Abstract
Many complementary and alternative products are used to treat wounds. The essential oil of Melaleuca alternifolia, tea tree oil, has proven antimicrobial and anti‐inflammatory properties, may be useful in methicillin‐resistant Staphylococcus aureus (MRSA) decolonisation regimens and is reputed to have ‘wound‐healing’ properties, but more data are required to support these indications. The primary aim of this uncontrolled case series was to assess whether a tea tree oil solution used in a wound cleansing procedure could decolonise MRSA from acute and chronic wounds of mixed aetiology. The secondary aim was to determine if the tea tree oil solution influenced wound healing outcomes. Nineteen participants with wounds suspected of being colonised with MRSA were enrolled in a pilot study. Seven were subsequently shown not to have MRSA and were withdrawn from the study. As many as 11 of the remaining 12 participants were treated with a water‐miscible tea tree oil (3·3%) solution applied as part of the wound cleansing regimen at each dressing change. Dressing changes were three times per week or daily as deemed necessary by the study nurse following assessment. One participant withdrew from the study before treatment. No participants were MRSA negative after treatment. After treatment had been implemented, 8 of the 11 treated wounds had begun to heal and reduced in size as measured by computer planimetry. Although this formulation and mode of delivery did not achieve the primary aim of the study, tea tree oil did not appear to inhibit healing and the majority of wounds reduced in size after treatment.
Keywords: Acute wound, Chronic wound, Melaleuca alternifolia, MRSA decolonisation, Tea tree oil, Wound healing
INTRODUCTION
Many complementary and alternative medicine products are sold over‐the‐counter and used in an ad hoc fashion on chronic and acute wounds. One such product is tea tree oil, the essential oil of the Australian native plant Melaleuca alternifolia. Tea tree oil enjoys remarkable popularity as a topical antimicrobial agent and, although it is marketed mainly for its well‐documented antibacterial, antifungal and antiviral properties, the oil also has anti‐inflammatory, analgesic, insecticidal and antipruritic properties (1). Tea tree oil has antibacterial activity against a wide range of bacteria including Staphylococcus aureus (including methicillin‐resistant S. aureus [MRSA]) (2), streptococci (3), coagulase negative staphylococci and coliforms (4). This spectrum of activity may explain in part anecdotal evidence supporting the application of products containing tea tree oil to colonised and infected wounds. The in vitro activity of tea tree oil against MRSA has been shown many times with minimum inhibitory concentrations ranging from 0·25% to 2% (2, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14). Despite this, there are no published data on the use of tea tree oil or its products to decolonise MRSA‐positive wounds other than those reported in a study by Dryden et al. (15) in which the decolonisation of wounds was a coincident finding. Similarly, while tea tree oil is often regarded and promoted as having wound healing properties, there are no published data on specific wound healing parameters, such as reductions in wound size or improved time to re‐epithelialisation.
The primary aim of this pilot study is to assess whether or not a tea tree oil solution used in a wound cleansing procedure could decolonise acute and chronic wounds of mixed aetiology containing MRSA. The secondary aim is to determine if the tea tree oil solution influenced wound healing.
METHODS
Study design and sample size
As there are no data on the decolonisation of MRSA‐containing wounds after tea tree oil treatment or on the rate of spontaneous decolonisation of wounds, an arbitrary level of decolonisation activity and a tolerable limit of error were set at 20% and 5%, respectively. The study was designed to choose between two hypotheses regarding MRSA decolonisation; that a tea tree oil solution is unlikely to be effective in 20% of participants or more or that a tea tree oil solution could be effective in 20% of participants or more. Depending on the number of treatment successes in a series of cases, the arbitrary level of activity would be supported or refuted (16). Participants were invited to participate and entered into the study and the number of consecutive treatment failures, that is failure to decolonise, was used to estimate whether the tea tree oil solution treatment fell above or below this threshold. The sample size that allowed acceptance of one of these hypotheses was 14. There was a <5% chance that treatment failure would happen 14 times in a row if the tea tree oil solution was truly effective 20% of the time. Conversely, there was a >95% chance that one or more treatment successes (MRSA elimination) would have occurred in 14 consecutive participants if the tea tree oil solution was effective at least 20% of the time. However, if there were 14 consecutive failures, the first hypothesis would be accepted. The chance that the tea tree oil solution may be rejected when it does have at least 20% effectiveness was <5%.
Recruitment
Participants were recruited from subjects receiving care from a metropolitan domiciliary nursing service for all acute or chronic wound types. Inclusion criteria for the participants were 18 years or older, able to give informed consent, receiving care for any wound type, previously known or suspected of having a wound colonised with MRSA (with no topical or systemic signs of infection) that was confirmed upon enrolment and that they would be available to participate in the study for the 12 weeks following enrolment. Exclusion criteria were a known allergy to tea tree oil, wound infection as evident by topical or systemic signs, pregnancy, lactation or non use of effective contraception in women of child bearing age, the presence of fistulas or cavities in the wound, current use of antibiotics or topical antimicrobial pharmaceutical or wound care products or prescribed use of occlusive dressings. Wound infection was an exclusion criterion as antibiotic therapy would follow. Before and after enrolment, wound infection was determined on the basis of clinical signs and symptoms. Post‐enrolment, wound swab results were also available to assist in the diagnosis of wound infection. The signs and symptoms of wound infection included pain, erythema, oedema, heat, purulence, friable granulation tissue, wound breakdown and malodour. Participants whose wounds became infected during the course of the study were withdrawn. Clients willing to participate in the study gave their informed, signed consent as required by the institutional review boards of the Silver Chain Nursing Association and The University of Western Australia.
Enrolment and initial assessment
If prospective participants met the inclusion and did not meet the exclusion criteria they were enrolled in the study. After enrolment, they had their wound swabbed to confirm MRSA colonisation and treatment with the tea tree oil solution began. If participants had multiple wounds in the same anatomical location, one wound was nominated as the primary wound for inclusion in the study, but all wounds were washed with the treatment product. Wound swabs were taken by removing the dressing and irrigating with sterile water to remove debris and exudates. Swabs were taken by passing the swab over the wound in a zig‐zag pattern while gently rotating the tip for at least one full rotation. Where necessary the swab tip was pre‐moistened with sterile water prior to swabbing. If available, granulation tissue was swabbed, or if not, the wound bed or freshly expressed pus. Necrotic tissue, slough and the wound perimeters were avoided.
Wound swabs were forwarded to the Division of Microbiology and Infectious Diseases at PathWest Laboratory Medicine WA, Nedlands for microscopy, culture and susceptibility testing. Wound swabs were plated onto the following non selective and selective media: blood agar, colistin–nalidixic acid agar, McConkey agar and laked blood agar supplemented with gentamicin (LBAG). Plates were incubated aerobically at 37°C for 24–48 h except the LBAG plates that were incubated anaerobically, also at 37°C for 24–48 h. Swabs were also cultured on oxacillin‐resistant screening agar base plates and incubated at 37°C for 48 h. Blue colonies were presumptively identified as S. aureus, sub‐cultured onto blood agar and the identity confirmed by a positive coagulase test. Susceptibility testing by disc diffusion methods according to Clinical Laboratory Standards Institute guidelines using a 30 µg cefoxitin disc was used to identify MRSA isolates (17, 18). S. aureus with zones of inhibition to cefoxitin ≥22 mm were reported as oxacillin/flucloxacillin susceptible and therefore methicillin‐susceptible S. aureus (MSSA). S. aureus with zones of inhibition to cefoxitin ≤21 mm were reported as oxacillin/flucloxacillin resistant and therefore MRSA. The growth or presence on microscopy of other major wound pathogens was also noted. Microorganisms other than MRSA detected by culture or viewed on microscopy were reported at species, genus or other level.
After enrolment, a digital photograph was taken and assessed by computer planimetry using the Advanced Medical Wound Imaging System (AMWIS), a validated digital planimetry tool (19). The same researcher conducted all the computer planimetry wound assessments.
The degree and frequency or occurrence of pain as reported by participants were recorded at the initial and subsequent wound assessments. Degree of pain was measured on a scale of 0–10 with 0 being no pain and 10 being extreme pain. Frequency or occurrence of pain was recorded as intermittent, constant, nocturnal, during cleansing or unknown.
Tea tree oil product
The product was a water‐miscible 10% v/v tea tree oil solution packaged in 25‐ml glass bottles (Novabac, Novasel Pty. Ltd.). The tea tree oil solution was formulated and provided by Novasel Pty. Ltd. (Mudgeeraba, Qld, Australia) and the tea tree oil complied with the international standard for ‘Oil of Melaleuca– terpinen‐4‐ol type’ (20).
Treatment
Wound dressings were removed, the wound washed with sterile water for irrigation (Baxter Healthcare Pty. Ltd., Midland, WA, Australia) and debrided if required. Two 25‐ml bottles of tea tree oil solution were opened and added to 100 ml of sterile water for irrigation, mixed by manual shaking and then used to irrigate and wash the wound. The final tea tree oil concentration in the wound wash solution was 3.3%. This concentration was chosen based on anecdotal evidence from Silver Chain Nursing Association staff and published data on the antimicrobial activity of tea tree oil (1) balanced with consideration of the lack of data about the effects of applying tea tree oil to large wounds. Where larger volumes of treatment product were required, additional bottles of tea tree oil product and sterile water were mixed, always with a 2:1 ratio, respectively. Excess tea tree oil solution was allowed to drain from the site and residual product was left on the wound for at least 5 minutes before the wound was dressed with a primary and secondary dressing. Antimicrobial agents (other than the tea tree oil solution) and antimicrobial or occlusive dressings were not used.
If required, wound swabs and wound data were always collected prior to application of the tea tree oil solution. The frequency of tea tree oil treatments varied for each participant and was governed by the frequency of dressing changes deemed necessary by the nurse following assessment of each wound, but dressings were changed at least weekly.
Subsequent assessments
Wound assessments were made every 2 weeks from weeks 2–12 and if infection occurred. Wound swabs for microbiological assessments were performed 3 weeks after the enrolment week (week 4) and again during week 12. Two swabs at least 24‐hours apart were taken both for week 4 and week 12 assessments. All swabs were taken at least 3 days after the last wash with tea tree oil solution. The last assessment for both the primary and secondary measures of efficacy was performed when the wound was completely healed or not later than 12 weeks. Participants in whom wounds had not healed continued to receive appropriate care until healing was complete. If participants withdrew from the study, the most recent swab result was used as the final MRSA status.
RESULTS
A total of 19 participants was enrolled into the study over a 9‐month period. Microbiological analysis of the initial swab confirmed that 12 (63·2%) were MRSA‐positive at enrolment. The seven participants who did not have wounds colonised with MRSA were colonised with methicillin‐sensitive S. aureus rather than the required MRSA and were withdrawn from the study. The flow of participants through the study is shown in Figure 1. Summary demographic data for the 12 MRSA positive participants are shown in Table 1. Summary treatment data for each participant are given in Table 2.
Figure 1.
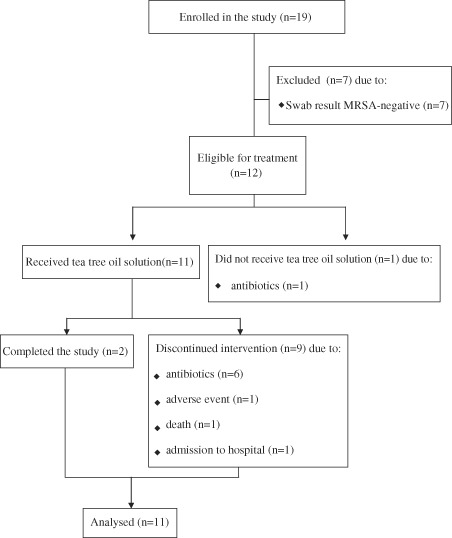
Flow diagram of participants through the study.
Table 1.
Summary demographic data for recruited participants with wounds subsequently confirmed to be MRSA positive (n = 12)
| Participant | Gender | Age (years) | Wound type | Pre‐existing wound duration |
|---|---|---|---|---|
| 2 | M | 85 | Leg ulcer mixed | 19 months |
| 5 | M | 66 | Leg ulcer venous | 12 months |
| 6 | F | 34 | Pressure ulcer | Unknown |
| 8 | F | 83 | Leg ulcer unclassified | 18 months |
| 10 | F | 91 | Leg ulcer unclassified | 6 months |
| 12 | F | 77 | Leg ulcer arterial | 3 months |
| 14 | F | 57 | Shingles | 3 weeks |
| 15 | F | 68 | Leg ulcer venous | 22 months |
| 16 | F | 95 | Leg ulcer venous | 5 months |
| 20 | M | 36 | Neuro‐ischaemic foot ulcer | 2 months |
| 21 | F | 72 | Surgical dehiscence | 8 months |
| 22 | F | 80 | Leg ulcer venous | 7 weeks |
MRSA, methicillin‐resistant Staphylococcus aureus.
Table 2.
Treatment summary data for recruited participants with wounds subsequently confirmed to be MRSA positive (n = 12)
| Participant | Withdrawal | No. of treatments (frequency) | Microorganisms (in addition to MRSA) * detected at | Wound size (mm2) † measurement at | Adverse events | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes/No | Week | Reason | Enrolment | Week 4 | Week 12 | Enrolment | Last measure (taken at week no.) [relative to size at enrolment] | |||
| 2 | Yes | 2 | Antibiotics | 8 (daily) | MSSA coliforms | None | nd | 1043 | 1427 (1) [137%] | No |
| 5 | Yes | 6 | Adverse event | 15 (3/week) | None | Citrobacter | nd | 512 | 68 (6) [13%] | Pain |
| 6 | Yes | 1 | Antibiotics | 0 | None | nd | nd | nd | nd | No |
| 8 | Yes | 3 | Death | 7 (3/week) | E. coli Candida sp. | nd | nd | 184 | 160 (1) [87%] | No |
| 10 | Yes | 2 | Hospitalised | 13 (daily) | P. aeruginosa | nd | nd | 2579 | 6383 (1) [247%] | No |
| 12 | Yes | 11 | Antibiotics | 30 (3/week) | None | None | nd | 54 | 62 (11) [115%] | No |
| 14 | Yes | 2 | Antibiotics | 3 (3/week) | None | nd | nd | 55 | 17 (2) [31%] | No |
| 15 | Yes | 8 | Antibiotics | 22 (3/week) | None | None | nd | 60 | 44 (8) [73%] | No |
| 16 | No | – | – | 35 (3/week) | None | None | S. maltophilia anaerobes | 594 | 485 (10) [82%] | Pain |
| 20 | Yes | 6 | Antibiotics | 35 (daily) | S. maltophilia | P. aeruginosa coliforms | nd | 1932 | 1073 (4) [56%] | No |
| 21 | Yes | 8 | Antibiotics | 8 (3/week) | None | None | nd | 3922 | 3447 (8) [88%] | Pain |
| 22 | No | – | – | 33 (3/week) | None | None | Gram‐positive bacilli | 17152 | 9947 (12) [52%] | No |
MRSA, methicillin‐resistant Staphylococcus aureus; MSSA, methicillin‐susceptible S. aureus; nd, not done; AMWIS, advanced medical wound imaging system.
Bold text indicates wounds that reduced in size.
*MRSA was detected on all initial and subsequent swabs.
†AMWIS area data.
Of the 12 participants confirmed to have MRSA, one was withdrawn before treatment because of the commencement of antibiotics. Eleven received at least one tea tree oil treatment. Of these, six were withdrawn because of the commencement of antibiotics and one each as result of an adverse event, death (unrelated to treatment) and admission to hospital. Two participants completed the 12‐week study.
An adverse event of pain was reported in 3 of the 12 MRSA‐positive participants resulting in withdrawal from the study by 1 participant (#5) who experienced pain during the cleansing procedure that may or may not have been because of the irrigation with the tea tree oil solution. The second adverse event of pain (in participant #16) was attributed to a compression bandage. The clinical consensus was that the pain was unrelated to the use of tea tree oil solution in the wound irrigation and cleansing procedure. The participant continued in the study and completed the 12 weeks of treatment. The other pain event (in participant #21) may or may not have been because of the irrigation with the tea tree oil solution. There were no other adverse events reported.
All 12 participants were still MRSA‐positive at their final available swab. This included one participant who was withdrawn before receiving tea tree oil treatment and was assumed to still be MRSA positive. Notable changes in the organisms cultured from the wound swabs included the disappearance of MSSA and coliforms in the week 4 cultures from one participant (#2) that were present at enrolment. A second participant (#8) whose enrolment swab grew coliforms (Escherichia coli) died during week 3 of her participation. No swabs were available to determine whether the coliforms remained. Her death was unrelated to her wounds or her participation in the study. Swabs from two participants (#5 and #20) in whom coliforms were not noted from the enrolment swab subsequently grew coliforms; in participant #5 Citrobacter sp. grew from one of the two swabs taken during week 4 and in participant #20 mixed coliforms were noted from the swabs taken during week 4. Finally, the abundant Gram‐positive bacilli seen on microscopy in the final swab specimen from participant #22 were characterised as coryneforms. Corynebacteria are commensal skin flora and their appearance in the wound is likely indicative of the healing status of this venous leg ulcer which had reduced in size by 48% during the 12 weeks of treatment with the tea tree oil solution.
Using area data from AMWIS 8 of the 12 participants (66.7%) had smaller wounds at the last measurement taken before withdrawal or completion, 3 (25%) had larger wounds and the change in 1 (8.8%) was unknown because she withdrew almost immediately after being enrolled as she was prescribed antibiotics. Wound photographs from three participants are shown in 2, 3, 4.
Figure 2.
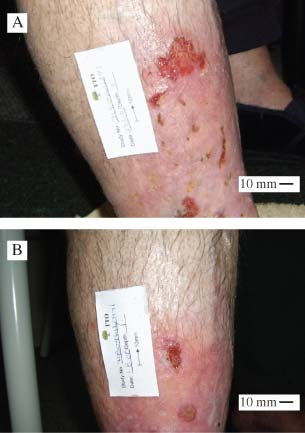
Initial and post‐treatment images of wounds for participant #5 who had a venous leg ulcer of 12 months duration. Images were taken on enrolment (A) and at week 6 (B).
Figure 3.
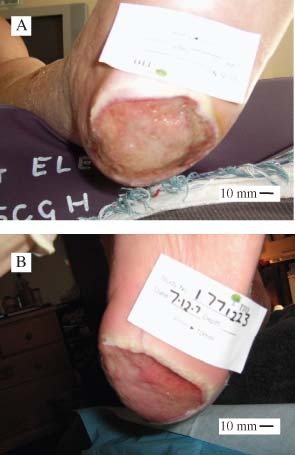
Initial and post‐treatment images of wounds for participant #20 who had a neuro‐ischaemic foot ulcer of 2 months duration. Images were taken on enrolment (A) and after 4 weeks treatment (B).
Figure 4.
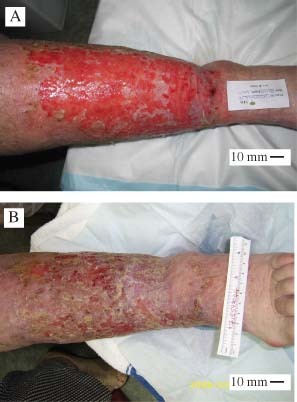
Initial and post‐treatment images of wounds for participant #22 who had a venous leg ulcer of 7 weeks duration. Images were taken on enrolment (A) and after trial completion in week 14 (B).
Of the eight wounds that were reduced in size after treatment with the tea tree oil solution, five were leg ulcers made up of four venous ulcers and one unclassified leg ulcer. The patient‐reported duration of these wounds ranged from 7 weeks to 22 months. The three wounds that were not leg ulcers and were reduced in size after treatment were herpes zoster lesions (shingles) of 3 weeks duration, a dehisced surgical wound of 8 months duration and a pressure ulcer of 2 months duration. Apart from the one wound that received no treatment, the three wounds that did not diminish in size during treatment were an arterial, an unclassified and a mixed venous/arterial leg ulcer of 3, 6 and 19 months in duration, respectively. These wounds increased in size to 136%, 247% and 115% of their original size.
Participant‐reported pain data are shown in Table 3. As many as 5 of the 11 participants who received at least one wash with the tea tree oil solution, reported a reduction in pain of two or more units over the course of their treatment and 4 reported increased pain levels (≥2).
Table 3.
Degree and frequency or occurrence of pain as indicated by the treated (n = 11) MRSA‐positive participants *
| Participant | Degree † and frequency/occurrence ‡ of pain at | |
|---|---|---|
| Enrolment | Final measurement | |
| 2 | 9, int | 6, int |
| 5 | 6, int | 10, const |
| 8 | 0, none | 10, cleans |
| 10 | 10, int | 9, const |
| 12 | 0, none | 0, none |
| 14 | 5, noct | 0, none |
| 15 | 0, none | 8, const |
| 16 | 5, cleans | 2, int |
| 20 | 0, none | 3, int |
| 21 | 6, int | 2, int |
| 22 | 8, int | 6, int |
Bold text indicates participants in whom pain reduced by ≥2 degrees on a scale of 1–10.
*Both measures are as reported by the participant.
†Degree of pain on a scale of 0–10 where 0 is no pain and 10 is extreme.
‡Frequency or occurrence of pain. Options were none, intermittent (int), constant (const), nocturnal (noct), only at time of wound cleansing (cleans) or unknown.
DISCUSSION
The primary outcome, decolonisation of MRSA‐positive wounds, was not achieved in any participant. As the target sample size of 14 consecutive treated participants was not achieved, the hypothesis that tea tree oil may be effective in decolonising 20% of participants or more cannot be accepted or refuted. Even so, our failure to eliminate MRSA from the wounds of any of the treated participants contrasts with an earlier report describing the use of a tea tree oil cream as part of an MRSA skin decolonisation regimen in which MRSA were cleared from 16 of 34 (46%) wounds treated with it compared with 8 of 24 wounds (31%) that were treated with silver sulfadiazine (15). However, the product used was a 10% tea tree oil cream applied to wounds once a day for 5 days. It is likely that the higher tea tree oil concentration and the leave‐on nature of the product used contributed to the greater success in decolonising MRSA compared with the results obtained in this pilot study with a lower concentration (3·3%) and a rinse‐off product. A study comparing 4% tea tree oil nasal ointment combined with 5% tea tree oil skin wash to 2% mupirocin ointment and Triclosan body wash also suggested that tea tree oil may be helpful in decolonising MRSA (21). More recently, a 5% tea tree oil body wash was part of a decolonisation regimen that significantly reduced the prevalence of MRSA carriage in nursing home residents and patients attending an MRSA clinic (22).
The secondary outcome (influence on wound healing) yielded some positive results with 8 of 11 treated participants having smaller wounds at the end of the study. Previous work evaluating a tea tree oil product as a wound treatment reported several negative outcomes including maceration of the wound and colonisation of wounds with multiple Gram‐negative organisms after the commencement of treatment (23). The product used in this earlier study, also designed to evaluate the use of tea tree oil in the management of chronic venous leg ulcers, was a 20% tea tree oil blemish gel and it was left in place under a dressing for up to a week. Wound maceration after using a leave‐on tea tree oil product has also been reported when tea tree oil ointment was applied to infected Hickman line sites (24). No maceration of the wound edges was observed in this pilot study and the widespread acquisition of Gram‐negative bacteria, such as Pseudomonas aeruginosa and coliforms reported previously (23) was not evident. Gram‐negative bacteria not previously detected in the initial swabs were detected later in only 3 of the 11 treated participants and coliforms noted on the initial swab were later absent in 1 of the 11 participants. The absence of wound edge maceration and the low‐level of Gram‐negative acquisition seen in this study suggest that they may be product‐specific effects and that presentation of the tea tree oil in an appropriate format may confer the benefits of tea tree oil without some of the previously observed problems.
Using AMWIS, a validated digital planimetry tool, wound size could be accurately estimated by computer planimetry which traced the perimeter. That 8 of the 11 treated wounds reduced in size according to this method of measurement is highly significant. Without controls, conclusions about the impact of tea tree oil on wound healing rates and reductions in wound size are impossible. All that can be said from this series is that the majority of treated wounds began to reduce in size including five wounds ranging in duration from 5 to 22 months. Data from in vitro work on the cytotoxicity of tea tree oil to human cell lines (25, 26) have led previously to suggestions that it may be unsuitable for use on wounds and that it may delay wound healing and increase scarring (27). The reductions in wound size seen in this series suggest that tea tree oil does not delay and may even promote wound healing because several long‐standing wounds began to heal once the tea tree oil solution was used.
When used as a wound irrigant in contact with wounds for a minimum of 5 minutes during wound cleansing and before dressing application, tea tree oil at a concentration of 3.3% did not decolonise MRSA‐positive wounds. However, when included in the wound irrigation and cleansing procedure, even this brief exposure allowed the healing of several previously chronic, non healing wounds to begin. This effect alone warrants further scrutiny. The low incidence of adverse effects attributable to tea tree oil also provides further support for the safety of topical application of tea tree oil products to open wounds. Although much further work is required, tea tree oil should continue to be considered and evaluated as a wound treatment product. It is possible that a higher concentration of tea tree oil formulated in a product designed to stay in contact with a wound, but not lead to peri‐wound maceration, may decolonise MRSA from wounds and enhance wound healing and this possibility should be investigated further.
This study was always intended to be a pilot study conducted as a prelude to a much larger study and, as such, it has provided much useful data, some of which contradicts previously published work. Recruitment for the study was difficult and many participants were lost because of the commencement of antibiotics, and other complications. Any subsequent clinical work should recruit participants with one type of wound that is colonised or critically colonised, but not infected with S. aureus, either methicillin‐susceptible or ‐resistant strains. A dosing study may be necessary to guide selection of the tea tree oil concentration required to not only promote healing, but also to decolonise S. aureus.
As a significant proportion of the chronic wounds to which a tea tree oil solution was briefly applied was reduced in size (8 of 11), and many after only short‐term exposure, further studies should be undertaken to investigate wound healing. This would best be performed in a clinical trial setting in which participants all have wounds of a similar type, such as venous leg ulcers. A control group of participants matched for wound type who receive no tea tree oil intervention would be ideal although recruiting sufficient numbers to have matched controls may prove difficult.
Additionally, the possibility remains that higher concentrations of tea tree oil left in contact with wounds for extended periods of time may decolonise MRSA. Discussions with wound product manufacturers regarding suitable product formats would be helpful and may lead to a wound dressing product that incorporates tea tree oil for this purpose.
ACKNOWLEDGEMENTS
This study was funded by the Rural Industries Research & Development Corporation (PRJ‐000822) and by Novasel Australia Pty. Ltd., Mudgeeraba, Qld, Australia. The substantial in‐kind contribution by Silver Chain Nursing Association is gratefully acknowledged. Tea tree oil product for this study was kindly donated by Novasel Australia Pty. Ltd.
REFERENCES
- 1. Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other properties. Clin Microbiol Rev 2006;19:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carson CF, Cookson BD, Farrelly HD, Riley TV. Susceptibility of methicillin‐resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. J Antimicrob Chemother 1995;35:421–24. [DOI] [PubMed] [Google Scholar]
- 3. Carson CF, Hammer KA, Riley TV. In‐vitro activity of the essential oil of Melaleuca alternifolia against Streptococcus spp. J Antimicrob Chemother 1996;37:1177–8. [DOI] [PubMed] [Google Scholar]
- 4. Hammer KA, Carson CF, Riley TV. Susceptibility of transient and commensal skin flora to the essential oil of Melaleuca alternifolia (tea tree oil). Am J Infect Control 1996;24:186–9. [DOI] [PubMed] [Google Scholar]
- 5. Nelson RRS. In‐vitro activities of five plant essential oils against methicillin‐resistant Staphylococcus aureus and vancomycin‐resistant Enterococcus faecium. J Antimicrob Chemother 1997;40:305–6. [DOI] [PubMed] [Google Scholar]
- 6. Elsom GKF, Hide D. Susceptibility of methicillin‐resistant Staphylococcus aureus to tea tree oil and mupirocin. J Antimicrob Chemother 1999;43:427–8. [DOI] [PubMed] [Google Scholar]
- 7. Chan CH, Loudon KW. Activity of tea tree oil on methicillin‐resistant Staphylococcus aureus (MRSA) [letter]. J Hosp Infect 1998;39:244–5. [DOI] [PubMed] [Google Scholar]
- 8. Brady A, Loughlin R, Gilpin D, Kearney P, Tunney M. In vitro activity of tea‐tree oil against clinical skin isolates of methicillin‐resistant and ‐sensitive Staphylococcus aureus and coagulase‐negative staphylococci growing planktonically and as biofilms. J Med Microbiol 2006;55:1375–80. [DOI] [PubMed] [Google Scholar]
- 9. La Plante KL. In vitro activity of lysostaphin, mupirocin, and tea tree oil against clinical methicillin‐resistant Staphylococcus aureus. Diagn Microbiol Infect Dis 2007;57:413–8. [DOI] [PubMed] [Google Scholar]
- 10. Mayaud L, Carricajo A, Zhiri A, Aubert G. Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Lett Appl Microbiol 2008;47:167–73. [DOI] [PubMed] [Google Scholar]
- 11. Hayes AJ, Markovic B. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem Toxicol 2002;40:535–43. [DOI] [PubMed] [Google Scholar]
- 12. Loughlin R, Gilmore BF, McCarron PA, Tunney MM. Comparison of the cidal activity of tea tree oil and terpinen‐4‐ol against clinical bacterial skin isolates and human fibroblast cells. Lett Appl Microbiol 2008;46:428–33. [DOI] [PubMed] [Google Scholar]
- 13. Davis A, O’Leary J, Muthaiyan A, Langevin M, Delgado A, Abalos A, Fajardo A, Marek J, Wilkinson B, Gustafson J. Characterization of Staphylococcus aureus mutants expressing reduced susceptibility to common house‐cleaners. J Appl Microbiol 2005;98:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banes‐Marshall L, Cawley P, Phillips CA. In vitro activity of Melaleuca alternifolia (tea tree) oil against bacterial and Candida spp. isolates from clinical specimens. Br J Biomed Sci 2001;58:139–45. [PubMed] [Google Scholar]
- 15. Dryden MS, Dailly S, Crouch M. A randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of MRSA colonisation. J Hosp Infect 2004;56:283–6. [DOI] [PubMed] [Google Scholar]
- 16. Gehan EA. The determination of the number of patients required in a preliminary and a follow‐up trial of a new chemotherapeutic agent. J Chron Dis 1961;13:346–53. [DOI] [PubMed] [Google Scholar]
- 17. Clinical and Laboratory Standards Institute . Performance standards for antimicrobial disk susceptibility tests: approved standard, 10th edn. M02‐A10. Wayne, Philadelphia, USA: Clinical and Laboratory Standards Institute, 2009.
- 18. Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing. Twentieth Informational Supplement M100‐S20. Wayne, Philadelphia, USA: Clinical and Laboratory Standards Institute, 2010. [Google Scholar]
- 19. Santamaria N, Carville K, Ellis I, Prentice J. The effectiveness of digital imaging and remote expert wound consultation on healing rates in chronic lower leg ulcers in the Kimberley region of Western Australia. Prim Intention 2004;12:62–4, 66–8, 70. [Google Scholar]
- 20. International Organisation for Standardisation. ISO 4730: oil of Melaleuca, terpinen‐4‐ol type (tea tree oil). Geneva: International Organisation for Standardisation, 2004. [Google Scholar]
- 21. Caelli M, Porteous J, Carson CF, Heller R, Riley TV. Tea tree oil as an alternative topical decolonisation agent for methicillin‐resistant Staphylococcus aureus. J Hosp Infect 2000;46:236–7. [DOI] [PubMed] [Google Scholar]
- 22. Bowler WA, Bresnahan J, Bradfish A, Fernandez C. An integrated approach to methicillin‐resistant Staphylococcus aureus control in a rural, regional‐referral healthcare setting. Infect Cont Hosp Epidemiol 2010;31:269–75. [DOI] [PubMed] [Google Scholar]
- 23. Chaudhuri A, Cogswell L, Quick CRG. A pilot evaluation of tea tree oil in the management of chronic venous leg ulcers. Phlebology 2005;20:134–7. [Google Scholar]
- 24. Gravett P. Aromatherapy treatment for patients with Hickman line infection following high‐dose chemotherapy. Int J Aromather 2001;11:18–9. [Google Scholar]
- 25. Söderberg TA, Johansson A, Gref R. Toxic effects of some conifer resin acids and tea tree oil on human epithelial and fibroblast cells. Toxicology 1996;107:99–109. [DOI] [PubMed] [Google Scholar]
- 26. Hayes AJ, Leach DN, Markham JL. In vitro cytotoxicity of Australian tea tree oil using human cell lines. J Essent Oil Res 1997;9:575–82. [Google Scholar]
- 27. Faoagali J, George N, Leditschke JF. Does tea tree oil have a place in the topical treatment of burns? Burns 1997;23:349–51. [DOI] [PubMed] [Google Scholar]


