Abstract
Malnutrition is associated with the delay or failure of healing. We assessed the effect of experimental malnutrition and early enteral feeding with standard diet or diet supplemented with arginine and antioxidants on the levels of mRNA encoding growth factors in acute, open wound healing. Standardised cutaneous dorsal wounds and gastrostomies for enteral feeding were created in malnourished (M, n = 27) and eutrophic control (E, n = 30) Lewis male adult rats. Both M and E rats received isocaloric and isonitrogenous regimens with oral chow and saline (C), standard (S) or supplemented (A) enteral diets. On post‐trauma day 7, mRNA levels of growth factor genes were analysed in wound granulation tissue by reverse transcription polymerase chain reaction (RT‐PCR). M(C) rats had significantly lower transforming growth factor β(TGF‐β 1) mRNA levels than E(C) rats (2·58 ± 0·83 versus 3·53 ± 0·57, P < 0·01) and in comparison with M(S) and M(A) rats (4·66 ± 2·49 and 4·61 ± 2·11, respectively; P < 0·05). VEGF and KGF‐7 mRNA levels were lower in M(A) rats than in E(A) rats (0·74 ± 0·16 versus 1·25 ± 0·66; and 1·07 ± 0·45 versus 1·79 ± 0·89, respectively; P≤ 0·04), but did not differ from levels in E(C) and M(C) animals. In experimental open acute wound healing, previous malnutrition decreased local mRNA levels of TGF‐β 1 genes, which was minimised by early enteral feeding with standard or supplemented diets.
Keywords: Antioxidants, Arginine, Enteral nutrition, Growth factors, TGF‐β1, Wound healing
Introduction
Growth factors actively participate in the organisation, coordination and mediation of all cellular processes involved in wound healing, such as cellular migration, angiogenesis, matrix synthesis, collagen deposition, formation of granulation and tissue remodelling 1, 2.
Transforming growth factor β (TGF‐β) has cell‐specific effects in this setting by influencing cellular proliferation, differentiation, metabolism and the extracellular matrix (ECM) 3, 4, 5, 6. TGF‐β 1 is the most abundant isoform in all tissues and cells, and its expression peaks earlier post‐wounding than TGF‐β 2 or TGF‐β 3 expression, suggesting a central role for this isoform in the acute phase of the healing process 7, 8.
TGF‐β 1 is crucial in wound healing because it influences fibroblast, collagen synthesis and ECM formation, and plays a critical role in wound strength by promoting the progressive replacement of collagen type III by collagen type I via its ECM remodelling properties 9, 10, 11.
Keratinocyte growth factor (KGF) stimulates reepithelialisation and mediates mesenchymal–epithelial interactions to promote epithelial proliferation and migration within the wounded area, which facilitates differentiation of new epidermis 12. Blood platelets secrete platelet‐derived growth factor (PDGF) when they adhere to traumatised tissues, which leads to an autocrine and paracrine amplifier effect 12. Angiogenesis in subjacent dermal endothelial cells is induced by vascular endothelial growth factor (VEGF), which is synthesised mainly by keratinocytes around the wound edge 12.
Wound healing is strongly dependent on the availability of energy, protein and micronutrients. Malnutrition is largely associated with a delay or failure of the healing process, but nutritional intervention can mitigate malnutrition and improve wound healing, mainly by increasing collagen deposition after trauma 3.
Specialised diets enriched with arginine and antioxidants have been advocated in this setting and associated with less morbidity and shorter hospital stays 3. Arginine may benefit the healing process by positively affecting microvascular and perfusion changes, protein synthesis, cellular proliferation and signalling via proline and polyamine synthesis, whereas antioxidants can contribute to healing by preventing or attenuating peroxidative damage 3, 13, 14, 15, 16.
We reasoned that growth factor synthesis during wound healing could be impaired in poor nutritional status, especially during the acute post‐trauma period, and that a specialised diet enriched with arginine and antioxidants would beneficially affect the molecular expression of these healing mediators. Therefore, the objective of this study was to assess the expression of growth factor genes in acute open wound healing under experimental malnutrition and early enteral feeding with standard and specialised (containing arginine, vitamins C and E, selenium and zinc) enteral diets.
Materials and methods
Animals
All animals received appropriate care throughout the experimental procedure, and the animal experimentation protocol was approved by the local ethics committee ‘Comissão de Ética para Análise de Projetos de Pesquisa do HCFMUSP’. We used 57 adult male isogenic Lewis rats that weighed 250–350 g. The animals were allowed free access to water and standard rat oral diet (AIN‐93M, Rhoster Indústria e Comércio, Brazil) in individual metabolic cages at room temperature for 10 days with regular light cycles before the experimental procedures in order to acclimatise the rats to our laboratory conditions.
Experimental malnutrition
After acclimation, the animals were randomly divided into two groups: eutrophic (E) and previously malnourished (M). The E rats were fed AIN‐93M ad libitum; M rats were submitted to dietary restriction, and received 50% of the mean amount of food consumed by E rats. After 14 days of dietary restriction, the M rats had a 12–15% reduction in body weight compared with their initial body weight.
Gastrostomy and cutaneous wounds
All rats were anaesthetised with an intraperitoneal injection of 100 mg/kg ketamine hydrochloride (Ketalar, Parke‐Davis, São Paulo, Brazil) and 2% xylazine (Rumpum, Bayer, São Paulo, Brazil). After abdominal and dorsal shaving, gastrostomy and four dorsal back full‐thickness skin excision wounds (1·3 cm diameter) were made, as described elsewhere 17. The dorsal muscular fascia was exposed (Fig. 1), and the wounds were left uncovered during the post‐trauma period.
Figure 1.
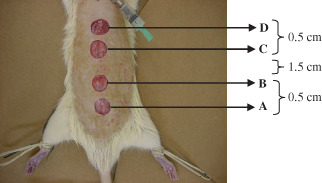
Surgically generated dorsal cutaneous wounds in Lewis rats.
Post‐trauma nutrition
Immediately following surgery, all rats were kept in a fasting state and received 5% dextrose at a rate of 0·3 ml/hour for 12 hours throughout the gastrostomy. After this period, the E and M groups were each randomly distributed into three different subgroups according to the nutritional treatment to be received (Table 1). All animals were fed orally with AIN‐93M chow (adjusted for specific total isocaloric and isonitrogenous intakes depending on the group allocation) and saline (0·9%), or two different liquid diets: the standard (Hiper Diet Multifiber, Nutricia, The Netherlands) or a specialised diet (Cubison, Nutricia, The Netherlands). The two enteral diets were isocaloric and isonitrogenous per volume, and had the same amount of dietary fibre, sodium, potassium, chlorine, calcium, magnesium, phosphorus, iron, fluorine, molybdenum, chromium, iodine, carotenoids, thiamine, niacin, pantothenic acid, biotin, choline, and vitamins A, D, K, B16, and B12. The specialised enteral diet had more arginine (0·003 versus 0 g/ml), zinc (0·02 versus 0·012 mg/ml), selenium (0·096 versus 0·057 µg/ml), vitamin E (0·075 versus 0·013 mg/ml) and vitamin C (0·38 versus 0·1 mg/ml) than the standard oral and enteral diets.
Table 1.
Experimental groups of isogenic Lewis rats, classified according to nutrition status, oral and/or enteral diet administered after gastrostomy and standardised cutaneous dorsal wound
| Groups | Animals (n) | Description |
|---|---|---|
| N(C) | 10 | Nourished; gastrostomy with saline + oral diet |
| N(S) | 10 | Nourished; gastrostomy with S + oral diet |
| N(A) | 10 | Nourished; gastrostomy with A + oral diet |
| M(C) | 09 | Malnourished; gastrostomy with saline + oral diet |
| M(S) | 09 | Malnourished; gastrostomy with S + oral diet |
| M(A) | 09 | Malnourished; gastrostomy with A + oral diet |
N, nourished; M, malnourished; C, saline; S, standard enteral diet; A, enteral diet supplemented with arginine and antioxidants.
Continuous infusion of saline and the enteral diets through the gastrostomy was performed until post‐trauma day 7 using a micropump infusion (Life Care, Abbott Laboratories, OH) and a graduated burette (Micro Soluset 150/160, Abbott Laboratories, Costa Rica). The infused saline and enteral diets were recorded daily. The oral dietary intake was measured daily by determining the difference between the amount of oral diet added and the residual diet found in the cages after 24 hours. The daily infused and consumed (total intake) energy, nitrogen, arginine, selenium, zinc, vitamin C and vitamin E were calculated.
Percentage body weight variation in the post‐trauma period
The variation in body weight during the post‐trauma period was calculated by considering the initial (surgery day) and final (post‐trauma day 7) body weight. Arithmetic means were calculated for each experimental group.
Granulation tissue sampling
On post‐trauma day 7, all rats were anaesthetised with intraperitoneal ketamine hydrochloride and xylazine. Wound tissue containing both epithelium and granulation tissue was collected from the proximal pair of wounds. Samples from granulation tissue collected from each rat were promptly identified, quickly frozen and stored in liquid nitrogen for further analysis of gene expression.
Molecular biology analysis
Total mRNA was extracted using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions and the methods described by Chomezynski and Sacchi 18. The relative quantification of the target genes was based on the endogenous β‐actin gene, as described by Pfaffl 19. The reactions were performed using Corbett Research Rotor‐Gene RG‐3000 equipment (Corbett Research, Sydney, Australia) and the SuperScript III Platinum SYBR Green One‐Step Quantitect Sybr Green RT‐PCR Kit (Invitrogen Life Technologies). Oligonucleotides were designed using the published mRNA sequence (available at www.ncbi.nlm.nih.gov/nucleotide), and primers were designed using the Primer 3 programme.
All primers were synthesised by Invitrogen Life Technologies. The oligonucleotide primers used were as follows: β‐actin (GenBank accession number C0985) sense primer: 5′‐TGT CAC CAA CTG GGA CGA TA‐3′, and antisense primer 5′‐GGG GTG TTG AAG GTC TCA AA‐3′; TGF‐β 1 (GenBank accession number A3546) sense primer: 5′‐ATA CGC CTG AGT GGC TGT CT‐3′, and antisense primer 5′‐TGG GAC TGA TCC CAT TGA TT‐3′; VEGF (GenBank accession number D4860) sense primer: 5′‐GCC CAT GAA GTG GTG AAG TT‐3′, and antisense primer 5′‐ACT CCA GGG CTT CAT CAT TG‐3′; PDGF‐α (GenBank accession number S0589) sense primer: 5′‐ATG CCT TGG AGA CAA ACC TG‐3′, and antisense primer 5′‐GTC AAG AAG TTG GCC GAT GT‐3′; and KGF‐7 (GenBank accession number J2745) sense primer: 5′‐CTG TGG CAG TTG TAA TTG TG‐3′, and antisense primer 5′‐ACA GGA AGC CCC TTT TGA TT‐3′.
Statistical analysis
The distributions of all data were tested for normality. Statistical analysis was then performed using analysis of variance (ANOVA) with the experimental factors of nutritional status (E versus M) and the two different enteral solutions delivered through the gastrostomy (S versus A). ANOVA was preceded by logarithm transformation of the mRNA levels, which was required for adequate mRNA level matching to normal profile data. When a significant interaction was found, post hoc analysis (Newman–Keuls test) was used to compare differences among independent groups. An alpha‐level of 0·05 was used to determine overall significance. Values are reported as mean ± SE.
Results
Total energy and nitrogen intake
For eutrophic animals, there was no difference in the total caloric intake among the experimental groups, but the A group ingested higher amounts of nitrogen than the C and S groups. For malnourished animals, there was a higher intake of calories in the C group than in the S and A groups, and a tendency towards lower nitrogen intake in the S group than in the C and A groups (P≥ 0·051). The data describing total calories and nitrogen intake among the experimental groups are described in Table 2.
Table 2.
Total of calories (kcal/day) and nitrogen intake (g/day) and percentage of body weight variation in post‐trauma period*
| Groups | Calories | Nitrogen | Body weight | |
|---|---|---|---|---|
| E | C | 53·31 ± 7·66 | 0·30 ± 0·04 | 0·40 ± 9·56 |
| S | 52·08 ± 5·27 | 0·30 ± 0·01 | −1·19 ± 3·17 | |
| A | 55·19 ± 6·43 | 0·42 ± 0·04† | 1·52 ± 4·68 | |
| M | C | 67·32 ± 12·23‡ | 0·38 ± 0·07 | 9·32 ± 7·98‡ |
| S | 54·35 ± 10·30 | 0·31 ± 0·06 | 1·15 ± 9·04 | |
| A | 48·29 ± 5·01 | 0·37 ± 0·04 | 2·03 ± 3·34 | |
E, eutrophic; M, malnourished; C, oral diet + saline; S, oral diet + standard enteral diet; A, oral diet + specialised enteral diet.
Data presented as mean ± standard deviation.
A > C and S (P≤ 0·000).
C > S and A (P≤ 0·036 for calories; P = 0·048 for body weight).
Arginine and antioxidant intake
For both studied nutritional status, animals from A group had a total intake of arginine, selenium, zinc, vitamins C and E that was higher than those from the C and S groups. However, there was no difference in the intake of these nutrients between the malnourished and eutrophic rats from A group. For both studied nutritional status, animals from the C and S groups were similar in terms of zinc intake, but the C group had a higher intake of arginine and vitamin E and a lower intake of selenium and vitamin C than the S group. The data describing total arginine and antioxidant intake among the experimental groups are shown in Table 3.
Table 3.
Total arginine and antioxidant daily intake in post‐trauma period*
| Groups | Arginine (g) | Selenium (µg) | Zinc (mg) | Vitamin C (mg) | Vitamin E (mg) | |
|---|---|---|---|---|---|---|
| N | C | 0·072 ± 0·008 | 0·003 ± 0·0003 | 0·56 ± 0·06 | 0·0 ± 0·0 | 1·20 ± 0·14 |
| S | 0·023 ± 0·003 | 2·345 ± 0·2169 | 0·67 ± 0·04 | 4·1 ± 0·4 | 0·92 ± 0·04 | |
| A | 0·412 ± 0·025 | 4·131 ± 0·2690 | 1·07 ± 0·06 | 16·6 ± 0·8 | 3·64 ± 0·21 | |
| M | C | 0·075 ± 0·010 | 0·003 ± 0·0004 | 0·58 ± 0·08 | 0·0 ± 0·0 | 1·25 ± 0·17 |
| S | 0·030 ± 0·004 | 2·115 ± 0·2261 | 0·68 ± 0·05 | 3·7 ± 0·4 | 0·98 ± 0·08 | |
| A | 0·403 ± 0·020 | 4·089 ± 0·2040 | 1·02 ± 0·04 | 16·1 ± 0·8 | 3·53 ± 0·18 | |
N, nourished; M, malnourished; C, oral diet + saline; S, oral diet + standard enteral diet; A, oral diet + specialised enteral diet.
Data presented as mean ± standard deviation. Arginine and all antioxidant for N and M groups: A > C; A > S (P < 0·001). Arginine and vitamin E: E(C) > E(S); M(C) > M(S) (p < 0.001). Selenium and vitamin C: E(C) < E (S); M(C) < M(S) (p < 0.001).
Percentage of body weight variation in the post‐trauma period
On post‐trauma day 7, most of the animals had gained weight compared with the first post‐trauma day. There were no significant differences in the percentage of body weight gain among the eutrophic animals. For malnourished animals, the C group had a higher weight gain compared with the A and S groups. The data regarding the percentage body weight variation among the experimental groups are described in Table 2.
Gene expression analysis
When we compared malnourished with eutrophic rats from control groups, malnutrition was associated with lower mRNA expression levels of TGF‐β 1. For the same nutritional status, malnourished rats from the control group also had lower expression levels of TGF‐β1 than malnourished animals treated with both studied enteral diets. For animals from A group, those malnourished had lower mRNA expression levels of VEGF and KGF‐7 than those eutrophic. The data describing growth factors mRNA expression are listed in Table 4.
Table 4.
Growth factor mRNA expression measurements in granulation tissue on post‐trauma day 7†
| Groups | TGF‐β 1 | VEGF | PDGF‐α | KGF‐7 | |
|---|---|---|---|---|---|
| Nourished | C | 3·53 ± 0·57 | 1·27 ± 0·97 | 1·45 ± 1·15 | 1·50 ± 0·72 |
| S | 4·06 ± 1·58 | 0·95 ± 0·46 | 1·46 ± 1·12 | 1·71 ± 1·16 | |
| A | 3·78 ± 1·85 | 1·25 ± 0·66 | 1·02 ± 0·45 | 1·79 ± 0·89 | |
| Malnourished | C | 2·58 ± 0·83* | 1·21 ± 0·70 | 1·24 ± 0·51 | 1·27 ± 0·78 |
| S | 4·66 ± 2·49 | 0·97 ± 0·37 | 0·97 ± 0·60 | 1·24 ± 0·50 | |
| A | 4·61 ± 2·11 | 0·74 ± 0·16* | 1·08 ± 0·62 | 1·07 ± 0·45* | |
| ANOVA | M(C) < N(C)* M(C) < M(S)** M(C) < M(A)** | M(A) < N(A)*** | No difference | M(A) < N(A)*** | |
P < 0·01;
P = 0·05;
P < 0·05.
C, oral diet + saline; S, oral diet + standard enteral diet; A, oral diet + specialised enteral diet.
Data expressed in arbitrary units (AU).
Discussion
Malnutrition is associated with delay or failure of healing, but little is known about the molecular mechanisms underlying this effect 6. This study is part of a large study that aimed to assess the effect of malnutrition and specialised enteral diet on wound healing. Partial data concerning tissue, cell and collagen alterations in wound healing were previously reported and the present data comprises the mechanistic approach of these alterations 17. In this study, we show that malnutrition was accompanied by decreased wound mRNA levels of TGF‐β 1, which was minimised by post‐trauma short‐term feeding with standard or supplemented enteral diets in experimental acute open wound healing.
We choose to assess growth factor gene expression in wounds under malnutrition by using experimental cutaneous open wounds in isogenic rats because this model enables us to verify all the phases of healing (reepithelialisation, formation of granulation tissue and wound contraction) that can be modulated by growth factors and minimises possible genetic variations between the animals that could influence gene expression 20, 21. We performed four cutaneous wounds, but only analysed the proximal pairs. The distal pair wounds were evaluated for cell and tissue markers that can be better observed after longer periods than 14 days, and which data were previous reported 17. In addition, the experimental malnutrition model, with an average weight loss of 15%, causes enough protein depletion to prioritise the use of supplied nutrients to wound healing, while avoiding a severe malnutrition status (>20% weight loss), in which organic maintenance can take precedence 22.
Malnutrition was associated with decreased TGF‐β 1 mRNA expression in wounds on post‐trauma day 7. TGF‐β 1 expression during wound healing was scrutinised previously in several reports. It is known that skin injury promotes an imbalance of TGF‐β1, 2 and 3 expression 24 hours post‐trauma, and also on the fifth to seventh day after trauma 9. However, to the best of our knowledge, this issue was not assessed under malnutrition status 23.
Nutritional deficiencies impact wound healing by impeding fibroblast proliferation, collagen synthesis and epithelialisation 24, 25. Animals with acute protein‐calorie malnutrition had reduced sponge hydroxyproline contents, indicating diminished wound collagen accumulation and decreased wound gene expression of type III, but not type I, collagen 26. We previously reported decreased wound gene expression of type III but not type I collagen, and an inhibition of the increase in the fibroblast cell contingent between post‐trauma days 7 and 14 in experimental acute open wound healing 17.
TGF‐β 1 is involved in ECM formation and subcutaneous administration of this growth factor in rats promotes reepithelialisation and proliferation of fibroblasts and keratinocytes, and increases deposition of collagen 9, 27. Increased mRNAs for collagen types I and III were observed in microdissected airways 1 week after intratracheal instillation of TGF‐β 1 in BALB/C mice 28. In a full‐thickness skin model, Yavuz et al. 29 demonstrated that the intact skin of diabetic rats had sparsely distributed regular collagen fibres in the granulation zone, and loss of the regular collagen fibre pattern associated with weak TGF‐β 1 expression when compared with healthy controls. The restoration of growth factor TGF‐β 1 expression by treating these animals with aminoguanidine improved wound healing and preserved collagen ultrastructure 29. Therefore, the reduction of wound TGF‐β 1 gene expression in malnourished rats that we observed may contribute to a better understanding of the molecular mechanisms involved in decreased wound collagen production associated with malnutrition during healing 17, 26.
In addition, the lack of consistent TGF‐β 1 mRNA levels under malnutrition may also be associated with the prolonged inflammatory response and impaired neovascularisation in wounds of nutritionally depleted animals previously reported by us and others 17, 30. Active TGF‐β 1 elicits the rapid chemotaxis of monocytes to the wound site 31. Besides to decreases in collagens III and I deposition, the exogenous addition of neutralising TGF‐β 1 antibodies to cutaneous wounds reduced the monocyte and macrophage inflammatory profile, as well as neovascularisation and fibronectin 10. These findings could traduce a delay of monocyte and macrophage recruitment to wound in the presence of reduced levels of TGF‐β1. Interestingly, TGF‐β knockout animals develop a severe wasting syndrome with an intense and prolonged inflammatory response in the granulation tissue 32. In 10‐day‐old TGF‐β knockout mouse models, wounds have characteristic inflammation, along with a slight decrease in granulation tissue 33.
It is worth noting that, although the total intake offered for all the animals was homogeneously isocaloric and isonitrogenous, there were higher ingestion of calories and nitrogen in M(C) and E(A), respectively, in comparison to the other experimental groups. However, these differences might not be significant enough to benefit the expression of TGF‐β 1 mRNA because M(C) had decreased TGF‐β 1 mRNA in relation to those malnourished groups fed with enteral diets; and E(A) did not change TGF‐β 1 mRNA in comparison to the other eutrophic groups.
In this study, nutritional therapy with commercially available enteral diets prevented the TGF‐β 1 mRNA depletion found in malnutrition states. These results confirm the importance of early nutritional therapy in the malnutrition states that occur after surgery or trauma 6.
The main difference between the two enteral diets used in the present study was supplementation with arginine and micronutrients that are thought to assist with the healing process. However, the introduction of these elements in the enteral formula failed to increase growth factor expression; importantly, it decreased VEGF and KGF expression in the malnourished animals.
The decreased mRNA levels of VEGF and KGF in malnourished animals fed with specialised enteral diet did not occur in the corresponding malnourished animals in the normal diet control group. Although supplementation was associated with diminished levels of VEGF and KGF mRNA in malnourished animals, this did not appear to jeopardise wound healing, because the previously reported data showed that on post‐trauma day 14 wound closure was not impaired in malnourished animals fed with specialised enteral diets in relation to those fed with oral control and standard enteral diets 17.
The current results do not support the hypothesis that arginine may have beneficial effects on the healing process by affecting microvascular and perfusion changes 34. Although dietary arginine supplementation was associated with increased protein synthesis, cellular proliferation, and signalling via proline and polyamine synthesis in other reports, environmental and dietary variables were not controlled in these reports, as they were in this experimental study 35, 36. The pool concentration of arginine is important in wound healing, particularly because it is a precursor of nitric oxide (NO). However, arginine levels did not appear to be affected by supplementation or previous nutritional status 37, 38. One possible explanation is that healing becomes the highest priority in injured animals, and therefore metabolic pools of amino acids are utilised by this process over other functions such as control of the inflammatory process.
In clinical practice, the use of enteral supplements/formulas containing arginine with other pharmaconutrients (n‐3 fatty acids and RNA) is currently recommended to patients undergoing elective major surgery to decrease infections, hospital stay and also to promote wound healing potentially, with a significant reduction in suture dehiscence, but without overall effect on mortality compared with standard care 39, 40, 41. In addition, a recent meta‐analysis concluded that this arginine‐supplemented enteral diet for patients undergoing elective surgery for gastrointestinal cancer is an effective and cost‐saving intervention 42. A more detailed evaluation of arginine supplementation in wound healing would be of interest in subsequent studies.
Vitamins and minerals can potentially enhance wound healing. Vitamins A, C and E; zinc; and selenium may be associated with the prevention or attenuation of peroxidative damage 3. Vitamin and mineral deficiencies are known to impair the normal wound healing process, and these deficiencies can be corrected with supplementation 3. Thus, new specialised diets enriched with arginine and antioxidants (vitamin C, E, selenium and zinc) have a potential role in improving healing in chronic cutaneous wounds and have been associated previously with lower morbidity and shorter lengths of hospital stay 40, 43, 44, 45. Supplementation with micronutrient antioxidants is currently advocated in some surgical patients. For example, post‐bariatric surgery patients with micronutrient levels below 50% of the recommended daily allowances may benefit from vitamin and oligoelement supplementation prior to body contouring surgery 46.
We did not observe any differences among nutritional status or post‐trauma treatment with regard to PDGF expression. This result may be associated with specific characteristics of PDGF, such as site of production and peak levels of expression. PDGF is mainly found in the epidermis, is produced early after trauma (around post‐trauma day 3, during the inflammatory phase), and stimulates chemotactic agents for neutrophils and the proliferation of fibroblasts 47. Despite the early peak of PDGF expression, we studied this growth factor because malnutrition could alter its kinetics.
This study has limitations that deserve to be discussed. The skin repair process was evaluated in rodents and similar results may therefore not be achieved in humans. In addition, the two different enteral diets evaluated are available for human treatment and were not designed to attend the specific nutritional needs of rodents to improve wound healing. Our skin wound model was acute and excisional, and similar results may not occur in other chronic and/or incisional models that may involve different mechanisms of wound repair. Growth factors are continuously released during wound healing, but we only studied molecular alterations on post‐trauma day 7 because this study was designed to evaluate molecular mechanisms that could be enrolled in tissues, cells and protein changes previously observed after 14 days of wound healing 17.
Taken together, the data of this study suggest that wound healing impairment in malnourished animals may be due in part to a local decrease in TGB‐β 1 expression that can be restored by early enteral nutrition. However, we failed to find any potential benefits to a specialised enteral diet that was enriched with arginine and antioxidants to increase wound growth factor expression. Previous studies reported that supplemented nutritional support improves cutaneous open wound healing; however, in light of our results, this may have been due to metabolic pathways that do not depend on the relative concentrations of the growth factors that we investigated 48. The strict conditions under which supplementation is beneficial should be explored in future studies.
Acknowledgements
The authors thank Daniel Giannella Neto, MD, PhD, who facilitated the molecular biology analysis included in this study at the Human Nutrition and Metabolic Diseases Laboratory. This study was funded by a grant from the Fundação de Apoio a Pesquisa do Estado de São Paulo (Fapesp 05/54185‐5) and supported by Nutricia Support Produtos Nutricionais Ltda (which provided standard and specialised enteral diets) and Abbott Laboratories (which provided life care micropumps and burettes).
CCA participated in the study design and conception, carried out all experimental assays, interpreted data and wrote the manuscript. RST performed data analysis and interpretation, and wrote the manuscript. RG participated in the acquisition and analysis of data, revised the manuscript critically and gave final approval to the version to be published. MMB participated in the study design and conception, oriented the acquisition and analysis of data, revised the manuscript critically and gave final approval to the version to be published. AFL participated in the study design, revised the manuscript critically and gave final approval to the version to be published. DNL participated in the design and conception of the study, oriented the experimental assays regarding surgical procedures, performed analysis and interpretation of the data, and revised and approved the manuscript.
References
- 1. Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am 1997;77:509–28. [DOI] [PubMed] [Google Scholar]
- 2. Phillips SJ. Physiology of wound healing and surgical wound care. ASAIO J 2000;46:S2–5. [DOI] [PubMed] [Google Scholar]
- 3. Arnold M, Barbul A. Nutrition and wound healing. Plast Reconstr Surg 2006;117:42S–58S. [DOI] [PubMed] [Google Scholar]
- 4. Jewell L, Guerrero R, Quesada AR, Chan LS, Garner WL. Rate of healing in skin‐grafted burn wounds. Plast Reconstr Surg 2007;120: 451–6. [DOI] [PubMed] [Google Scholar]
- 5. Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease‐related malnutrition. Clin Nutr 2008;27:5–15. [DOI] [PubMed] [Google Scholar]
- 6. Campos AC, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care 2008;11: 281–8. [DOI] [PubMed] [Google Scholar]
- 7. Roberts AB. Transforming growth factor‐beta: activity and efficacy in animal models of wound healing. Wound Repair Regen 1995;3: 408–18. [DOI] [PubMed] [Google Scholar]
- 8. Frank S, Madlener M, Werner S. Transforming growth factors beta1, beta2, and beta3 and their receptors are differentially regulated during normal and impaired wound healing. J Biol Chem 1996;271: 10188–93. [DOI] [PubMed] [Google Scholar]
- 9. O’Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol 1997;29:63–78. [DOI] [PubMed] [Google Scholar]
- 10. Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF‐beta 1 and TGF‐beta 2 or exogenous addition of TGF‐beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995;108:985–1002. [DOI] [PubMed] [Google Scholar]
- 11. Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Gramates P, Deuel TF. Transforming growth factor beta reverses the glucocorticoid‐induced wound‐healing deficit in rats: possible regulation in macrophages by platelet‐derived growth factor. Proc Natl Acad Sci U S A 1989;86:2229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldman R. Growth factors and chronic wound healing: past, present, and future. Adv Skin Wound Care 2004;17:24–35. [DOI] [PubMed] [Google Scholar]
- 13. Barbul A, Fishel RS, Shimazu S, Wasserkrug HL, Yoshimura NN, Tao RC, Efron G. Intravenous hyperalimentation with high arginine levels improves wound healing and immune function. J Surg Res 1985;38:328–34. [DOI] [PubMed] [Google Scholar]
- 14. Shi HP, Efron DT, Most D, Tantry US, Barbul A. Supplemental dietary arginine enhances wound healing in normal but not inducible nitric oxide synthase knockout mice. Surgery 2000;128:374–8. [DOI] [PubMed] [Google Scholar]
- 15. Witte MB, Barbul A, Schick MA, Vogt N, Becker HD. Upregulation of arginase expression in wound‐derived fibroblasts. J Surg Res 2002;105:35–42. [DOI] [PubMed] [Google Scholar]
- 16. Shi HP, Most D, Efron DT, Witte MB, Barbul A. Supplemental L‐arginine enhances wound healing in diabetic rats. Wound Repair Regen 2003;11:198–203. [DOI] [PubMed] [Google Scholar]
- 17. Alves CC, Torrinhas RS, Giorgi R, Brentani MM, Logullo AF, Arias V, Mauad T, da Silva LF, Waitzberg DL. Short‐term specialized enteral diet fails to attenuate malnutrition impairment of experimental open wound acute healing. Nutrition 2010;26:873–9. [DOI] [PubMed] [Google Scholar]
- 18. Chomezynski P, Sacchi N. The single‐step method of RNA isolation by acid guanidinium thiocyanate‐phenol‐chloroform extraction: twenty‐something years on. Nat Protoc 2006;1:581–5. [DOI] [PubMed] [Google Scholar]
- 19. Pfaffl MW, Georgieva TM, Georgiev IP, Ontsouka E, Hageleit M, Blum JW. Real‐time RT‐PCR quantification of insulin‐like growth factor (IGF)‐1, IGF‐1 receptor, IGF‐2, IGF‐2 receptor, insulin receptor, growth hormone receptor, IGF‐binding proteins 1, 2 and 3 in the bovine species. Domest Anim Endocrinol 2002;22:91–102. [DOI] [PubMed] [Google Scholar]
- 20. Galiano RD, Michaels J, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 2004;12:485–92. [DOI] [PubMed] [Google Scholar]
- 21. Festing MF. Laboratory animal genetics and genetic quality control. In: Hau J, Van Hoosier GL Jr, editors. Handbook of laboratory and animal science. 2nd edn, Vol. 1. Life Science – CRS Press, 2003: 174–203. [Google Scholar]
- 22. Demling RH. Nutrition, anabolism, and the wound healing process: an overview. Eplasty 2009;9:e9. [PMC free article] [PubMed] [Google Scholar]
- 23. Klass BR, Grobbelaar AO, Rolfe KJ. Transforming growth factor beta1 signalling, wound healing and repair: a multifunctional cytokine with clinical implications for wound repair, a delicate balance. Postgrad Med J 2009;85:9–14. [DOI] [PubMed] [Google Scholar]
- 24. Kavalukas SL, Barbul A. Nutrition and wound healing: an update. Plast Reconstr Surg 2011;127:38S–43S. [DOI] [PubMed] [Google Scholar]
- 25. Tsuda K, Nakatani T, Sugama J, Okuwa M, Sanada H. Influence of the timing of switching a protein‐free to a protein‐containing diet on the wound healing process in a rat all‐layer skin defect. Int Wound J 2010;7:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schäffer MR, Tantry U, Ahrendt GM, Wasserkrug HL, Barbul A. Acute protein‐calorie malnutrition impairs wound healing: a possible role of decreased wound nitric oxide synthesis. J Am Coll Surg 1997;184:37–43. [PubMed] [Google Scholar]
- 27. Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor‐β1 induces α‐smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kenyon NJ, Ward RW, McGrew G, Last JA. TGF‐beta1 causes airway fibrosis and increased collagen I and III mRNA in mice. Thorax 2003;58:772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yavuz D, Tuğtepe H, Cetinel S, Uyar S, Kaya H, Haklar G, Civelek S, Deyneli O, San T, Burçak G, Akalin S. Collagen ultrastructure and TGF‐beta1 expression preserved with aminoguanidine during wound healing in diabetic rats. Endocr Res 2005;31:229–43. [DOI] [PubMed] [Google Scholar]
- 30. Otranto M, Souza‐Netto I, Aguila MB, Monte‐Alto‐Costa A. Male and female rats with severe protein restriction present delayed wound healing. Appl Physiol Nutr Metab 2009;34:1023–31. [DOI] [PubMed] [Google Scholar]
- 31. Wahl SM, Hunt DA, Wakefield LM, McCartney‐Francis N, Wahl LM, Roberts AB, Sporn MB. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A 1987;84:5788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kobayashi S, Yoshida K, Ward JM, Letterio JJ, Longenecker G, Yaswen L, Mittleman B, Mozes E, Roberts AB, Karlsson S, Kulkarni AB. Beta 2‐microglobulin‐deficient background ameliorates lethal phenotype of the TGF‐beta 1 null mouse. J Immunol 1999;163: 4013–9. [PubMed] [Google Scholar]
- 33. Brown RL, Ormsby I, Doetschman TC, Greenhalgh DG. Wound healing in the transforming growth factor‐beta‐deficient mouse. Wound Repair Regen 1995;3:25–36. [DOI] [PubMed] [Google Scholar]
- 34. Flynn NE, Meininger CJ, Haynes TE, Wu G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother 2002;56:427–38. [DOI] [PubMed] [Google Scholar]
- 35. Barbul A, Lazarou SA, Efron DT, Wasserkrug HL, Efron G. Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery 1990;108:331–6. [PubMed] [Google Scholar]
- 36. Kirk SJ, Hurson M, Regan MC, Holt DR, Wasserkrug HL, Barbul A. Arginine stimulates wound healing and immune function in elderly human beings. Surgery 1993;114:155–9. [PubMed] [Google Scholar]
- 37. Debats IB, Wolfs TG, Gotoh T, Cleutjens JP, Peutz‐Kootstra CJ, van der Hulst RR. Role of arginine in superficial wound healing in man. Nitric Oxide 2009;21:175–83. [DOI] [PubMed] [Google Scholar]
- 38. Brandt CT, Leite CR, Manhaes‐de‐Castro FM, Macedo EM, Silva RP, Castro CM. Nitric oxide monocyte production levels in patients with the hepatosplenic form of schistosomiasis mansoni who underwent splenectomy, ligature of the left gastric vein and auto implantation of spleen tissue in the major omentum. Acta Cir Bras 2006;21:285–90. [DOI] [PubMed] [Google Scholar]
- 39. Sánchez Álvarez C, Zabarte Martínez de Aguirre M, Bordejé Laguna L, Metabolism and Nutrition Working Group of the Spanish Society of Intensive Care Medicine and Coronary units. Guidelines for specialized nutritional and metabolic support in the critically‐ill patient: update. Consensus SEMICYUC‐SENPE: gastrointestinal surgery. Nutr Hosp 2011;26(2 Suppl):41–5. [DOI] [PubMed] [Google Scholar]
- 40. Waitzberg DL, Saito H, Plank LD, Jamieson GG, Jagannath P, Hwang TL, Mijares JM, Bihari D. Postsurgical infections are reduced with specialized nutrition support. World J Surg 2006;30:1592–604. [DOI] [PubMed] [Google Scholar]
- 41. Drover JW, Dhaliwal R, Weitzel L, Wischmeyer PE, Ochoa JB, Heyland DK. Perioperative use of arginine‐supplemented diets: a systematic review of the evidence. J Am Coll Surg 2011;212:385–99. [DOI] [PubMed] [Google Scholar]
- 42. Mauskopf JA, Candrilli SD, Chevrou‐Séverac H, Ochoa JB. Immunonutrition for patients undergoing elective surgery for gastrointestinal cancer: impact on hospital costs. World J Surg Oncol 2012;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heyland DK, Tranmer JE, Kingston General Hospital ICU Research Working Group. Measuring family satisfaction with care in the intensive care unit: the development of a questionnaire and preliminary results. J Crit Care 2001;16:142–9. [DOI] [PubMed] [Google Scholar]
- 44. Beale RJ, Bryg DJ, Bihari DJ. Immunonutrition in the critically ill: a systematic review of clinical outcome. Crit Care Med 1999;27:2799–805. [DOI] [PubMed] [Google Scholar]
- 45. Desneves KJ, Todorovic BE, Cassar A, Crowe TC. Treatment with supplementary arginine, vitamin C and zinc in patients with pressure ulcers: a randomized controlled trial. Clin Nutr 2005;24:979–87. [DOI] [PubMed] [Google Scholar]
- 46. Agha‐Mohammadi S, Hurwitz DJ. Nutritional deficiency of post‐bariatric surgery body‘contouring patients: what every plastic surgeon should know. Plast Reconstr Surg 2008;122:604–13. [DOI] [PubMed] [Google Scholar]
- 47. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–70. [DOI] [PubMed] [Google Scholar]
- 48. Naderpour M, Rad JS, Ayat E, Mesgari M, Farahani RM, Roshangar L, Tubbs RS, Shoja MM. Dietary L‐arginine and cutaneous wound healing. Ital J Anat Embryol 2008;113:135–42. [PubMed] [Google Scholar]


