Abstract
A meta‐analysis and systematic review assessing randomised controlled trials (RCTs) was sought to determine whether subcutaneous injection of insulin with hypertonic glucose promotes healing in postoperative incisions with aseptic fat liquefaction. We searched the Cochrane library, Pubmed, EMBASE, National Science Digital Library (NSDL) and China Biological Medicine Database (CBMdisc) for literature published from 1 January 1990 to 30 September 2011. RCTs that evaluated subcutaneous injection of insulin with hypertonic glucose as a treatment for postoperative wound with fat liquefaction were sought. Wound healing was the primary endpoint. Jadad score and Cochrane Collaboration's tool were used for assessing quality of studies and risk of bias. We abstracted data regarding time to wound healing, cost and adverse effects. The random‐effects inverse variance model was used for all analyses using weighted mean difference and 95% confidence interval. Eight trials (414 participants) were identified that met the inclusion criteria. Subcutaneous injection of insulin with hypertonic glucose significantly reduces time to healing by 6·33 days compared with conventional drainage, with less cost. There was no report concerning adverse effects. Subcutaneous injection of insulin with hypertonic glucose may improve the healing process in postoperative wounds with aseptic fat liquefaction.
Keywords: Fat liquefaction, Hypertonic glucose, Insulin, Postoperative, Wound
INTRODUCTION
Aseptic fat liquefaction, as a main cause of prolonged healing of aseptic post‐surgical incision, is the necrosis of adipose tissue without infection and exhibits an incidence of 0·52–1·11% in all postoperative wounds 1, 2, 3. It is more common in older or overweight patients complicated with diabetes or malnutrition 4, 5, 6, which, besides the disease and operation, increases stress and economic burdens on patients and their families. Many preclinical and clinical interventions have shown their efficacy in promoting wound healing, including topical application of honey, hormones, insulin‐zinc, negative pressure therapy, low‐level laser energy, antibiofilm, microRNA and statins 7, 8, 9, 10, 11, 12, 13, 14. There has been anecdotal and sporadic evidence of insulin in promoting problematic wound healing over decades (15). However, data from clinical studies in English literature are still limited.
In this meta‐analysis, we investigated into the therapeutic efficacy of the subcutaneous application of insulin with hypertonic glucose in post‐surgical wound healing with aseptic fat liquefaction, and reviewed related literature.
METHODS
Sources
We searched Medline, EMBASE, Cochrane Library, National Science Digital Library (NSDL), and China Biological Medicine Database (CBMdisc) for relevant randomised controlled trials (RCTs) aiming to promote the healing of postoperative wounds with fat liquefaction by subcutaneous application of insulin. Queries included articles published from 1 January 1990 to 30 September 2011 in English and Chinese peer‐reviewed publications (including abstracts). Keywords used were ‘subcutaneous’ (or ‘regional’), ‘insulin’, ‘glucose’, ‘operation’ (or ‘surgery’ or ‘surgical’ or ‘postoperative’ or ‘post‐surgical’) and ‘wound’ (or ‘incision’) and ‘fat liquefaction’ (or ‘necrosis’). We also hand‐searched bibliographies of original studies, reviews (including meta‐analyses) and relevant conference abstracts, and contacted some investigators directly. The date last searched was 15 October 2011.
Study selection
Two authors independently selected relevant studies, extracted data and assessed trial quality by means of modified Jadad score (Table 1). The modified Jadad used is an 8‐item scale designed to assess randomisation, blinding, withdrawals/dropouts, inclusion/exclusion criteria, adverse effects and statistical analysis. The score for each article can range from 0 (lowest quality) to 8 (highest quality). Scores of 4 to 8 represent high quality 16, 17. The Cochrane Collaboration's tool for assessing risk of bias (18) was also referred to address potential bias (Table 2). When necessary, supplementary study information was obtained by contacting the RCT authors. Questionable RCTs were confirmed by discussion with a third author. Inclusion criteria: the diagnosis of fat liquefaction of post‐surgical wounds was clear; patients were without wound infection, fever or inflammation (indicated by blood leukocyte and postoperative morbidity; also refer to Figure 1). We abstracted data about study design and methods, inclusion and exclusion criteria, patient characteristics, treatment methods and comparative dosage regimens, patient outcomes, cost and adverse events.
Table 1.
Clinical comparison of patients in insulin + hypertonic glucose and control groups
| RCT | Treatment | Operation performed | IG † (n) | C ‡ (n) | Time/cost to healing | Clinical indices | Jadad Score § | |||
|---|---|---|---|---|---|---|---|---|---|---|
| IG † | C ‡ | IG (d) | C (d) | IG | C | |||||
| Liu 2001 (22) | 50% glucose 20 ml + 8 IU insulin | Saline | Obstetrical and gynecological | 13 | 12 | 9·46 ± 4·96 | 17·50 ± 7·51 | − | − | 3/6 |
| Ding 2006 (23) | 50% glucose 20 ml + 3 IU insulin infrared physiotherapy | Saline + infrared physiotherapy | C‐section | 25 | 20 | 3·51 ± 0·08 | 10·33 ± 2·42 | − | − | 3/6 |
| Butterfly‐shaped adhesive | Butterfly‐shaped adhesive | |||||||||
| Hu 2008 (24) | 50% glucose 20 ml + 4 IU insulin | Saline + secondary suture | General surgery | 20 | 18 | 13·30 ± 2·80 | 18·40 ± 8·00 | − | − | 3/6 |
| Butterfly‐shaped adhesive | Butterfly‐shaped adhesive | |||||||||
| Yang 2008 (25) | 50% glucose 20 ml + 8 IU insulin | Saline + secondary suture | Gynecological surgery | 37 | 23 | 10·40 ± 1·20 | 17·40 ± 2·20 | 4·1 ± 2·0 ‖; | 3·8 ± 2·2 ‖; | 3/6 |
| Butterfly‐shaped adhesive | ||||||||||
| 108·3 ± 27·1 ¶ | 607 ± 57·4 ¶ | 37·8 ± 3·4 †† | 38·2 ± 3·0 †† | |||||||
| Chen 2008 (26) | 50% glucose 20 ml + 5 IU insulin | Saline | C‐section | 33 | 20 | 3·36 ± 0·13 | 9·58 ± 1·42 | − | − | 3/6 |
| Butterfly‐shaped adhesive | Butterfly‐shaped adhesive | |||||||||
| Zh 2009 (27) | 50% glucose 20 ml + 2 IU insulin | Saline | C‐section | 28 | 20 | 9·80 ± 2·30 | 13·90 ± 5·80 | − | − | 2/5 |
| Butterfly‐shaped adhesive | Butterfly‐shaped adhesive | |||||||||
| Wu 2010 (28) | 50% glucose 20 ml + 20 U insulin infrared physiotherapy | Saline + infrared physiotherapy | Obstetrical and gynecological | 20 | 20 | 9·2 ± 1·7 | 15·3 ± 3·9 | − | − | 3/6 |
| Butterfly‐shaped adhesive | Butterfly‐shaped adhesive | |||||||||
| Lei 2011 (29) | 50% glucose 20 ml + 5 U insulin | Saline | Obstetrical and gynecological | 76 | 29 | 3·4 ± 0·1 | 9·6 ± 1·4 | − | − | 2/5 |
| Butterfly‐shaped adhesive | Butterfly‐shaped adhesive | |||||||||
There were no differences in incidence of diabetes, severe anaemia and cough after operation between the insulin + hypertonic glucose group and the control group of enrolled studies.
†Insulin + hypertonic glucose.
‡Control.
§Jadad Score (3 items/6 items).
¶Cost, unit in RMB, shown by mean ± SD.
‖;Thickness of abdominal fat (cm).
††Age.
* P < 0·05 for comparisons of ¶, P > 0·05 for comparison of ‖; and ††.
Table 2.
General information of enrolled RCTs (according to the Cochrane Collaboration's tool for assessing risk of bias)
| Sequence generation | Allocation concealment | Blinding of participants, personnel and assessors | Incomplete outcome data | Selective outcome reporting | Other sources of bias | |
|---|---|---|---|---|---|---|
| Liu 2001 (22) | Random table | Unclear | Measurement not influenced by lack of blinding | No missing outcome data | All pre‐specified outcomes reported | No |
| Ding 2006 (23) | Random table | Unclear | Measurement not influenced by lack of blinding | No missing outcome data | All pre‐specified outcomes reported | No |
| Hu 2008 (24) | Computer random number generator | Unclear | Measurement not influenced by lack of blinding | No missing outcome data | All pre‐specified outcomes reported | No |
| Yang 2008 (25) | Coin tossing | Unclear | Measurement not influenced by lack of blinding | No missing outcome data | All pre‐specified outcomes reported | No |
| Chen 2008 (26) | Computer random number generator | Unclear | Measurement not influenced by lack of blinding | No missing outcome data | All pre‐specified outcomes reported | No |
| Zhou 2009 (27) | Unclear † | Unclear | Measurement not influenced by lack of blinding | No missing outcome data | All pre‐specified outcomes reported | No |
| Wu 2010 (28) | Computer random number generator | Unclear | Measurement not influenced by lack of blinding | No missing outcome data | All pre‐specified outcomes reported | No |
| Lei 2011 (29) | Unclear † | Unclear | Measurement not influenced by lack of blinding | No missing outcome data | All pre‐specified outcomes reported | No |
†High risk.
Figure 1.
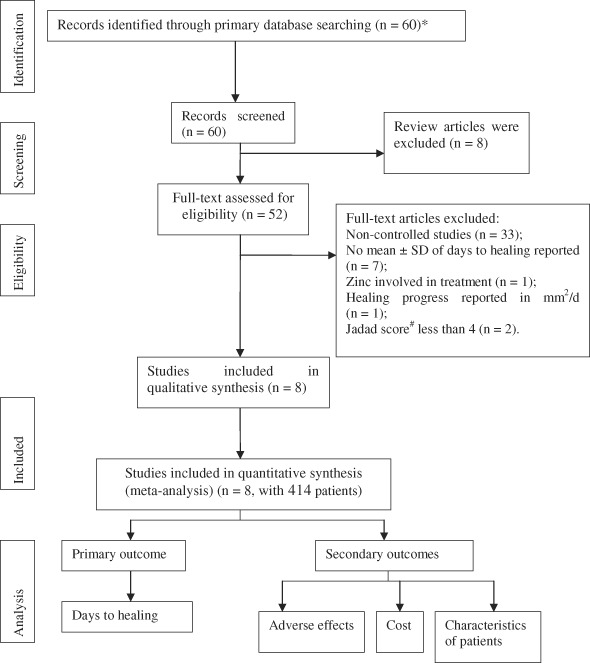
Flow chart of study recruitment and data selection. *Only studies from peer‐reviewed journals or conferences with a modified Jadad score of above 4 (6‐item scale) were included; studies that did not meet the criteria set in the search section were excluded; studies without enough information about patients' clinical conditions were excluded. Authors were further contacted if their names appeared more than once in the included studies to rule out duplicate data. #6‐item Jadad score.
For primary outcome, we estimated the time from initial treatment to wound healing. Secondary outcomes included thickness of abdominal fat, cost of treatment, patient characteristics and adverse effects. In the included RCTs, the diagnosis of fat liquefaction of a postoperative wound should meet all of the following four criteria (19):
-
1
Developed 3–7 days after operation. No subjective symptoms except yellow exudate;
-
2
Open or pseudo closure of surgical incision, with fat droplets in exudate;
-
3
No redness or tenderness presented, and no signs of necrosis along the incision and subcutaneous tissue;
-
4
Large amount of fat droplets from exudates observed under microscope, and no growth of bacteria after three consecutive bacterial cultures.
Statistical analyses were conducted using Review Manager version 5·0 software (Cochrane Collaboration). Pooled weighted mean difference (WMD) and 95% confidence intervals (CIs) were determined by choice between the fixed‐effects or random‐effects model of inverse variance method, whichever was most conservative (20) (showing less efficacy, with a higher P value, and the random‐effects model qualified for this purpose) and shown in forest plot. The latter is a graphical display designed to illustrate the relative strength of treatment effects in multiple quantitative scientific studies addressing the same question (21). Statistical between‐study heterogeneity was assessed by I 2 test (22) and χ 2 test. Publication bias was assessed by funnel plot 23, 24. An asymmetric funnel indicates a relationship between treatment effect and study size, suggesting the possibility of either publication bias or a systematic difference between smaller and larger studies (‘small study effects'), or the use of an inappropriate effect measure. For all the tests performed, statistical significance was achieved if the P value was < 0·05 (for overall effect of intervention) or < 0·10 (for heterogeneity test, due to the small number of RCTs) (20).
RESULTS
Eight RCTs were included and analysed quantitatively 25, 26, 27, 28, 29, 30, 31, 32 (characteristics in Table 2, selection process in Figure 1), including 414 cases. All wounds were closed by the time of healing. According to diagnosis criteria, efficacy of insulin with hypertonic glucose was evaluated according to days to healing for comparison purpose. Debridement and wash with disinfectant were performed before intervention. Solution of insulin and hypertonic glucose mixture was given by subcutaneous injection along both sides of incision. Solution in control groups was given by flushing. All studies determined wound healing by visualisation of skin regeneration and closure of the incision. Funnel plot indicating selection bias was shown in figure of the corresponding comparison.
Primary outcomes
According to healing criterion, the efficacy of subcutaneous injection of insulin and hypertonic glucose indicated by WMD [95% CI] was −6·33 [−6·66, −5·99] (P < 0·01 for overall effect), with no heterogeneity (P = 0·48, I 2 = 0%, Figure 2).
Figure 2.
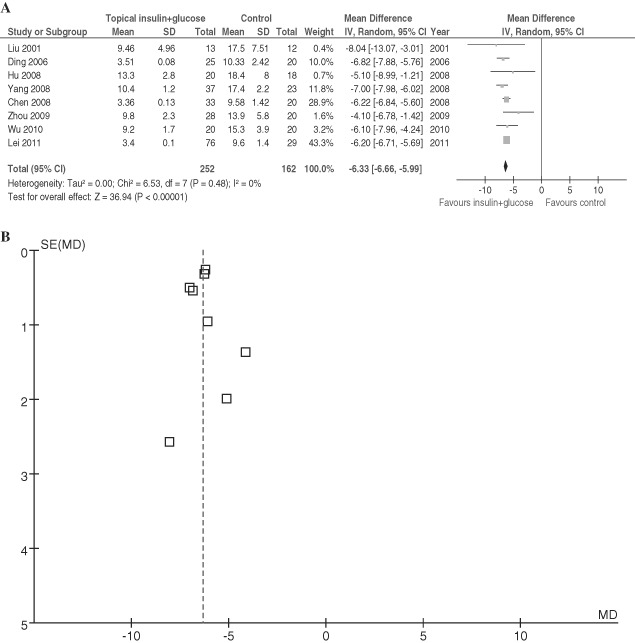
Comparison of subcutaneous injection of insulin with hypertonic glucose and conventional drainage in postoperative patients with incision fat liquefaction. (A) Forest plot. (B) Funnel plot of enrolled studies for A. Vertical line indicates no difference between compared treatments. Horizontal lines show 95% CIs. Squares indicate point estimates, and the size of the squares indicates the weight of each study in the meta‐analysis. IV Random, random‐effects inverse variance model; CI, confidence interval.
Secondary outcomes
Although in individual studies it was mentioned that there was no significant difference between the two groups in clinical characteristics of patients, such as age and degree of obesity, only one study (28) reported the above items quantitatively (Table 1). There was no report of adverse effects in any of the included studies.
DISCUSSION
Factors contributing to fat liquefaction in postoperative wounds include:
-
1
Obesity, with thick abdominal subcutaneous fat (33). 43–75% of wound fat liquefaction occurs in overweight patients (34).
-
2
Unnecessary and overuse of electrotome in surgery (35). Heat generated by the electrotome may cause thermal injury in superficial subcutaneous adipose tissue, leading to its partial degeneration. Meanwhile, because of thermal coagulation and thrombosis in adipose tissue capillaries, blood supply diminishes, causing aseptic necrosis of fat tissue and more exudation. Therefore, for general surgeries, its application should be as gentle and quick as possible.
-
3
Prolonged exposure of the incision, along with mechanical irritation, such as compression and forceps clipping, is prone to cause more oxidation, decomposition and aseptic inflammation of fatty tissue, and finally fat liquefaction (36).
-
4
Old age and chronic diseases, such as anaemia, hypoproteinaemia and diabetes.
Role of insulin and hypertonic glucose in wound healing
Wound healing is a complicated biological process involving chemotaxis, angiogenesis and neovascularisation that comprise synthesis of extracellular matrix proteins and remodelling of tissues. The physiological properties of insulin suggest its potential favourable role in wound healing because of its stimulation of individual cell growth as well as anabolism of the organism as a whole 37, 38, 39. Glucose combined with insulin may also promote protein synthesis, inhibit protein degradation, increase anti‐inflammation capacity of local tissue, promote wound healing and reduce skin scarring. As much as 50% glucose is hypertonic, which may inhibit bacterial growth, prevent oedema of granulation tissue and stimulate its growth. Meanwhile, a butterfly‐shaped adhesive may eliminate dead space by avoiding damage to the problematic wound caused by conventional suture stitches, and thus speed healing (28).
It has been shown pre‐clinically that subcutaneous injection of insulin in mice led to regional longer vessels with more branches, along with increased numbers of associated alpha‐smooth muscle actin‐expressing cells, suggesting the appropriate differentiation and maturation of the new vessels. Also found was that insulin stimulates human microvascular endothelial cell migration and tube formation (38). Insulin has also been reported to have beneficial effects on cell proliferation and protein metabolism in skin donor site wound (40). When topically applied to incision wounds, insulin accelerates re‐epithelialisation and stimulates ‘maturation’ of the healing tissue (41). One clinical study has shown the efficacy of topical application of insulin (indicated by healing rate, mm2/day) in the treatment of non infected acute and chronic extremity wounds regardless of baseline wound size (42). In another study, wounds treated with topical insulin (without hypertonic glucose) healed 2·4 ± 0·8 days faster than the wounds treated with saline (P < 0·001) (43).
Safety of subcutaneous application of insulin combined with hypertonic glucose
Although none of the included studies reported hypoglycaemia, insulin should be used with caution in patients with hypoglycaemia, acute hepatitis, liver cirrhosis, haemolytic jaundice, pancreatitis and nephritis. In our analysis, no secondary wound infection was reported in the insulin–glucose group, showing the antiinfectious efficacy of hypertonic glucose.
Influencing factors
In our analysis, the dose of insulin ranged from 2 to 20 IU. Ideally, we may analyse the efficacy of insulin by dosage so that the optimal dose of insulin can be determined. However, because of the limited number of studies and patients in each study, a stratified analysis would not be much more convincing. According to our data, there does not appear to be a clear dose‐dependant improvement of healing within this range. It has been reported in each enrolled study that there were no differences in the rate of diabetes, severe anaemia, and cough after operation between the insulin–glucose group and control group, which excluded their influence on the result of our analysis. All enrolled studies were carried out in China because there were no qualified studies available elsewhere. The result of incision healing was seldom influenced by patients' in‐hospital activity, and the treatment could not be blinded because of daily wound care by doctors and nurses; therefore blinding was not considered as a bias‐raising factor in our analysis. The concealment of allocation was unclear in all studies, because not much attention has been paid to the notion of allocation concealment in China, and even if the authors carried out the allocation concealment, they did not report. As an effective method to eliminate bias in randomisation process, the notion of concealment should be reinforced among clinical researchers. There might also be possible bias based on individual physician skills and hospital conditions. As were shown by funnel plots, the estimated WMD is likely biased in favour of the insulin and hypertonic glucose treatment due to publication bias. More RCTs of higher quality and larger size are needed for further investigations and more convincing results.
In conclusion, our meta‐analysis suggests that subcutaneous injection of insulin along with hypertonic glucose is effective in the management of aseptic postoperative wounds with fat liquefaction without significant adverse effects or complications, and therefore might be recommended, especially to obese and old patients undergoing operations. Prophylactic application of insulin and hypertonic glucose might be recommended to patients with risk factors contributing to fat liquefaction in postoperative wounds.
ACKNOWLEDGEMENTS
This systemic review was carried out using the recommendations and protocols of the Cochrane Collaboration. The authors would like to thank Dr. Allen Nicholson, Dr. James Bloxton (both from Temple University), Dr. Patricia Dillon (LaSalle University) and Dr. Joshua Breslau (Princeton University) for their critical review and generous support. No funding source was involved. The authors have neither conflict of interest nor competing interest concerning this paper.
ZS, LM and HW contributed equally to this paper.
REFERENCES
- 1. Sun Z, Sun Y, Cao J, Wang L, Tian M, Zhang Y, Liu J, Wang P. Cause analysis and clinical management of postoperative wound complications after total knee arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2009;23:644–7. [PubMed] [Google Scholar]
- 2. Liang J, Wei X. Investigation of related factors in 60 cases of obstetrical and gynecological abdominal incision with fat liquefaction. Henan J Surg 2007;13:37–8. [Google Scholar]
- 3. Chen H. Analysis of 28 cases of obstetrical and gynecological abdominal incision with fat liquefaction. J Clin Exp Med 2006;5:1413. [Google Scholar]
- 4. Sarsam SE, Elliott JP, Lam GK. Management of wound complications from cesarean delivery. Obstet Gynecol Surv 2005;60:462–73. [DOI] [PubMed] [Google Scholar]
- 5. Mangrulkar S, Khair PS. Comparison of healing of surgical wounds between diabetics and non‐diabetics. J Indian Med Assoc 2009;107:765–70. [PubMed] [Google Scholar]
- 6. Wissing U, Unosson M, Lennernäs MA, Ek AC. Nutritional intake and physical activity in leg ulcer patients. J Adv Nurs 1997;25:571–8. [DOI] [PubMed] [Google Scholar]
- 7. Jull AB, Rodgers A, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2008;CD005083. [DOI] [PubMed] [Google Scholar]
- 8. Ashcroft GS, Greenwell‐Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol 1999;155:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang XJ, Wu X, Wolf SE, Hawkins HK, Chinkes DL, Wolfe RR. Local insulin‐zinc injection accelerates skin donor site wound healing. J Surg Res 2007;142:90–6. [DOI] [PubMed] [Google Scholar]
- 10. Egemen O, Ozkaya O, Ozturk MB, Aksan T, Orman C, Akan M. Effective use of negative pressure wound therapy provides quick wound‐bed preparation and complete graft take in the management of chronic venous ulcers. Int Wound J 2011. DOI: 10.1111/j.1742-481X.2011.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neira R, Arroyave J, Ramirez H, Ortiz CL, Solarte E, Sequeda F, Gutierrez MI. Fat liquefaction: effect of low‐level laser energy on adipose tissue. Plast Reconstr Surg 2002;110:912–22. [DOI] [PubMed] [Google Scholar]
- 12. Baffoni M, Bessa LJ, Grande R, Di Giulio M, Mongelli M, Ciarelli A, Cellini L. Laser irradiation effect on Staphylococcus aureus and Pseudomonas aeruginosa biofilms isolated from venous leg ulcer. Int Wound J 2011. DOI: 10.1111/j.1742-481X.2011.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing: promotive role of miR‐21 in fibroblast migration. Int Wound J 2011. DOI: 10.1111/j.1742-481X.2011.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farsaei S, Khalili H, Farboud ES. Potential role of statins on wound healing: review of the literature. Int Wound J 2011. DOI: 10.1111/j.1742-481X.2011.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson JM, Baines R, Babu ED, Kelley CJ. A role for topical insulin in the management problematic surgical wounds. Ann R Coll Surg Engl 2008;90:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 17. Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cogn Disord 2001;12:232–6. [DOI] [PubMed] [Google Scholar]
- 18. The Cochrane Collaboration's tool for assessing risk of bias. http://www.ohg.cochrane.org/forms/Risk%20of%20bias%20assessment%20tool.pdf [Accessed 8 June 2011]. [DOI] [PMC free article] [PubMed]
- 19. Su Y, Liu X. Obstetric & gynecologic surgery. Beijing: People's Medical Publishing House, 2001. [Google Scholar]
- 20. The Cochrane Collaboration open learning material. http://www.cochrane‐net.org/openlearning/HTML/mod12‐3.htm [Accessed 20 June 2011].
- 21. Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ 2001;322:1479–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huedo‐Medina TB, Saínchez‐Meca J, Marín‐Martínez F, Botella J. Assessing heterogeneity in meta‐analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206. [DOI] [PubMed] [Google Scholar]
- 23. Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta‐analysis. Eval Health Prof 2001;24:126–51. [DOI] [PubMed] [Google Scholar]
- 24. Sterne JA, Egger M. Funnel plots for detecting bias in metaanalysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–55. [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Chu M. Treatment of abdominal incision by hypertonic glucose energy mixture. Mod Med Health 2001;17:201. [Google Scholar]
- 26. Ding L. Hypertonic glucose combined with insulin treatment of abdominal incision fat liquefaction. Mod Pract Med 2006;18:573. [Google Scholar]
- 27. Hu M, Cao H, Li J. Experience of hypertonic glucose‐insulin treatment of abdominal incision with fat liquefaction. Shaanxi Medical J 2008;37:1393. [Google Scholar]
- 28. Yang Y, Shen H, Weng T, Li X, Tang H. The efficacy of butterfly‐shaped adhesive combined with insulin and hyperosmotic glucose solution for treating in patients with abdominal incision undesirable healing. China Practical Medicine 2008;3:13–14. [Google Scholar]
- 29. Chen Y. Treatment of abdominal incision with fat liquefaction after cesarean section. Chin J Rural Med Pharm 2008;15:31–2. [Google Scholar]
- 30. Zhou W, Yang B, Peng X, Su Y. Squeeze liquid discharge with hypertonic glucose plus insulin in the treatment of liquefaction of abdominal wound. Guide China Medicine 2009;7:90–1. [Google Scholar]
- 31. Wu Y, Yu F, Liang D, Han Y. Prevention and treatment of abdominal incision by hypertonic glucose and insulin. MedInformation 2010;5:1048–9. [Google Scholar]
- 32. Lei D. Efficacy of hypertonic glucose with insulin in the treatment of fat liquefaction of abdominal incision after gynecologic surgery. Chin Community Doctors 2011;13:62–3. [Google Scholar]
- 33. Avram AS, Avram MM, James WD. Subcutaneous fat in normal and diseased states: 2. Anatomy and physiology of white and brown adipose tissue. J Am Acad Dermatol 2005;53:671–83. [DOI] [PubMed] [Google Scholar]
- 34. He Y. Analysis of 26 cases of obstetrical and gynecological abdominal incision with fat liquefaction. Mod Med Health 2007;23:2612. [Google Scholar]
- 35. Ji GW, Wu YZ, Wang X, Pan HX, Li P, Du WY, Qi Z, Huang A, Zhang LW, Zhang L, Chen W, Liu GH, Xu H, Li Q, Yuan AH, He XP, Mei GH. Experimental and clinical study of influence of high‐frequency electric surgical knives on healing of abdominal incision. World J Gastroenterol 2006;12:4082–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang H. Analysis of 53 cases of obstetrical and gynecological abdominal incision with fat liquefaction. J Med Theor Pract 2009;22:1344–5. [Google Scholar]
- 37. Apikoglu‐Rabus S, Izzettin FV, Turan P, Ercan F. Effect of topical insulin on cutaneous wound healing in rats with or without acute diabetes. Clin Exp Dermatol 2010;35:180–5. [DOI] [PubMed] [Google Scholar]
- 38. Liu Y, Petreaca M, Martins‐Green M. Cell and molecular mechanisms of insulin‐induced angiogenesis. J Cell Mol Med 2009;13:449–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tuvdendorj D, Zhang XJ, Chinkes DL, Aarsland A, Kulp GA, Jeschke MG, Herndon DN. Intensive insulin treatment increases donor site wound protein synthesis in burn patients. Surgery 2011;149:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang XJ, Meng C, Chinkes DL, Herndon DN. Beneficial effects of insulin on cell proliferation and protein metabolism in skin donor site wound. J Surg Res 2009;168:e155–61. [DOI] [PubMed] [Google Scholar]
- 41. Liu Y, Petreaca M, Yao M, Martins‐Green M. Cell and molecular mechanisms of keratinocyte function stimulated by insulin during wound healing. BMC Cell Biol 2009;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rezvani O, Shabbak E, Aslani A, Bidar R, Jafari M, Safarnezhad S. A randomized, double‐blind, placebo‐controlled trial to determine the effects of topical insulin on wound healing. Ostomy Wound Manage 2009;55:22–8. [PubMed] [Google Scholar]
- 43. Greenway SE, Filler LE, Greenway FL. Topical insulin in wound healing: a randomised, double‐blind, placebo‐controlled trial. J Wound Care 1999;8:526–8. [DOI] [PubMed] [Google Scholar]


