Abstract
Infections of contaminated or colonised acute or chronic wounds remain a grave risk for patients even today. Despite modern surgical debridement concepts and antibiotics, a great need exists for new therapies in wound management. Since the late 1990s, advantageous effects of negative pressure wound therapy (NPWT) have been combined with local antiseptic wound cleansing in the development of NPWT with instillation (NPWTi). This article summarises the current scientific knowledge on this topic. MEDLINE literature searches were performed on the subject of negative pressure wound and instillation therapy covering publications from the years 1990 to 2013 (36 peer‐reviewed citations) and regarding randomised controlled trials (RCTs) covering wound care with bone involvement (27 publications) or soft‐tissue wounds without bone participation (11 publications) from 2005 to 2012. The use of NPWTi in the therapy of infected wounds appears to be not yet widespread, and literature is poor and inhomogeneous. However, some reports indicate an outstanding benefit of NPWTi for patients, using antiseptics such as polyhexanide (concentration 0·005–0·04%) and acetic acid (concentration 0·25–1%) in acute and chronic infected wounds and povidone‐iodine (10% solution) as prophylaxis in contaminated wounds with potential viral infection. Soaking times are recommended to be 20 minutes each, using cycle frequencies of four to eight cycles per day. Additionally, the prophylactic use of NPWTi with these substances can be recommended in contaminated wounds that cannot be closed primarily with surgical means. Although first recommendations may be given currently, there is a great need for RCTs and multicentre studies to define evidence‐based guidelines for an easier approach to reach the decision on how to use NPWTi.
Keywords: Antiseptics, Contamination, Instillation, Negative pressure wound therapy, Vacuum‐assisted closure
Introduction
Contamination or infection of wounds is a relevant clinical problem and an unpleasant burden for many patients who have undergone burn trauma, injuries or surgeries 1. Pain, discomfort and a prolonged hospital stay for the patient and substantial economic costs for the health care system are the main consequences 2.
As contaminations or bacterial infections of the wounds impair wound healing 3, it is important to know that 10–16% of hospital‐acquired infections are wound infections 4; the highest mortality rate among surgical patients (77%) is due to wound infections 5, making them the most common surgical complication 6.
There are various reasons for wound infections. In hospitals, they are frequently caused by pathogenic germs, extended operation periods, poor hygiene of the staff, poor compliance or a weak immune system of the patient 7, 8, 9. Therefore, the main focus of attention in wound care is to prevent wound infection and to promote wound healing.
Beyond surgical procedures, antibiotics have been established as firm components in the fight against wound infections. However, because of excessive use of antibiotics, bacterial resistance has become an important problem 10, 11. The declining effectiveness of antibiotics caused by multiresistant germs 12 shows the urgent need for alternative therapies to discover, prevent and treat wound infections.
Negative pressure wound therapy (NPWT)
In the late 1980s, NPWT was simultaneously developed in the USA and Germany as a method for the treatment of acute and chronic wounds and has ever since enjoyed increasingly widespread clinical use over the past years 13, 14, 15. NPWT (V.A.C.® Therapy; KCI USA, Inc., San Antonio, TX) has been regarded as an established wound care method for routine clinical use since the mid‐to‐late 1990s. Soon after, the range of indications was extended to chronic wounds (e.g. leg ulcers and decubitus ulcers). Since 2000, there has been a reported increase in clinical uses for NPWT (Figure 1). This includes severe dermatological syndromes, problematic wounds in vascular and plastic surgery and enterocutaneous and lymphocutaneous fistulas and open abdomens in visceral surgery. In trauma surgery, the range of uses has extended to implant infections in the fields of endoprosthetics and spinal surgery. After 2000, burn injuries (e.g. burns of the hand and the fixation of skin substitutes) were found to be ideally treatable by NPWT. In visceral and thoracic surgery, NPWT use was extended from the management of septic wounds or defect regions on the body surface to problematic wound conditions deep in body cavities (e.g. bronchial stump insufficiency and pancreatic trauma).
Figure 1.
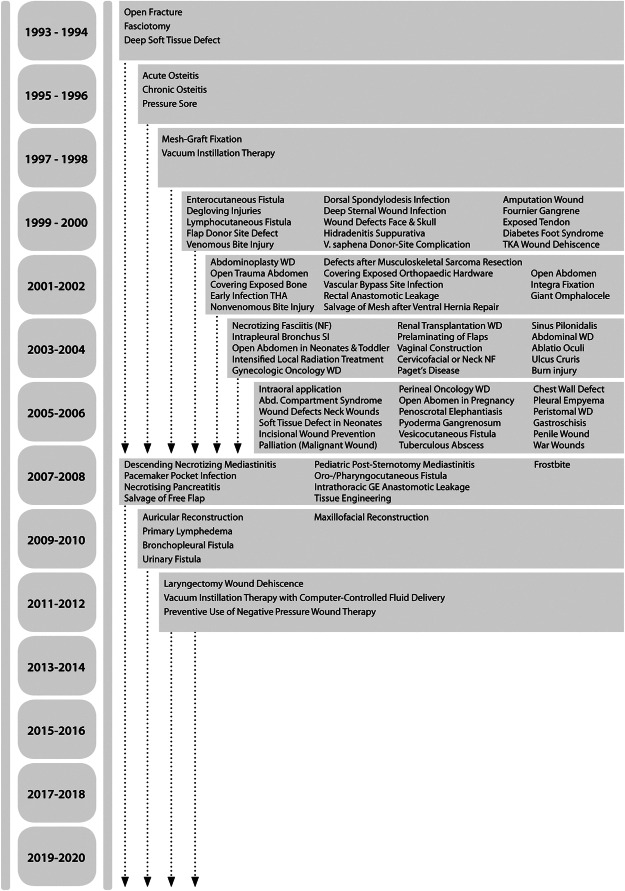
Development of the spectrum of indications for negative pressure wound therapy (NPWT) from 1993 to 2013. The assigned time is based on the date of publication. NPWT with instillation (NPWTi) is described for the first time in 1998. WD, wound dehiscence; THA, total hip arthroplasty; TKA, total knee arthroplasty; SI, stump insufficiency. (Reprinted with permission from Dr Christian Willy.)
Principle and effect of NPWT
The principle of NPWT involves extending the usually narrowly defined suction effect of drainage across the entire area of the wound cavity or surface using a reticulated open‐cell foam that fits the contour of the wound (Figure 2A and B). To prevent air leakage, wound and foam are hermetically sealed with an airtight adhesive polyurethane drape that is permeable to water vapour, transparent and bacteria proof (Figure 2C). A suction pad (T.R.A.C.™ Pad connector) is applied over a small hole made in the drape (Figure 2D) and is then connected to a vacuum source by a tube (Figure 2E and F).
Figure 2.
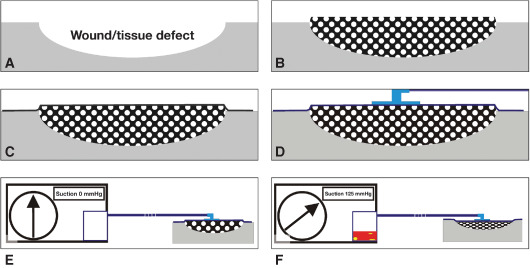
Functional principles of the negative pressure wound therapy (NPWT). (A, B) The wound (A) and a foam that has been fitted to the wound geometry, cut to size and placed inside the wound (B). (C, D) The wound is sealed airtight with a thin adhesive drape (C); similarly, with the attached suction pad (trac‐pad connector) including the drainage tube (D). (E) The wound is hermetically sealed with a thin adhesive drape and connected to the vacuum source by means of the attached suction pad (suction strength 0 mmHg). (F) Suction strength 125 mmHg. The foam has collapsed and the exudate collection reservoir is already partly filled. (Reprinted with permission from Dr Christian Willy.)
The following mechanisms of action can be considered as main effects on wound healing and thus potential clinical benefits of NPWT 16, 17, 18:
Reduction of the wound area secondary to the negative pressure that acts on the foam and pulls together the edges of the wound (wound retraction).
Promotion of granulation tissue formation in an optimally moist wound milieu induced by cellular microdeformations.
Continuation of effective wound cleansing (i.e. removal of small tissue debris by suction) after adequate primary surgical debridement of the wound.
Reliable, continuous removal of wound exudate (and consequently, fewer dressing changes) within a closed hygienic system.
Pressure‐related reduction of interstitial oedema with consecutive improvement of microcirculation (i.e. better nutritive perfusion).
NPWT with instillation (NPWTi)
NPWTi (V.A.C. Instill® Wound Therapy; V.A.C. VeraFlo™ Therapy; KCI USA, Inc.) is a modification of the conventional NPWT as adjunctive treatment in the management of acute and chronic wound infections, combining the benefits of NPWT with the addition of controlled delivery of topical solutions (such as cleansers, antiseptics and antibiotics) to the wound bed. This modification involves the retrograde instillation of substances into the sealed wound via an additional tubing system while the vacuum pump is paused. The foam is thus impregnated with the instilled fluid. The substance is allowed to soak for a user‐selected period of time (e.g. 20 minutes) after which the therapy unit resumes its suction and the remaining fluid is removed. This process can be repeated as often as required. In this manner, the topical solution comes into contact with the entire area between the foam and the wound surface.
Literature and recommendations for NPWTi
A literature search was subsequently performed in the MEDLINE database with medical keywords (Medical Subject Headings/MeSH) and free text. An additional survey was conducted in the Cochrane Library database. Limitations were set as well. The first filter concerned what the studies actually covered: only Cochrane Reviews, controlled clinical‐empirical studies, meta‐analysis, randomised controlled trials (RCTs) and systematic reviews were examined. Second, the publication period was limited to 2005–2012, and third, only publications in German and English were considered. The individual terms of keywords and free‐text searches are listed explicitly in the appendix lists (Appendix). Selection and evaluation of the literature implied was founded upon the criteria of evidence‐based medicine. They were based on one randomisation.
Another literature search was done in the MEDLINE database looking for all publications on the subject of NPWTi covering publications from the years 1990 to 2013.
Evidence‐based main experiences
NPWTi review
The total number of hits with duplicators was 2260 in the MeSH search and 2986 in the free‐text search. The results of the literature search were divided into subjects. All results were subjected to a test of relevance, taking into account the title and abstract information. Two‐hundred ninety publications met the criteria of relevance in the first inspection. They were then checked a second time in a full‐text analysis. After completing the full‐text review, 80 studies were included, the contents extracted and their statements/recommendations of evidence were checked. Summaries from the individual RCTs revealed 27 articles covering wound care with bone involvement and 11 articles covering wound care with soft‐tissue wounds without bone participation.
Concerning soft‐tissue wounds with bone involvement, NPWTi was performed in several studies with larger patient groups with newly infected soft‐tissue and orthopaedic injuries 19, 20, 21.
Gabriel et al. in 2008 published results for 15 patients with complex infected wounds. They were treated with NPWTi using silver nitrate and compared to the control group, which was treated with moist gauze bandages. The study showed that NPWTi patients required significantly fewer days of treatment (9·9 ± 4·3 compared with 36·5 ± 13·1 days, P < 0·001) and experienced earlier wound healing (13·2 ± 6·8 compared with 29·6 ± 6·5 days, P < 0·001) compared with the control group. The authors concluded that treatment with NPWTi ‘…can reduce costs’ 19.
Timmers et al. in 2009 21 demonstrated the usefulness of NPWTi in patients with osteomyelitis of the pelvis or lower extremities in a retrospective case–control cohort study. In addition to systemic antibiotic therapy, patients received either NPWTi using polyhexanide or were additionally treated with gentamicin‐polymethacrylate beads. The rate of infections was significantly reduced in the NPWTi group compared with the usual control group [3/30 (10%) versus 55/93 (58·5%), P < 0·0001].
The MEDLINE search for NPWTi identified a total of 36 peer‐reviewed citations in the literature on 7 March 2013 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, with a total of 154 documented patients. Although the technical basis of the therapy was established before 1996, the first publication mentioning instillation therapy was released only in 1998 27. The first commercially available version of an NPWTi system was introduced in 2003 (V.A.C. Instill® Wound Therapy). The annual publication rate has significantly increased since the year 2000, whereas the vast majority of publications – more than 80% – are case reports, case series or expert opinions (Level 4 or 5 according to the Oxford Centre for Evidence‐Based Medicine, as of May 2001). Recorded applications included antiseptics such as polyhexanide, 12·5% Dakin's solution and acetic acid solution, and different antibiotics (e.g. vancomycin, gentamicin, tobramycin, doxycycline and cephalosporins), Ringer's lactate and local anaesthetics as well as even insulin. However, in most articles, there was no further information regarding the instillation time, soak (exposure) time or the vacuum duration phase.
Irrigation review
Also, the literature contains significant evidence to indicate that sterile tap water is equal to NaCl irrigation in cleaning contaminated acute wounds, including those with open fractures (with respect to the incidence of infections). Additionally, washing with soap or antibiotic rinses show no advantage over NaCl flushing. Povidone‐iodine, however, shows significantly better rates of infection prevention at the surgical site for spinal surgery compared with NaCl rinses 55. Compared with Dermacyn® (Oculus Innovative Sciences, Petaluma, CA), povidone‐iodine had significantly poor results when applied for the prevention of infection on sternal wounds.
After reviewing the literature for the treatment of soft‐tissue wounds without bone participation, polyhexanide leads to faster wound healing in mesh graft‐treated burns compared with povidone‐iodine and silver nitrate 56. Compared with a saline solution, tap water is cheaper and just as safe and effective (incidence of wound infection is the same or lower with tap water) 57. In the absence of potable tap water, boiled and cooled water or distilled water can be used as wound cleanser as well. However, there is no evidence that cleansing wounds promotes healing or reduces infection risk, per se 58, 59, 60.
A systemic review by Nicks et al. in 2010 studied the treatment of acute wounds and examined studies comparing saline and diluted 1% povidone‐iodine solution for wound cleansing. There was no difference in the rate of infection 61, 62, 63. Another study recommended tap water or a saline solution for irrigation and decontamination with burns (n = 24) 64. Antiseptic solutions such as povidone‐iodine, chlorhexidine and hydrogen oxide, however, are toxic to the total tissue and can prevent wound healing 65. Warm body temperature (32–37°C) and 0·5% and 1·0% taurolidine‐Ringer solutions for debridement and 4% taurolidine gel for topical treatment of infections are alternatives. They cause no detectable cytotoxicity and do not negatively impact wound healing. However, there were no RCTs in the selected period that met the inclusion criteria for an octenidine and taurolidine application for the treatment of acute wounds.
In summary, the current literature shows an advantage of using polyhexanide for the treatment of soft‐tissue injuries without bone involvement compared with povidone‐iodine and silver nitrate. Tap water also appeared to have no disadvantages in comparison to NaCl and 1% povidone‐iodine irrigation.
Recommendations
As a result of the paucity of evidence from well‐designed RCTs, the selection of antiseptic agents for an NPWTi application must be based on a comparative assessment of all available efficacy, effectiveness and tolerability data from a wide variety of sources ranging from in vitro tests to RCTs and also including meta‐analyses. The type of wound and the condition of the patient must be taken into account as well.
Up until now, RCTs have been conducted only to a limited extent on this topic. The majority of studies compare an active substance with a control (e.g. NaCl or Ringer's) solution. Only a few studies compare different active substances. For this reason, an evaluation of antiseptic agents must be based on a comparative analysis of all available data. This comprehensive approach shall help to provide relevant evidence on how to use NPWTi and which antiseptics should be used in different clinical situations.
NPWTi: acute and chronic infections
The antiseptic solution used with NPWTi has to be suitable for deep penetrating wounds and should not cause oedematous inflammation or necrosis, in case of insufficient drainage of the fluid. According to current knowledge, polyhexanide and acetic acid are the first‐choice antiseptic solutions when using adjunctive NPWTi for acute and chronic infected wounds after surgical debridement (Table 1). Figure 3 illustrates an example of a clinical application of NPWTi.
Table 1.
Institutional recommendations on the concentration, fluid delivery, soak time and cycle frequency of polyhexanide, acetic acid and povidone‐iodine for use with NPWTi
| Solution | Concentration | Fluid delivery | Soak time | Cycle frequency |
|---|---|---|---|---|
| Polyhexanide | Concentration of 0·1% polyhexanide or 0·04% polyhexanide. On cartilage at the wound ground, the effective concentration of polyhexanide should be reduced to 0·005% to avoid adverse effects. | Amount of instilled fluid depends on wound volume. User‐selected instillation volume is controlled by the device. | 20 minutes | 4–8 times per day |
| Acetic acid | Concentrations between 0·25% and 1% are recommended. | Amount of instilled fluid depends on wound volume. User‐selected instillation volume is controlled by the device. | 20 minutes | 4–8 times per day |
| Povidone‐iodine | A combination of ethanol/propan‐2‐ol and povidone‐iodine [e.g. available as 100 ml solution, 3·24 g povidone‐iodine (this means 0·324%), 38·9 g isopropanol and 38·9 g ethanol] is the first‐choice antiseptic in the treatment of stabbing wounds at risk of infection with HBV, HCV or HIV and should be applied after bleeding has been encouraged. The same applies to the initial treatment of bite wounds. | Amount of instilled fluid depends on wound volume. User‐selected instillation volume is controlled by the device. | 20 minutes | 4–8 times per day |
NPWTi, negative pressure wound therapy with instillation; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Figure 3.
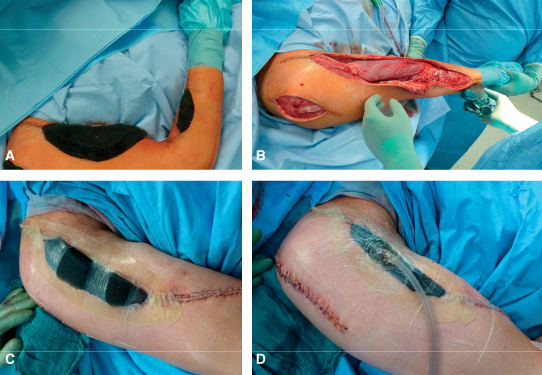
Example of a clinical application of negative pressure wound therapy with instillation (NPWTi) in a patient with necrotising fasciitis (instillation with polyhexanide 0·02%). (A) Reticulated open‐cell foam (V.A.C. VeraFlo™ Dressing; KCI USA, Inc., San Antonio, TX) fitted to the wounds; (B) well‐perfused clean soft tissue after second look and two intervals with vacuum instillation therapy; (C) reticulated open‐cell foam fitted to the upper arm wound, sealed in the first step with two drape strips, with skin protected by hydrocolloid dressing; (D) double lumen SensaTRAC‐pad (V.A.C. VeraT.R.A.C.™ Pad; KCI USA, Inc.) with closed wound.
NPWTi: prophylaxis in contaminated surgical extremity wounds
It is to be considered that, in spite of modern aseptic surgery and antibiotic treatment, infection rates of surgical wounds in all four classes (‘clean’, ‘clean‐contaminated’, ‘contaminated’ and ‘dirty’) are unsatisfactorily high. Before routine use of prophylactic antibiotics, infection rates were 1–2% or less for clean wounds, 6–9% for clean‐contaminated wounds, 13–20% for contaminated wounds and about 40% for dirty wounds 66. With the introduction of a routine prophylactic antibiotic use, infection rates in US hospitals were reported in 1991 by the National Nosocomial Infection Surveillance system 67 as: clean 2·1%, clean‐contaminated 3·3%, contaminated 6·4% and dirty 7·1% 68. However, there are still considerable variations in each class according to the type of surgery performed 69.
The presence of devitalised tissue and tissue with a damaged angio‐microarchitecture is an ideal culture medium for bacteria. A usually extensive contamination of wounds at the time of injury necessitates adequate irrigation and debridement. Literature indicates no clear support for additives given to irrigation fluids in extremity wounds, thus supporting the role of normal saline, sterile water and even potable water, if other fluids are not available 70. Even recent guidelines for combat injuries recommend the irrigation of wounds (only) with normal saline under low pressure (bulb syringe or equivalent) without additives to remove gross contamination 71, 72. Also, other sources support the assumption that today, worldwide, a routinely prophylactic use of antiseptics in contaminated surgical extremity wounds is not common 73. There is no controversy that irrigation is a key component to prevent an infection after open fracture, as it serves to decrease bacterial load and remove foreign bodies. However, currently, there is no consensus regarding the optimal approach to irrigate open fracture wounds during the initial operative procedure. Even the selection of the type of irrigating fluid remains controversial.
The aforementioned guideline for combat injuries 71 that is primarily based on the work of Murray et al. in 2011 must currently be considered the latest recommendation concerning the use of irrigation fluids 70, 72. However, when analysing the studies that support this guideline and those that led to the recommendation not to use irrigation fluid additives, it is obvious that today's recommended antiseptic solutions, such as acetic acid, polyhexanide or povidone‐iodine, were not analysed sufficiently 74, 75, 76.
In this context, the result of a survey performed by Petrisor et al. in 2008 is interesting 73. The authors asked physicians (a total of 676 surgeons) about their routinely used irrigation solution for open fracture wounds; 70.5% favoured normal saline alone. Over half of the respondents did not experience that either bacitracin or soap solution provided advantages over saline alone, but that iodine and chlorhexidine were more effective than saline alone. Furthermore, an estimated low efficiency of soap solution is supported by preliminary data of the FLOW study, which shows no differences in infection rates between the use of surfactant in comparison to that of saline 76. Thus, it is quite astonishing that the largest study performed so far is about testing particularly saline versus the surfactant castile soap (multicentre RCT, surgeons at clinical sites in North America, Europe, Australia and Asia, planned recruitment of 2280 patients). This large, multicentre collaborative project started in 2010 and should provide insights into the ideal irrigation strategies. Currently available modern antiseptics will be not used in this study 77. However, there are also a few studies supporting the prophylactic use of antiseptic substances (e.g. polyhexanide, povidone‐iodine, betadine solution and superoxidised water) to lower the rate of surgical site infections 78, 79, 80, 81 (Table 2). Despite the paucity of evidence from further well‐designed studies, the recommendation for the prophylactic use of antiseptic agents in critically contaminated wounds is justified. However, there is a need to do everything possible and reasonable to reduce the patient's risk, especially from wounds vulnerable to a surgical site infection. In this context, it would be necessary to define which ‘contaminated’ wounds signify indications for the prophylactic use of antiseptic agents in NPWTi and which antiseptics should be used (as mentioned above, same agents – polyhexanide and acetic acid – are recommended (Table 1).
Table 2.
Studies supporting the prophylactic use of antiseptic solutions for wound care
| Authors | Model | Study design/solutions tested | Benefits | Toxic effects |
|---|---|---|---|---|
| Kokavec and Fristakova 80 | Human/orthopaedic paediatric operations at proximal femur, hip and pelvis regions | RCT: 3·5% solution of betadine, povidone‐iodine (group 1) versus no betadine irrigation (group 2) | No infection in group: 1. Two superficial wound infections in group. 2. Need of prophylactic intraoperative irrigation of wounds in the hip and pelvis regions | None |
| Fournel et al. 79 | Human/surgical wounds | Meta‐analysis of n = 25, RCT: 1% povidone‐iodine (PVI) solution | Intraoperative PVI application significantly decreased the SSI rate (P = 0·003) | None |
| Mohd et al. 81 | Human/median sternotomy wound | RCT: Dermacyn superoxidised water versus povidone‐iodine | Incidence of sternotomy wound infection was 5·7% in Dermacyn® group and 15·6% in povidone‐iodine group (P = 0·033) | No superoxidised water‐related complication was identified |
| Becerro de Bengoa Vallejo et al. 78 | Human/nail avulsion surgery | RCT: 0·9% saline solution versus 0·2% nitrofurazone versus 0·1% polyhexanide | All three intraoperative irrigation methods reduced the total bacterial load, but polyhexanide was significantly more effective | None |
RCT, randomised controlled trial; SSI, surgical site infection.
Prophylactic NPWTi use in non‐infected wounds
Based on the currently available literature, no indisputable evidence‐based recommendations may be given. However, some implications can still be drawn 82, 83. Usually, the clinical use pertains to traumatic and more or less contaminated wounds. Traumatic wounds that can be closed primarily, either with or without drainage, without the risk of infection do not constitute as an indication for NPWTi. Wounds in which a revision surgery (second look) is planned owing to a real or suspected high contamination level and/or the critical perfusion of the wound edges after the first debridement, or wounds that simply cannot be closed, should be treated by NPWTi using an antiseptic when an increased infection risk exists. The extent of the infection risk cannot be calculated exactly but at best be estimated.
The following can be used as an aid towards deciding if the indications for a second look exist for the primarily non‐infected traumatic wounds:
Heavily contaminated penetrating injuries (blast injury, mine injuries, stab and gunshot wounds; thus, mostly wounds caused by violence and war as well as by humanitarian catastrophes like earthquakes)
Severe traumatic wounds [e.g. open fractures with severe contamination, extended degloving injury (Morel‐Lavallee) and soft‐tissue defects]
Cat, dog or human bite wounds.
It must be considered that a difficult hygienic situation in association with the social or occupational environment, patients' age being greater than 80 years or less than 1 year, their nutritional status, possible drug abuses, the immunological situation and comorbidities can modify the risk of an infection 82.
Conclusion and final remarks
The prophylactic use of NPWTi in non‐infected wounds is still not widespread 71, 72. However, especially in view of an expected >40% infection rate of contaminated traumatic wounds, there is still enough scientific‐based information supporting the prophylactic use of antiseptics without any information about any clinically relevant adverse effects. It is believed that it is possible to provide first temporary recommendations on the use of an antiseptics‐based NPWTi based on implications from the currently available literature.
A review of the existing literature supports polyhexanide and acetic acid as antiseptic substances most suitable for NPWTi in acute and chronic infections and povidone‐iodine as prophylaxis in potentially viral contaminated wounds. Also, when non‐infected wounds cannot be closed primarily with surgical means, NPWTi can be prophylactically applied using polyhexanide, acetic acid or povidone‐iodine, particularly in contaminated wounds.
Further studies on the level of evidence of RCTs and multicentre studies are immediately required to establish clear criteria for evidence‐based guidelines to ease decision‐making regarding the most efficient use of NPWTi in favour of injured patients with contaminated wounds or those suffering from infections after surgical interventions.
Acknowledgements
Dr CW presented as a faculty member during the 2012 and 2013 International Surgical Wound Forum (ISWF), an annual educational event sponsored by Kinetic Concepts, Inc. (KCI). He is the guest editor for this KCI‐funded educational supplement based on faculty presentations at 2012 and 2013 ISWF sessions related to wound care strategies with a focus on use of negative pressure wound therapy with instillation (i.e. V.A.C. Instill® Wound Therapy and V.A.C. VeraFlo™ Therapy; KCI, San Antonio, TX). KCI assisted with editorial review of manuscript. Dr DAB and Dr CSP report no conflict of interests or financial relationship with KCI.
Search terms Medical subject headings
((((((((((((((((((((((“Anti‐Infective Agents, Local” [Mesh] OR “Anti‐Infective Agents, Local” [Pharmacological Action]) OR “Sodium Chloride” [Mesh]) OR (“Lactic Acid”[Mesh] OR “Lac≠ .tates” [Mesh])) OR (“Pharmaceutical Solutions” [Mesh] OR “Solutions”[Mesh] OR “Pharmaceu tical Solutions” [Pharmacological Action])) OR “Povidone‐Iodine”[Mesh]) OR “Povidone” [Mesh]) OR (“Iodine”[Mesh] OR “Iodides”[Mesh])) OR “Biguanides”[Mesh]) OR “Chlorhexidine” [Mesh]) OR “Sodium Hypochlorite”[Mesh]) OR “Hydrogen Peroxide”[Mesh]) OR (“Acetic Acid” [Mesh] OR “Acetates”[Mesh])) OR “Nitrofurazone” [Mesh]) OR “Bacitracin”[Mesh]) OR “Polymyxin B” [Mesh]) OR “Gentamicins”[Mesh]) OR “Negative‐Pressure Wound Therapy”[Mesh]) OR “Anesthesia, Local”[Mesh]) OR “Surgical Sponges”[Mesh]) OR “Polymethyl Methacrylate” [Mesh]) OR (“Bone Cements”[Mesh] OR “Bone Cements” [Pharmacological Action])) OR “Honey”[Mesh]) OR “Debridement”[Mesh] OR (“Anti‐Infective Agents” [Mesh] OR “Anti‐Infective Agents” [Pharmacological Action])
((((((((((((((((“Abdominal Injuries”[Mesh]) OR “Burns”[Mesh]) OR “Negative‐Pressure Wound Therapy” [Mesh]) OR “Surgical Wound Dehiscence”[Mesh]) OR “Wounds, Guns‐hot”[Mesh]) OR “Wound Healing”[Mesh]) OR “Wound Infection”[Mesh]) OR “Surgical Wound Infection”[Mesh]) OR “Wounds and Injuries”[Mesh]) OR “Soft Tissue Injuries”[Mesh]) OR “Fractures, Bone” [Mesh]) OR “Wounds, Stab”[Mesh]) OR “Wound Closure Techniques”[Mesh]) OR “Debridement”[Mesh]) OR “Surgical Stapling” [Mesh]) OR “Wounds, Nonpenetrating”[Mesh]) OR “Skin Diseases, Infectious”[Mesh]
#1 AND #2 Filters activated: Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Controlled Clinical Trial, Meta‐Analysis, Multicenter Study, Randomized Controlled Trial, Systematic Reviews, Publication date from 2005/01/01 to 2012/12/31, Humans, English, German
Free text
contaminated wounds OR gun shot OR degloving injury OR morel lavallee OR soft tissue wound and open fracture OR abdominal wound plus no colonic and small bowel injury OR groin infection after arterial bypass OR infection of a joint OR war soft tissue wound OR infection of a body cavity OR clean wounds OR bypass operation OR osteosynthesis OR joint replacement OR intraabdominal operation OR complex contaminated plus infected soft tissue wounds OR open fracture OR acute osteomyelitis OR early infection OR penetrating abdominal trauma OR acute wound OR cartilage infection OR joint infection OR fracture OR open abdomen OR surgical wound OR surgical site infection OR burn wound OR wound infection OR wound infections OR wound contamination OR injury OR wound infection plus drug therapy OR wounds and injuries OR anti bacterial agents plus therapeutic use OR wound infection plus prevention and control OR wound healing plus drug effects OR intraoperative care plus method
ringer lactate OR isopropanol OR povidone iodine OR cadexomer iodine OR polyhexanide OR biguanides OR octenidine OR chlorhexidine OR taurolidine OR sodium hypochlorite OR dakin OR hydrogen peroxide OR acetic acid OR nitrofurazone OR neomycin sulfate polymyxin b OR gentamycin sulfate OR vacuum instillation OR local anaesthesia OR gentamycin sponges OR pmma‐chain OR antibiotic beads OR antibiotic‐spacers OR antibiotic solutions OR honey OR sugar OR pressure jet lavage OR hydrogen debridement OR ultrasound assisted device OR tap water OR microcyn OR antiseptics OR anti bacterial agents plus local OR antimicrobial agents plus local OR topical drug therapy OR anti‐infective agents plus local OR anti‐ infective agents plus topical OR silver OR medihoney OR antisepsis
#1 AND #2 Filters activated: Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Controlled Clinical Trial, Meta‐Analysis, Multicenter Study, Randomized Controlled Trial, Systematic Reviews, Publication date from 2005/01/01 to 2012/12/31, Humans, English, German
Cochrane Library search
MeSH‐search: (Anti‐Infective Agents):ti,ab,kw
(Wounds and Injuries):ti,ab,kw
#1 AND #2
Back DA, Scheuermann‐Poley C, Willy C. Recommendations on negative pressure wound therapy with instillation and antimicrobial solutions – when, where and how to use: what does the evidence show?.
References
- 1. ŐMeara S, Al‐Kurdi D, Ologun Y, Ovington LG. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2008;20(Suppl 1):CD003557. [DOI] [PubMed] [Google Scholar]
- 2. Knops AM, Storm‐Versloot MN, Mank AP, Ubbink DT, Vermeulen H, Bossuyt PM, Goossens A. Factors influencing long‐term adherence to two previously implemented hospital guidelines. International J Qual Health Care 2010;22:421–9. [DOI] [PubMed] [Google Scholar]
- 3. Ovington L. Bacterial toxins and wound healing. Ostomy Wound Manage 2003;49(7A Suppl):8–12. [PubMed] [Google Scholar]
- 4. Singhal H, Kaur K, Zammit C. Wound Infection. URL http://emedicinecom/med/topic2422htm%3E. [accessed on 15 September 2009]
- 5. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999;20:250–78, quiz 79–80. [DOI] [PubMed] [Google Scholar]
- 6. Wilson AP, Gibbons C, Reeves BC, Hodgson B, Liu M, Plummer D, et al. Surgical wound infection as a performance indicator: agreement of common definitions of wound infection in 4773 patients. BMJ 2004;329:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hacker SM. Common infections of the skin. Characteristics, causes, and cures. Postgrad Med 1994;96:43–6, 49–52. [PubMed] [Google Scholar]
- 8. Janda JM, Abbott SL, Brenden RA. Overview of the etiology of wound infections with particular emphasis on community‐acquired illnesses. Eur J Clin Microbiol Infect Dis 1997;16:189–201. [DOI] [PubMed] [Google Scholar]
- 9. Nichols RL. Surgical wound infection. Am J Med 1991;91(3B):54S–64S. [DOI] [PubMed] [Google Scholar]
- 10. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 1999;12:147–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devi PS, Rao PS, Shivananda PG. Characterization, antibiotic susceptibility pattern and detection of beta‐lactamases in Enterococci. Indian J Pathol Microbiol 2002;45:79–82. [PubMed] [Google Scholar]
- 12. Joint Formulary Committee . British National Formulary: London, UK. v. 43. 2002. [DOI] [PMC free article] [PubMed]
- 13. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76, discussion 77. [PubMed] [Google Scholar]
- 14. Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg 1993;96:488–92. [PubMed] [Google Scholar]
- 15. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 16. Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanisms of action of vacuum assisted closure: more to learn. Surgery 2009;146:40–51. [DOI] [PubMed] [Google Scholar]
- 17. Orgill DP, Bayer LR. Update on negative‐pressure wound therapy. Plast Reconstr Surg 2011;127(Suppl 1):105S–15S. [DOI] [PubMed] [Google Scholar]
- 18. Willy C, editor. Experimental basis I – review of the literature. The theory and practice of vacuum therapy. Scientific basis, indications for use, case reports, practical advice. Ulm: Lindqvist Publishing, 2006. [Google Scholar]
- 19. Gabriel A, Shores J, Heinrich C, Baqai W, Kalina S, Sogioka N, Gupta S. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 2008;5:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lehner B, Fleischmann W, Becker R, Jukema GN. First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study. Int Orthop 2011;35:1415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Timmers MS, Graafland N, Bernards AT, Nelissen RG, van Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen 2009;17:278–86. [DOI] [PubMed] [Google Scholar]
- 22. Zelen CM, Stover B, Nielson D, Cunningham M. A prospective study of negative pressure wound therapy with integrated irrigation for the treatment of diabetic foot ulcers. Eplasty 2011;11:e5. [PMC free article] [PubMed] [Google Scholar]
- 23. Allen D, Labarbera LA, Bondre IL, Lessing MC, Rycerz AM Jr, Kilpadi DV, Collins BA, Perkins J, McNulty AK. Comparison of tissue damage, cleansing and cross‐contamination potential during wound cleansing via two methods: lavage and negative pressure wound therapy with instillation. Int Wound J 2012. Aug 21. doi: 10.1111/j.1742‐481X.2012.01073.x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brem MH, Blanke M, Olk A, Schmidt J, Mueller O, Hennig FF, Gusinde J. [The vacuum‐assisted closure (V.A.C.) and instillation dressing: limb salvage after 3 degrees open fracture with massive bone and soft tissue defect and superinfection]. Unfallchirurg 2008;111:122–5. [DOI] [PubMed] [Google Scholar]
- 25. Chien SH, Tan WH, Hsu H. New continuous negative‐pressure and irrigation treatment for infected wounds and intractable ulcers. Plast Reconstr Surg 2008;122:318, author reply 9. [DOI] [PubMed] [Google Scholar]
- 26. D'Hondt M, D'Haeninck A, Dedrye L, Penninckx F, Aerts R. Can vacuum‐assisted closure and instillation therapy (VAC‐Instill therapy) play a role in the treatment of the infected open abdomen? Tech Coloproctol 2011;15:75–7. [DOI] [PubMed] [Google Scholar]
- 27. Fleischmann W, Russ M, Westhauser A, Stampehl M. [Vacuum sealing as carrier system for controlled local drug administration in wound infection]. Unfallchirurg 1998;101:649–54. [DOI] [PubMed] [Google Scholar]
- 28. Fujiwara M, Matsushita Y, Fukamizu H. Negative pressure therapy with irrigation for an infected digit: a preliminary report. Hand Surg 2011;16:99–103. [DOI] [PubMed] [Google Scholar]
- 29. Gabriel A. Integrated negative pressure wound therapy system with volumetric automated fluid instillation in wounds at risk for compromised healing. Int Wound J 2012;9(Suppl 1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gupta S. Sterilizing chronic wounds with negative pressure therapy: the role of antibiotic irrigation. Wound Repair Regen 2000;9:171. [Google Scholar]
- 31. Harada A, Nakamura Y, Fukumori K, Nagata T, Iguro Y. Negative pressure wound therapy was useful in treating empyema with bronchopleural fistula. Kyobu Geka 2010;63:1039–43. [PubMed] [Google Scholar]
- 32. Jerome D. Advances in negative pressure wound therapy: the VAC instill. J Wound Ostomy Continence Nurs 2007;34:191–4. [DOI] [PubMed] [Google Scholar]
- 33. Kirr R, Wiberg J, Hertlein H. [Clinical experience and results of using the V.A.C. instill therapy in infected hip‐ and knee prosthetics]. Zentralbl Chir 2006;131(Suppl 1):S79–82. [DOI] [PubMed] [Google Scholar]
- 34. Kiyokawa K, Takahashi N, Rikimaru H, Yamauchi T, Inoue Y. New continuous negative‐pressure and irrigation treatment for infected wounds and intractable ulcers. Plast Reconstr Surg 2007;120:1257–65. [DOI] [PubMed] [Google Scholar]
- 35. Kurihara C, Nishimura T, Kinoshita O, Kawata M, Hisagi M, Kyo S, Ono M. Successful treatment of mediastinitis after ventricular assist device implantation with rerouting of the outflow vascular prosthesis. J Artif Organs 2011;14:155–8. [DOI] [PubMed] [Google Scholar]
- 36. Labler L, Trentz O. V.A.C. instillation: in vitro model. Part 1. Biomed Tech (Berl) 2005;50:413–8. [DOI] [PubMed] [Google Scholar]
- 37. Labler L, Trentz O. V.A.C. instillation: in vitro model. Part 2. Biomed Tech (Berl) 2006;51:30–7. [DOI] [PubMed] [Google Scholar]
- 38. Lee SS, Chang KP, Lai CS, Lin SD. Does continuous negative‐pressure and irrigation treatment really rinse the whole closed wound? Plast Reconstr Surg 2008;122:319–20, author reply 20‐1. [DOI] [PubMed] [Google Scholar]
- 39. Lehner B, Bernd L. [V.A.C.‐instill therapy in periprosthetic infection of hip and knee arthroplasty]. Zentralbl Chir 2006;131(Suppl 1):S160–4. [DOI] [PubMed] [Google Scholar]
- 40. Matsushita Y, Fujiwara M, Nagata T, Noda T, Fukamizu H. Negative pressure therapy with irrigation for digits and hands: pressure measurement and clinical application. Hand Surg 2012;17:71–5. [DOI] [PubMed] [Google Scholar]
- 41. McNulty AK, Nguyen K. What is the benefit of instillation therapy? Int J Low Extrem Wounds 2010;9:68–9. [DOI] [PubMed] [Google Scholar]
- 42. Moch D, Fleischmann W, Russ M. [The BMW (biosurgical mechanical wound treatment) in diabetic foot]. Zentralbl Chir 1999;124(Suppl 1):69–72. [PubMed] [Google Scholar]
- 43. Moch D, Fleischmann W, Westhauser A. [Instillation vacuum sealing‐‐report of initial experiences]. Langenbecks Arch Chir Suppl Kongressbd 1998;115:1197–9. [PubMed] [Google Scholar]
- 44. Participants . V.A.C. instill therapy – indications and technical applications. Abstracts of the First V.A.C. Instill Symposium. Heidelberg, Germany. November 21, 2008. Infection 2008;37(Suppl 1):3–45. [DOI] [PubMed] [Google Scholar]
- 45. Plikaitis CM, Molnar JA. Subatmospheric pressure wound therapy and the vacuum‐assisted closure device: basic science and current clinical successes. Expert Rev Med Devices 2006;3:175–84. [DOI] [PubMed] [Google Scholar]
- 46. Raad W, Lantis JC 2nd, Tyrie L, Gendics C, Todd G. Vacuum‐assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int Wound J 2010;7:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riepe G, Schneider M. [V.A.C. instill – first experiences on an inflammatory process of the proximal femur 3 years after hip‐joint operation]. Zentralbl Chir 2006;131(Suppl 1):S157–9. [DOI] [PubMed] [Google Scholar]
- 48. Rycerz AM, Slack P, McNulty AK. Distribution assessment comparing continuous and periodic wound instillation in conjunction with negative pressure wound therapy using an agar‐based model. Int Wound J 2013;10:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schintler MV, Grohmann M, Donia C, Aberer E, Scharnagl E. Management of an unfortunate triad after breast reconstruction: pyoderma gangrenosum, full‐thickness chest wall defect and Acinetobacter baumannii infection. J Plast Reconstr Aesthet Surg 2010;63:e564–7. [DOI] [PubMed] [Google Scholar]
- 50. Scimeca CL, Bharara M, Fisher TK, Giovinco N, Armstrong DG. Novel use of doxycycline in continuous‐instillation negative pressure wound therapy as "wound chemotherapy". Foot Ankle Spec 2010;3:190–3. [DOI] [PubMed] [Google Scholar]
- 51. Scimeca CL, Bharara M, Fisher TK, Kimbriel H, Mills JL, Armstrong DG. Novel use of insulin in continuous‐instillation negative pressure wound therapy as "wound chemotherapy". J Diabetes Sci Technol 2010;4:820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Streubel PN, Stinner DJ, Obremskey WT. Use of negative‐pressure wound therapy in orthopaedic trauma. J Am Acad Orthop Surg 2012;20:564–74. [DOI] [PubMed] [Google Scholar]
- 53. Wolvos T. Wound instillation–the next step in negative pressure wound therapy. Lessons learned from initial experiences. Ostomy Wound Manage 2004;50:56–66. [PubMed] [Google Scholar]
- 54. Wolvos T. Wound instillation with negative pressure wound therapy. Ostomy Wound Manage 2005;51(2A Suppl):21S–6S. [PubMed] [Google Scholar]
- 55. Chang FY, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Can povidone‐iodine solution be used safely in a spinal surgery? Eur Spine J 2006;15:1005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Daeschlein G, Assadian O, Bruck JC, Meinl C, Kramer A, Koch S. Feasibility and clinical applicability of polihexanide for treatment of second‐degree burn wounds. Skin Pharmacol Physiol 2007;20:292–6. [DOI] [PubMed] [Google Scholar]
- 57. Moscati RM, Mayrose J, Reardon RF, Janicke DM, Jehle DV. A multicenter comparison of tap water versus sterile saline for wound irrigation. Acad Emerg Med 2007;14:404–9. [DOI] [PubMed] [Google Scholar]
- 58. Fernandez R, Griffiths R. Water for wound cleansing. Cochrane Database Syst Rev 2008;1:CD003861. [DOI] [PubMed] [Google Scholar]
- 59. Fernandez R, Griffiths R. Water for wound cleansing. Cochrane Database Syst Rev 2012;2:CD003861. [DOI] [PubMed] [Google Scholar]
- 60. Hall S. A review of the effect of tap water versus normal saline on infection rates in acute traumatic wounds. J Wound Care 2007;16:38–41. [DOI] [PubMed] [Google Scholar]
- 61. Chisholm CD, Cordell WH, Rogers K, Woods JR. Comparison of a new pressurized saline canister versus syringe irrigation for laceration cleansing in the emergency department. Ann Emerg Med 1992;21:1364–7. [DOI] [PubMed] [Google Scholar]
- 62. Khan MN, Naqvi AH. Antiseptics, iodine, povidone iodine and traumatic wound cleansing. J Tissue Viability 2006;16:6–10. [DOI] [PubMed] [Google Scholar]
- 63. Watt BE, Proudfoot AT, Vale JA. Hydrogen peroxide poisoning. Toxicol Rev 2004;23:51–7. [DOI] [PubMed] [Google Scholar]
- 64. Cartotto RC, Peters WJ, Neligan PC, Douglas LG, Beeston J. Chemical burns. Can J Surg 1996;39:205–11. [PMC free article] [PubMed] [Google Scholar]
- 65. Nicks BA, Ayello EA, Woo K, Nitzki‐George D, Sibbald RG. Acute wound management: revisiting the approach to assessment, irrigation, and closure considerations. Int J Emerg Med 2010;3:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Conroy BP, Anglen JO, Simpson WA, Christensen G, Phaup G, Yeager R, Gainor BJ. Comparison of castile soap, benzalkonium chloride, and bacitracin as irrigation solutions for complex contaminated orthopaedic wounds. J Orthop Trauma 1999;13:332–7. [DOI] [PubMed] [Google Scholar]
- 67. Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, Banerjee SN, Edwards JR, Tolson JS, Henderson TS, Hughes JM. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med 1991;91(3B):152S–7S. [DOI] [PubMed] [Google Scholar]
- 68. Costagliola M, Agrosi M. Second‐degree burns: a comparative, multicenter, randomized trial of hyaluronic acid plus silver sulfadiazine vs. silver sulfadiazine alone. Curr Med Res Opin 2005;21:1235–40. [DOI] [PubMed] [Google Scholar]
- 69. Creanor S, Barton A, Marchbank A. Effectiveness of a gentamicin impregnated collagen sponge on reducing sternal wound infections following cardiac surgery: a meta‐analysis of randomised controlled trials. Ann R Coll Surg Engl 2012;94:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Murray CK, Obremskey WT, Hsu JR, Andersen RC, Calhoun JH, Clasper JC, Whitman TJ, Curry TK, Fleming ME, Wenke JC, Ficke JR. Prevention of infections associated with combat‐related extremity injuries. J Trauma 2011;71(2 Suppl 2):S235–57. [DOI] [PubMed] [Google Scholar]
- 71. Hospenthal DR, Murray CK, Andersen RC, Bell RB, Calhoun JH, Cancio LC, Cho JM, Chung KK, Clasper JC, Colyer MH, Conger NG, Costanzo GP, Crouch HK, Curry TK, D'Avignon LC, Dorlac WC, Dunne JR, Eastridge BJ, Ficke JR, Fleming ME, Forgione MA, Green AD, Hale RG, Hayes DK, Holcomb JB, Hsu JR, Kester KE, Martin GJ, Moores LE, Obremskey WT, Petersen K, Renz EM, Saffle JR, Solomkin JS, Sutter DE, Tribble DR, Wenke JC, Whitman TJ, Wiesen AR, Wortmann GW. Guidelines for the prevention of infections associated with combat‐related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma 2011;71(2 Suppl 2):S210–34. [DOI] [PubMed] [Google Scholar]
- 72. Hospenthal DR, Murray CK, Andersen RC, Bell RB, Calhoun JH, Cancio LC, Cho JM, Chung KK, Clasper JC, Colyer MH, Conger NG, Costanzo GP, Crouch HK, Curry TK, D'Avignon LC, Dorlac WC, Dunne JR, Eastridge BJ, Ficke JR, Fleming ME, Forgione MA, Green AD, Hale RG, Hayes DK, Holcomb JB, Hsu JR, Kester KE, Martin GJ, Moores LE, Obremskey WT, Petersen K, Renz EM, Saffle JR, Solomkin JS, Sutter DE, Tribble DR, Wenke JC, Whitman TJ, Wiesen AR, Wortmann GW. Executive summary: guidelines for the prevention of infections associated with combat‐related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma 2011;71(2 Suppl 2):S202–9. [DOI] [PubMed] [Google Scholar]
- 73. Petrisor B, Jeray K, Schemitsch E, Hanson B, Sprague S, Sanders D, Bhandari M. Fluid lavage in patients with open fracture wounds (FLOW): an international survey of 984 surgeons. BMC Musculoskelet Disord 2008;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stevenson J, McNaughton G, Riley J. The use of prophylactic flucloxacillin in treatment of open fractures of the distal phalanx within an accident and emergency department: a double‐blind randomized placebo‐controlled trial. J Hand Surg Br 2003;28:388–94. [DOI] [PubMed] [Google Scholar]
- 75. Anglen JO. Comparison of soap and antibiotic solutions for irrigation of lower‐limb open fracture wounds. A prospective, randomized study. J Bone Joint Surg Am 2005;87:1415–22. [DOI] [PubMed] [Google Scholar]
- 76. Petrisor B, Sun X, Bhandari M, Guyatt G, Jeray KJ, Sprague S, Tanner S, Schemitsch E, Sancheti P, Anglen J, Tornetta P, Bosse M, Liew S, Walter S. Fluid lavage of open wounds (FLOW): a multicenter, blinded, factorial pilot trial comparing alternative irrigating solutions and pressures in patients with open fractures. J Trauma 2011;71:596–606. [DOI] [PubMed] [Google Scholar]
- 77. Investigators F. Fluid lavage of open wounds (FLOW): design and rationale for a large, multicenter collaborative 2 x 3 factorial trial of irrigating pressures and solutions in patients with open fractures. BMC Musculoskelet Disord 2010;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Becerro de Bengoa Vallejo R, Losa Iglesias ME, Cervera LA, Fernandez DS, Prieto JP. Efficacy of intraoperative surgical irrigation with polihexanide and nitrofurazone in reducing bacterial load after nail removal surgery. J Am Acad Dermatol 2011;64:328–35. [DOI] [PubMed] [Google Scholar]
- 79. Fournel I, Tiv M, Soulias M, Hua C, Astruc K, Aho Glele LS. Meta‐analysis of intraoperative povidone‐iodine application to prevent surgical‐site infection. Br J Surg 2010;97:1603–13. [DOI] [PubMed] [Google Scholar]
- 80. Kokavec M, Fristakova M[. Efficacy of antiseptics in the prevention of post‐operative infections of the proximal femur, hip and pelvis regions in orthopedic pediatric patients. Analysis of the first results]. Acta Chir Orthop Traumatol Cech 2008;75:106–9. [PubMed] [Google Scholar]
- 81. Mohd AR, Ghani MK, Awang RR, Su Min JO, Dimon MZ. Dermacyn irrigation in reducing infection of a median sternotomy wound. Heart Surg Forum 2010;13:E228–32. [DOI] [PubMed] [Google Scholar]
- 82. Dissemond J, Assadian O, Gerber V, Kingsley A, Kramer A, Leaper DJ, Mosti G, Piatkowski de Grzymala A, Riepe G, Risse A, Romanelli M, Strohal R, Traber J, Vasel‐Biergans A, Wild T, Eberlein T. Classification of wounds at risk and their antimicrobial treatment with polihexanide: a practice‐oriented expert recommendation. Skin Pharmacol Physiol 2011;24:245–55. [DOI] [PubMed] [Google Scholar]
- 83. Willy C, Scheuermann‐Poley C, Krapohl BD, Back DA. A small view back, some reflections and final recommendation. In: Willy C, editor. Antiseptics in surgery – update 2013, 1st edn. Berlin: Lindqvist Books Publishing, 2013:203–19. [Google Scholar]


