Abstract
Hidradenitis suppurativa is a cutaneous, chronic, recurrent inflammatory disease. Here, we report the case of a 66‐year‐old man who had hidradenitis suppurativa in the buttocks. He suffered from diabetes mellitus. In the past, he had perianal abscesses. Because of improper treatment of furuncle infections in the buttocks, skin ulcers formed, which worsened and resulted in multiple fistulas. The skin lesion surface was large and the infection was severe. After wound debridement treatment, topical negative pressure and nutritional support were given. After one and a half months, the wound healed with split‐thickness skin grafting. In a 2‐year follow‐up, there was no evidence of hidradenitis suppurativa recurrence.
Keywords: Buttocks, Hidradenitis suppurativa, Skin graft, Topical negative pressure
Introduction
Hidradenitis suppurativa, also known as Verneuil's disease, is a chronic, recurrent, inflammatory disease. The disease affects skin areas rich in terminal hairs and apocrine sweat glands 1, mostly in scalp and axillary region, which is also called perifolliculitis capitis abscedens et suffodiens, followed by the groin, buttocks, perineum and submammary fold 2. The disease is characterised by formation of abscesses. Initially, the skin lesions are painful, red, hard nodules that eventually suppurate and become skin ulcers or sinus tracts. While these skin lesions do heal, recurrence is common and severe scar tissues form usually. On the basis of the extent of the skin lesions and the severity of the scar tissues, Hurley categorised hidradenitis suppurativa into three phases: phase I, characterised by single or multiple abscess formations with no fistulas or scars; phase II, characterised by one or more recurrent abscesses with sinus tract formations and scars; and phase III, characterised by multiple deep fistulas and abscesses. When the disease is in phase III, there is secondary infection and skin ulceration, which contribute to chronic hyponomous abscesses 3. The aetiology of the disease is unclear and its pathogenesis is very complex. Hidradenitis suppurativa may result from an immune response of the body. Because of its long course, the treatment of the disease is difficult. The disease is refractory even after wound healing. In hidradenitis suppurativa, Staphylococcus aureus and Staphylococcus epidermidis are common pathogenic bacteria. Hidradenitis suppurativa, perifolliculitis capitis and acne conglobata have similar pathogenesis; these diseases affect sweat glands, hair follicles, sebaceous glands and other skin appendages 4. Altogether, these diseases are known as the follicular occlusion triad.
Case report
A 66‐year‐old male patient was hospitalised as a result of recurrent abscesses for 7 months in the bilateral buttocks. Eight years prior, he had suffered from perianal abscess resection. Six years prior, the perianal abscess recurred and treatment was provided. Seven months prior to hospitalisation, he had a furuncle infection in the left buttock. The furuncle was red, hard and painful. In spite of treatment provided at home, the furuncle infection diffused to the entire left buttock and to part of the right buttock. When the abscess ruptured, an ulcer appeared and several sinus tracts and fistulas formed. Malodorous pus discharged over the cribriform skin. For the past 20 years, he had been suffering from diabetes mellitus and was taking oral hypoglycaemic drugs. He had no history of hepatitis, tuberculosis or similar infectious diseases; additionally, he had no family history of these diseases.
At the time of admission, the patient was conscious but had a wasting appearance. His body temperature was 37·5–38°C. The skin in the left and right buttocks was dark. The sinus tracts and fistulas, which differed in size, were visible on the surface of the buttocks. The abscesses had penetrated the subcutaneous tissue (Figure 1) and were painful to him. Lightly pressing the abscesses resulted in a purulent malodorous bloody discharge. No abnormalities were found in the anus. The blood analyses results were 15·4 × 109 white blood cells/l, in which neutrophils accounted for 80%, lymphocytes 13%, oeosinophil granulocyte 3%, 69 g haemoglobin/l and 4·9 mmol glucose/l. The urine and stool analyses and the hepatic and renal functions were normal. There were no abnormalities in the concentrations of serum immunoglobulin or complement proteins. The purulent bloody discharge from the abscesses was cultured for bacteria. The bacterial analysis showed the presence of Klebsiella pneumonia and Proteus mirabilis. On the basis of antibiotic sensitivity tests, it was found that K. pneumonia was sensitive to amikacin, ciprofloxacin, gentamicin and cotrimoxazole; P. mirabilis was sensitive to ampicillin, gentamicin, amikacin, cefazolin and piperacillin. The tuberculin skin and treponema pallidum haemagglutination test results were negative. A histological examination showed the presence of epidermal hyperplasia, dermal infiltration of inflammatory cells, multinucleated giant cells, granulomatous changes and a small abscess formation (Figure 2).
Figure 1.
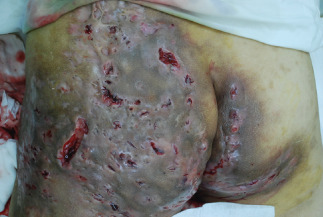
Hidradenitis suppurativa in buttocks. Dark brown skin ulcers were visible in the entire left buttock and part of the right buttock. Yellow bloody discharge was observed from numerous skin lesions.
Figure 2.
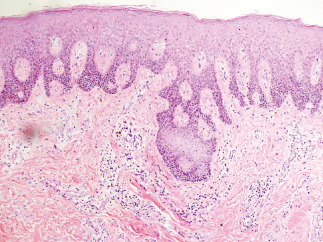
Histological examination of the skin lesions in a patient with hidradenitis suppurativa. The examination showed the presence of epidermal hyperplasia, dermal infiltration of inflammatory cells, multinucleated giant cells, granulomatous changes and a small abscess formation (HE staining, ×40).
Wound treatment
Under local anaesthesia, the abscess in the bilateral buttock was incised. The abscess cavity was canalized and fenestrated each other, which was characteristic of a hyponomous abscess (Figure 3). Sinus tracts, fistulas, granulation tissue and proliferative scar tissue were incised and removed. Hydrogen peroxide and saline were used to wash the wound. A topical negative pressure (TNP) treatment was given. TNP material (VSD, Wuhan VSD Medical Science & Technology Co., Ltd., Wuhan, China) consisted of a 15 × 10 × 0·5 cm polyvinyl formal foam, a 20 × 15 cm polyethylene amine ethyl film and a 30‐cm long silicone drainage tube with holes in one end. The wound was filled with polyvinyl medical foam and sealed with polyethylene amine ethyl film. The drainage tube was connected to a vacuum suction device; the drainage negative pressure was maintained between −16 and −40 kPa. Gentamicin and saline were instilled into the wound using the drainage tube. After 7 days, the negative pressure suction device was replaced and the wound was examined. The wound was cleaner; however, there was still some necrotic tissue and discharge. Hence, a wound debridement treatment was given again. The patient was also given iron supplements and insulin injections. After four TNP treatments, the wound became clean and moist. No purulent discharge was detected and a normal granulation tissue formation was observed. The wound was treated with a split‐thickness skin graft. After 1 week, the skin graft healed (Figure 4). He remained in the hospital for one and a half months.
Figure 3.
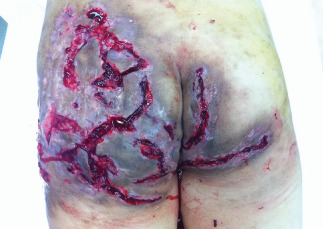
A hyponomous abscess.
Figure 4.
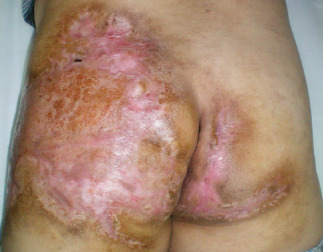
A split‐thickness skin graft was performed following four topical negative pressure (TNP) treatments. The skin graft survived 2 weeks post‐surgery.
TNP treatments may affect the ambulation of patients. When the patient had to get out of bed, the TNP was temporarily closed. In a 2‐year follow‐up of the patient, there was no evidence of hidradenitis suppurativa recurrence.
Discussion
Hidradenitis suppurativa is an inflammatory disease. This disease has a high rate of recurrence, thereby causing personal distress and economic burden. Patients experience a lot of pain, which affects their mobility and daily activities. The disease has a long course; granulation tissue grows slowly and wounds are difficult to heal. After wound healing, new pus appears usually, resulting in the recurrence of the abscess. Finally, purulent discharges are malodorous and not easily tolerated by patients.
For the treatment of mild to moderate hidradenitis suppurativa, tissue‐saving surgical procedures could be enough 5. However, in severe cases of hidradenitis suppurativa, aggressive wound debridement and adequate drainage treatments are required 6. Most of the skin undergoes degeneration and necrosis. Furthermore, cell viability is poor. Alternatively, cells might be in a state of anhydrobiosis 7. Any abscess cavities and sinus tracts in the subcutaneous dermis should be excised as much as possible. Only then TNP treatment must be given. Based on the results of antibiotic sensitivity tests, certain antibiotics can be instilled into the wound, thereby reducing inflammation and favouring wound healing 8. Negative pressure drainage promotes the growth of granulation tissue and keeps the wound fresh. Wounds should be treated with a split‐thickness skin graft.
TNP has several advantages. TNP allows a continuous drainage from the abscess, thereby allowing the necrotic tissue to be removed in a timely manner and reducing the absorption of toxins by the body. The semi‐permeable biological film has a single direction of ventilation. While gases resulting from the necrotic tissue can penetrate the film, air and bacteria from the outside environment cannot penetrate the film. This ensures that the wound is isolated and protected from the outside environment; toxin exposure and cross‐infection are effectively prevented 9. Additionally, TNP promotes blood circulation close to the wound, which promotes the growth of granulation tissue and prepares the wound for follow‐up treatments 10, 11. Vacuum sealing drainage can be maintained for 7–10 days without the need of changing dressings, which minimises any patient discomfort and reduces medical staff workload. Thus, TNP is an effective treatment for complex chronic wounds 12.
When applying TNP, it is important to consider the following factors. First, wound debridement treatment should be carefully performed; this can reduce the duration of TNP treatment. Second, during negative pressure drainage, necrotic discharge might block the drainage tube. Saline drip irrigation is effective in preventing this blockage. Furthermore, saline drip irrigation can also reduce abscess discharge. Third, negative pressure suction for a long period of time can harden the sponge dressing, thereby compromising the water permeability and air permeability of the sponge. Thus, bacteria could easily reproduce. In this condition, the negative pressure suction device should be replaced every 7–10 days. Finally, any other diseases of the patients should be aggressively treated, especially diabetes mellitus, which affects wound healing. It is crucial to maintain a positive nitrogen balance through the consumption of dietary proteins.
Conclusion
Hidradenitis suppurativa is a severe infectious disease that results in sinus tracts and fistulas. In this disease, wound debridement treatment is necessary. Necrotic tissue should be incised to control the infection. Furthermore, the wound should undergo surgical procedures as required. The use of TNP following wound debridement can improve the condition of the wound by accelerating wound healing; this reduces hospitalisation and increases the success of surgical procedures.
References
- 1. Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol 2012;26:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel J, Habtgiorgis D, Cervellione KL, Bagheri F. Multiple recurrent abscesses on the face and buttocks of a 21‐year‐old man. Clin Infect Dis 2009;48:914–5. [DOI] [PubMed] [Google Scholar]
- 3. Wollina U, Tilp M, Meseg A, Schönlebe J, Heinig B, Nowak A. Management of severe anogenital acne inversa (hidradenitis suppurativa). Dermatol Surg 2012;38:110–7. [DOI] [PubMed] [Google Scholar]
- 4. Varshney N, Al Hammadi A, Sam H, Watters AK. Perifolliculitis capitis abscedens et suffodiens in an 18‐year‐old Aboriginal Canadian patient: case report and review of the literature. J Cutan Med Surg 2007;11:35–9. [DOI] [PubMed] [Google Scholar]
- 5. van der Zee HH, Prens EP, Boer J. Deroofing: a tissue‐saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol 2010;63:475–80. [DOI] [PubMed] [Google Scholar]
- 6. Rompel R, Petres J. Long‐term results of wide surgical excision in 106 patients with hidradenitis suppurativa. Dermatol Surg 2000;26:638–43. [DOI] [PubMed] [Google Scholar]
- 7. Balik E, Eren T, Bulut T, Büyükuncu Y, Bugra D, Yamaner S. Surgical approach to extensive hidradenitis suppurativa in the perineal/perianal and gluteal regions. World J Surg 2009;33:481–7. [DOI] [PubMed] [Google Scholar]
- 8. Jacobs F, Metzler G, Kubiak J, Röcken M, Schaller M. New approach in combined therapy of perifolliculitis capitis abscedens et suffodiens. Acta Derm Venereol 2011;91:726–7. [DOI] [PubMed] [Google Scholar]
- 9. Thompson JT, Marks MW. Negative pressure wound therapy. Clin Plast Surg 2007;34:673–84. [DOI] [PubMed] [Google Scholar]
- 10. McNulty AK, Schmidt M, Feeley T, Villanueva P, Kieswetter K. Effects of negative pressure wound therapy on cellular energetics in fibroblasts grown in a provisional wound (fibrin) matrix. Wound Repair Regen 2009;17:192–9. [DOI] [PubMed] [Google Scholar]
- 11. Egemen O, Ozkaya O, Ozturk MB, Aksan T, Orman Ç, Akan M. Effective use of negative pressure wound therapy provides quick wound‐bed preparation and complete graft take in the management of chronic venous ulcers. Int Wound J 2012;9:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee DL, Ryu AY, Rhee SC. Negative pressure wound therapy: an adjuvant to surgical reconstruction of large or difficult skin and soft tissue defects. Int Wound J 2011;8:406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


