 75·1 (11·8) | 73·5 (12·2) |
| BMI, mean (SD) | 31·0 (7·2) | 28·9 (8·2) | 30·0 (7·8) |
| Duration years, mean (SD) | 2·8 (4·2) | 2·9 (5·1) | 2·8 (4·7) |
| Ankle/Brachial Index (ABI), mean (SD) | 1·1 (0·2) | 1·1 (0·2) | 1·1 (0·2) |
| Recurrency, yes/no (%) | 50/50 | 55/45 | 52/48 |
| Ulcer area cm2, mean (SD) | 15·4 (14·1) | 14·5 (13·4) | 15·0 (13·7) |
| Local signs, at least 3 out of 5 (%) | 61 (65) | 54 (62) | 115 (64) |
| Presence of pain (%) | 46 (49) | 46 (53) | 92 (51) |
| Presence of erythema (%) | 65 (69) | 59 (68) | 124 (69) |
| Presence of local oedema (%) | 49 (52) | 42 (48) | 91 (50) |
| Presence of odour (%) | 17 (18) | 16 (18) | 33 (18) |
| Presence of exudate (%) | 93 (99) | 85 (98) | 178 (98) |
Figure 1.
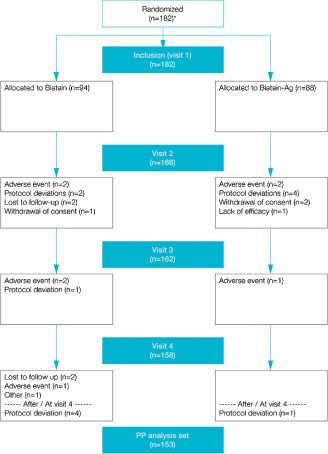
Flowchart of subject disposition. Asterisk indicates a total of 182 subjects who were randomised in the study; however, one patient was in systematic antibiotic treatment (shown after code breaking, Biatain‐Ag group) and was erroneously enrolled, hence the intention to treat (ITT) analysis set comprised 181 subjects.
Wound area reduction and rate of healing
The mean relative reduction in wound area 6 weeks after visit 1 was 35% for Biatain and 42% for Biatain‐Ag and the estimated treatment difference was 11 percent point (P = 0·0853) taking baseline area, age and BMI as covariates into account and fixed effects: gender, ulcer age, effect of country and treatment into account. A strong significant effect of country was observed (P < 0·0001) indicating that the level of relative ulcer area reduction differed between countries. The effect of silver treatment sustained over time (Figure 2) and at 10 weeks visit the estimated treatment difference between Biatain and Biatain‐Ag increased to 13·6 percent point with a significant country effect (P < 0·0001).
Figure 2.
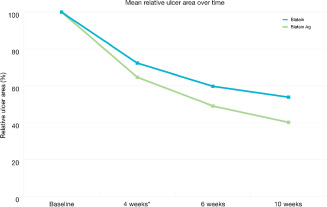
Mean relative reduction in ulcer area over time. Asterisk indicates that the relative reduction at 4 weeks was only adjusted for effect of baseline and treatment.
The ulcer healing rate was determined by the Gilman rate 26. After 6 weeks the mean Gilman rate was 0·53 mm/week for the Biatain group, whereas it was 0·67 mm/week for the Biatain‐Ag group—a difference of 0·14 mm/weeks. The estimated treatment difference taking baseline area, age and BMI as covariates into account and fixed effects: gender, ulcer age < 6 months, effect of country and treatment into account was 0·23 mm/week (P = 0·0852). Also here the effect of country was significant (P = 0·0015).
The number of complete healed wounds during the 6‐week blinded period were 3 (out of 94) for the Biatain group and 7 (out of 87) in the Biatain‐Ag group.
To clarify the importance of the confounding effect of country, baseline characteristics were compared for the three largest included data sets, France (n = 75), Germany (n = 52) and Denmark (n = 43) (data not shown) and it was evident that the French patients differed significantly from the others with respect to parameters which are connected to a poor prognosis: patients were older, had a significant history of venous thrombosis, and their ulcers were significantly larger, of longer duration and more often recurrent (Figure 3). The mean relative reduction in ulcer area in the French group of participants was 22% for Biatain and 44% for Biatain‐Ag with an estimated significant treatment difference of 22 percent point (P = 0·023) after 6 weeks treatment. The estimated treatment difference was increased after 10 weeks to 30 percent points (P = 0·026).
Figure 3.
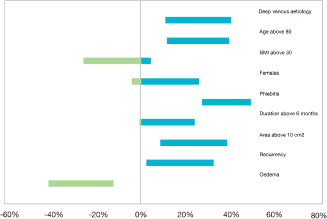
Differences in distribution of baseline characteristics (France versus all others) given as 95% CI. The figure illustrates, for each dichotomous characteristic (such as ‘deep venous aetiology’ or ‘age above 80’), the difference between the percentages of French and ‘all other’ subjects with that is ‘age above 80’. The difference can, in principle be anything between −100% and 100%. The difference is equipped with a 95% confidence bar, centred at the observed difference. If the bar is monochrome, red or blue, one group (France or ‘all others’) has a larger prevalence than the other. A red bar shows that a given characteristic is significantly more frequent in France, whereas a blue marks that a given characteristic is statistically more frequent in the group of ‘all others’. The mixed coloured bar indicates that the difference between the groups is non significant.
The Gilman healing rate was also significant after 6 weeks treatment in the French group of participants. For Biatain the Gilman healing rate was 0·33 mm/week and for the Biatain‐Ag group it was 0·63 mm/week (double as fast) (P = 0·0021). Taking baseline area, age and BMI as covariates into account and fixed effects: gender, ulcer age < 6 months, effect of country and treatment into account the estimated treatment difference in Gilman healing rate was 0·41 mm/week (P = 0·0221) in favour of Biatain‐Ag.
Assessment of local inflammatory signs and evaluation of the properties of the dressing
The frequency of patients reporting at least three out of five pre‐defined local inflammatory signs (pain, odour, erythema, oedema and exudate) were equal in both the groups after 6 and 10 weeks treatment. Also in the subgroup of French participants there was no significant difference between the two treatment groups.
The properties of the dressings were evaluated on a scale from 0 to 10. The participants were asked (i) How easy is the removal of the dressing, (ii) Ability to absorb exudate, (iii) How easy is the dressing to apply, (iv) Patient comfort with the dressing on, (v) Pain at removal of the dressing. On questions (i)–(iv), the scores were in the range 8·5–9·4 with no statistical significance between the two treatment groups. For the last question (pain at removal of the dressing) the score was ≤1·6 indicating minimal pain at removal. The results were as expected as the two dressings are similar except for the silver coating in Biatain‐Ag.
Discussion
In industrialised countries, 1–3% of the population suffer from a non healing wound, which counts for a significant part of the health care budget 27, 28. There is, consequently, increasing interest in the quality of available evidence for the effectiveness of specific interventions and dressing materials used in wound healing 29, 30. However, despite recent advances in wound care, venous leg ulcers continue to be a challenging problem both for patients and the public health. Several studies suggest that the probability for chronic wounds to heal properly may be limited when the bacterial load exceeds a certain level of contamination 14, 31, 32, 33, 34, 35, 36, 37, 38, 39. Moreover, ‘non healing’ ulcers fail to progress through the normal pattern of wound repair and remain in a chronic and persistent inflammatory state 13, 40, 41. These considerations have led to the use of treatment with silver in chronic wounds that fail to heal or when a negative local impact of bacterial colonisation is suspected. Silver‐impregnated dressings are typically used for a limited number of weeks. This practice reflects the fact that if such dressings are effective in removing excessive ulcer inflammation then, as soon as the process is complete, they ought to be removed to ensure appropriate wound treatment 42. In this study, highly stagnating ulcers requiring sufficient exposure to silver was anticipated and therefore the treatment period was fixed for 6 weeks. The effect of silver as support in the healing process has been investigated by others for both 4 19, 21, 43, 44, 45 and 6 weeks 22, 25 and more recently, the safety and effectiveness of a silver foam dressing was evaluated over a 9‐week treatment period 18. None of the studies experienced toxic issues when using different forms of silver‐releasing dressings.
The ITT data set consisted of 181 randomised participants. No differences between treatment groups regarding demographics and baseline characteristics were seen. However, country wise a discrepancy was evident. The French patients differed significantly from the others with respect to parameters that are associated with poor healing prognosis; patients were older, had significant history of venous thrombosis, and their ulcers were larger, of longer duration and more often recurrent 46, 47. This country‐wise difference was quite unexpected as the procedures for enrolment were identical for all five countries. However, the French subgroup is composed of elder subjects with older ulcers; this could be due to differences in the national referral systems, that is French patients are treated by their own physician for a longer period before being admitted to dedicated wound care centres. The demographic profile of the French participants is remarkable similar to that of another French study by Lazareth et al. testing a contact layer silver dressing 44. In the study by Lazareth et al., the relative reduction of ulcer area after 4 weeks of treatment with silver‐releasing dressing was significantly higher than that with the control dressing. These results are comparable with the results obtained with a lower number of patients (n = 75) in the French data set in this study. For the total data, the trend was the same for the relative reduction though not significant (P = 0·0852). At the 10 weeks visit the relative reduction was 46% for the Biatain group and 60% for the Biatain‐Ag group, and the estimated treatment difference showed increase in effect compared with the results after 6 weeks treatment. This indicates that the effect of silver appears to continue at least for 4 weeks after the treatment. The results from the French data set confirmed these observations as the estimated treatment difference in relative reduction at the 10 weeks visit was significant, in favour of Biatain‐Ag.
In this study the Gilman rate was used to evaluate the rate of healing at 4, 6 and 10 weeks visits. The Gilman rate has been used in a number of studies reviewed by Donahue et al. 48. Although the Gilman rate was tested only in a small number of participants in each study, they suggested that a healing rate of 0·50 mm/week during the first 3 to 4 weeks can predict healing. In this study the mean Gilman rate after 6 weeks treatment was 0·53 mm/week for the Biatain group, whereas it was 0·67 mm/week for the Biatain‐Ag group. When the same analysis was applied on the French data set the estimated treatment difference after 6 weeks treatment was 0·41 mm/week (P = 0·0221).
Conclusion
This study suggests a superior performance of Biatain‐Ag compared with the non silver‐releasing dressing with Biatain. Although the relative reduction in ulcer area after 6 weeks of treatment was not significant for the whole data set, the sub‐group of patients, who had significantly older and larger ulcers, had a significant relative reduction in ulcer area when treated with Biatain‐Ag.
Acknowledgements
The authors would like to thank all the investigators for their contribution to the study. Per Settergren Sørensen and Carsten Henrik Wachmann (Larix Aps, Denmark) conducted the statistical analysis. Birte Petersen Jakobsen provided expert comments to the manuscript. Zenia M. Størling (Trial Form Support Aps, Denmark) contributed with medical writing of the manuscript. The study was financially supported by Coloplast A/S, Denmark.
References
- 1. Mekkes JR, Loots MA, Van Der Wal AC, Bos JD. Causes, investigation and treatment of leg ulceration. Br J Dermatol 2003;148:388–401. [DOI] [PubMed] [Google Scholar]
- 2. Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994;31:49–53. [DOI] [PubMed] [Google Scholar]
- 3. Senet P. Local treatment of venous leg ulcers. Phlebolymphology 2010;17:87–94. [Google Scholar]
- 4. Watson JM, Kang'ombe AR, Soares MO, Chuang LH, Worthy G, Bland JM, Iglesias C, Cullum N, Torgerson D, Nelson EA, VenUS III Team. Use of weekly, low dose, high frequency ultrasound for hard to heal venous leg ulcers: the VenUS III randomised controlled trial. BMJ 2011;342:d1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen KE, Franken CPM, Gad P, Larsen AM, Larsen JR, Franciscus PA, van Neer A, Vuerstaek J, Wuite J, Martino HA. A randomized, controlled study to compare the effectiveness of two foam dressings in the management of lower leg ulcers. Ostomy Wound Manage 2002;48:34–41. [Google Scholar]
- 6. Karlsmark T, Agerslev RH, Bendz SH, Larsen JR, Roed‐Petersen J, Andersen KE. Clinical performance of a new silver dressing, Contreet Foam, for chronic exuding venous leg ulcers. J Wound Care 2003;12:351–354. [DOI] [PubMed] [Google Scholar]
- 7. Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420–1425. [DOI] [PubMed] [Google Scholar]
- 8. Moore K. Using wound area measurement to predict and monitor response to treatment of chronic wounds. J Wound Care 2005;14:229–232. [DOI] [PubMed] [Google Scholar]
- 9. Prince S, Dodds SR. Use of ulcer size and initial responses to treatment to predict the healing time of leg ulcers. J Wound Care 2006;15:299–303. [DOI] [PubMed] [Google Scholar]
- 10. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen 2004;12:163–168. [DOI] [PubMed] [Google Scholar]
- 11. Skene AI, Smith JM, Dore CJ, Charlett A, Lewis JD. Venous leg ulcers: a prognostic index to predict time to healing. BMJ 1992;305:1119–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies CE, Hill KE, Newcombe RG, Stephens P, Wilson MJ, Harding KG, Thomas DW. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Repair Regen 2007;15:17–22. [DOI] [PubMed] [Google Scholar]
- 13. Trengove NJ, Bielefeldt‐Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non‐healing and healing chronic leg ulcers. Wound Repair Regen 2000;8:13–25. [DOI] [PubMed] [Google Scholar]
- 14. Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997;77:637–650. [DOI] [PubMed] [Google Scholar]
- 15. Lansdown AB. Silver. I: its antibacterial properties and mechanism of action. J Wound Care 2002;11:125–130. [DOI] [PubMed] [Google Scholar]
- 16. Leaper DJ. Silver dressings: their role in wound management. Int Wound J 2006;3:282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leaper D, Drake R. Should one size fit all? An overview and critique of the VULCAN study on silver dressings. Int Wound J 2011;8:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harding K, Gottrup F, Jawieå A, Mikosiåski J, Twardowska‐Saucha K, Kaczmarek S, Sopata M, Shearman C, Pieronne A, Kommala D. A prospective, multi‐centre, randomised, open label, parallel, comparative study to evaluate effects of AQUACEL((R)) Ag and Urgotul((R)) Silver dressing on healing of chronic venous leg ulcers. Int Wound J 2012;9:285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jãrgensen B, Price P, Andersen KE, Gottrup F, Bech‐Thomsen N, Scanlon E, Kirsner R, Rheinen H, Roed‐Petersen J, Romanelli M, Jemec G, Leaper DJ, Neumann MH, Veraart J, Coerper S, Agerslev RH, Bendz SH, Larsen JR, Sibbald RG. The silver‐releasing foam dressing, Contreet Foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J 2005;2:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lazareth I, Ourabah Z, Senet P, Cartier H, Sauvadet A, Bohbot S. Evaluation of a new silver foam dressing in patients with critically colonised venous leg ulcers. J Wound Care 2007;16:129–132. [DOI] [PubMed] [Google Scholar]
- 21. Mãnter KC, Beele H, Russell L, Crespi A, Grãchenig E, Basse P, Alikadic N, Fraulin F, Dahl C, Jemma AP. Effect of a sustained silver‐releasing dressing on ulcers with delayed healing: the CONTOP study. J Wound Care 2006;15:199–206. [DOI] [PubMed] [Google Scholar]
- 22. Wunderlich U, Orfanos CE. Behandlung der ulcera cruris venosa mit trockenen wundauflagen, phasenübergreifende anwendung eines silberimprägnierten Aktivkohle‐xerodressings. Der Hautartz 1991;42:446–450. [PubMed] [Google Scholar]
- 23. Vanscheidt W, Lazareth I, Routkovsky‐Norval C. Safety evaluation of a new ionic silver dressing in the management of chronic ulcers. Wounds 2003;15:371–378. [Google Scholar]
- 24. Eklãf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, Meissner MH, Moneta GL, Myers K, Padberg FT, Perrin M, Ruckley CV, Smith PC, Wakefield TW, American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40:1248–1252. [DOI] [PubMed] [Google Scholar]
- 25. Humbert P. Ulcères de jambe présentant des signes locaux d'infection: intérêt du pansement Biatain Argent [Leg ulcers showing local signs of infection: benefits of the Biatain Argent dressing]. Journal de la société francaise et francophone des plaies et cicatrisations 2006;XI(March):52. [Google Scholar]
- 26. Gilman T. Wound outcomes: the utility of surface measures. Int J Low Extrem Wounds 2004;3:125–132. [DOI] [PubMed] [Google Scholar]
- 27. Gottrup F. A specialized wound‐healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am J Surg 2004;187(A):38S–43S. [DOI] [PubMed] [Google Scholar]
- 28. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health‐care providers in Europe. J Wound Care 2009;18:154–161. [DOI] [PubMed] [Google Scholar]
- 29. Horkan L, Stansfield G, Miller M. An analysis of systematic reviews undertaken on standard advanced wound dressings in the last 10 years. J Wound Care 2009;18:298–304. [DOI] [PubMed] [Google Scholar]
- 30. Sultan MJ, McCollum C. Don't waste money when dressing leg ulcers. Br J Surg 2009;96:1099–1100. [DOI] [PubMed] [Google Scholar]
- 31. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Browne AC, Vearncombe M, Sibbald RG. High bacterial load in asymptomatic diabetic patients with neurotrophic ulcers retards wound healing after application of Dermagraft. Ostomy Wound Manage 2001;47:44–49. [PubMed] [Google Scholar]
- 33. Ebright JR. Microbiology of chronic leg and pressure ulcers: clinical significance and implications for treatment. Nurs Clin North Am 2005;40:207–216. [DOI] [PubMed] [Google Scholar]
- 34. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–96. [DOI] [PubMed] [Google Scholar]
- 35. Gilliland EL, Nathwani N, Dore CJ, Lewis JD. Bacterial colonisation of leg ulcers and its effect on the success rate of skin grafting. Ann R Coll Surg Engl 1988;70:105–108. [PMC free article] [PubMed] [Google Scholar]
- 36. Jones SG, Edwards R, Thomas DW. Inflammation and wound healing: the role of bacteria in the immuno‐regulation of wound healing. Int J Low Extrem Wounds 2004;3:201–208. [DOI] [PubMed] [Google Scholar]
- 37. Madsen SM, Westh H, Danielsen L, Rosdahl VT. Bacterial colonization and healing of venous leg ulcers. APMIS 1996;104:895–899. [DOI] [PubMed] [Google Scholar]
- 38. Trengove NJ, Stacey MC, McGechie DF, Mata S. Qualitative bacteriology and leg ulcer healing. J Wound Care 1996;5:277–280. [DOI] [PubMed] [Google Scholar]
- 39. Xu L, McLennan SV, Lo L, Natfaji A, Bolton T, Liu Y, Twigg SM, Yue DK. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care 2007;30:378–380. [DOI] [PubMed] [Google Scholar]
- 40. Eming SA, Koch M, Krieger A, Brachvogel B, Kreft S, Bruckner‐Tuderman L, Krieg T, Shannon JD, Fox JW. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res 2010;9:4758–4766. [DOI] [PubMed] [Google Scholar]
- 41. Agren MS, Eaglstein WH, Ferguson MW, Harding KG, Moore K, Saarialho‐Kere UK, Schultz GS. Causes and effects of the chronic inflammation in venous leg ulcers. Acta Derm Venereol Suppl (Stockh) 2000;210:3–17. [PubMed] [Google Scholar]
- 42. Cutting K, White R, Edmonds M. The safety and efficacy of dressings with silver—addressing clinical concerns. Int Wound J 2007;4:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dimakakos E, Katsenis K, Kalemikerakis J, Arkadopoulos N, Mylonas S, Arapoglou V, Tsiganis T, Kotsis T. Infected venous leg ulcers: management with silver‐releasing foam dressing. Wounds 2009;21:4–8. [PubMed] [Google Scholar]
- 44. Lazareth I, Meaume S, Sigal‐Grinberg ML, Combemale P, Guyadec TL, Zagnoli A. The role of a silver releasing lipido‐colloid contact layer in venous leg ulcers presenting inflammatory signs suggesting a heavy bacteria colonization: results of a randomized controlled study. Wounds 2008;20:158–166. [PubMed] [Google Scholar]
- 45. Meaume S, Vallet D, Morere MN, Teot L. Evaluation of a silver‐releasing hydroalginate dressing in chronic wounds with signs of local infection. J Wound Care 2005;14:411–419. [DOI] [PubMed] [Google Scholar]
- 46. Margolis DJ, Gross EA, Wood CR, Lazarus GS. Planimetric rate of healing in venous ulcers of the leg treated with pressure bandage and hydrocolloid dressing. J Am Acad Dermatol 1993;28:418–421. [DOI] [PubMed] [Google Scholar]
- 47. Moffatt CJ, Doherty DC, Smithdale R, Franks PJ. Clinical predictors of leg ulcer healing. Br J Dermatol 2010;162:51–58. [DOI] [PubMed] [Google Scholar]
- 48. Donohue K, Falanga V. Healing rate as a prognostic indicator of complete healing: a reappraisal. Wounds 2003;15:71–76. [Google Scholar]


