Abstract
The aim of this study was to prove the effectiveness of MatriDerm® combined with skin grafting versus skin grafting alone in post‐traumatic wounds treatment. At the Department of Plastic and Reconstructive Surgery of the University of Rome Tor Vergata, we treated 60 patients: 30 patients with dermal substitutes (MatriDerm®) combined with autologous skin graft and 30 with skin graft alone. Two weeks after the first treatment, 95% of wounds treated with MatriDerm® and skin graft showed a re‐epithelisation, whereas it was 75–80% in the control group. We used the Manchester Scar Scale (MSS) and patient's self‐estimation scale to assess the outcomes. Mann–Whitney U test was performed for the five items of the MSS and the results were combined to those of patient's self‐estimation scale and the re‐epithelialisation percentage to test the significance between the two groups. These data confirm the evidence of the clinical use of MatriDerm® technology in the healing of soft tissue wounds and prove the effectiveness of combining MatriDerm® and skin grafting for the first time. Furthermore, we observed a percentage reduction of wound contraction and in the same time an improvement of elasticity, quality of scars tissue and dermal architecture.
Keywords: Dermal substitute, Post‐traumatic wounds, Skin grafting
INTRODUCTION
The treatment of full‐thickness post‐traumatic wounds is a multilayer approach: orthopaedic, micro‐vascular and finally plastic surgery. The gold standard coverage for these wounds is bioengineering substitute, free flaps and autologous skin grafting (1). However, poor skin quality and scar contracture occur frequently and are well‐known problems in split‐grafted areas. Dermal substitute is an appropriate way to minimise scar contraction and to optimise the quality of the grafted area in strained regions with loss of function and with high requirements of elasticity, pliability and stability (2).
A cell‐based wound coverage with keratinocytes and fibroblasts on the basis of a commercially available dermal substitute (MatriDerm®, Kollagen/Elastin matrix) was generated, in order to treat wide wounds (3). This scaffold has already be proven to be suitable for a single‐stage grafting procedure without the disadvantage of skin grafting rejection (4).
MATERIALS AND METHODS
Between 2007 and 2009, we treated 60 patients (38 males, 22 females, age ranging from 30 to 70 years old) with full‐thickness post‐traumatic skin defects. All wounds were localised on the inferior limbs (Figure 1). Exclusion criteria were arterial, venous or diabetic wounds. In our study, we treated 30 patients with MatriDerm® combined with autologous skin graft, and 30 patients with autologous skin graft alone. The two groups were homogeneous because the patients were randomly allocated in the treated or in the control group. Our protocol was based on clinical evaluation, wound examination, swab culture, instrumental examination (echo–colour‐Doppler) of the lower limbs and photographs were taken with a follow‐up time at: 0, 2 weeks and 1, 3, 6 months and 1 year. Each patient was examined considering the efficiency of main organs (heart, liver, kidney and lungs) and possible concomitant pathology.
Figure 1.
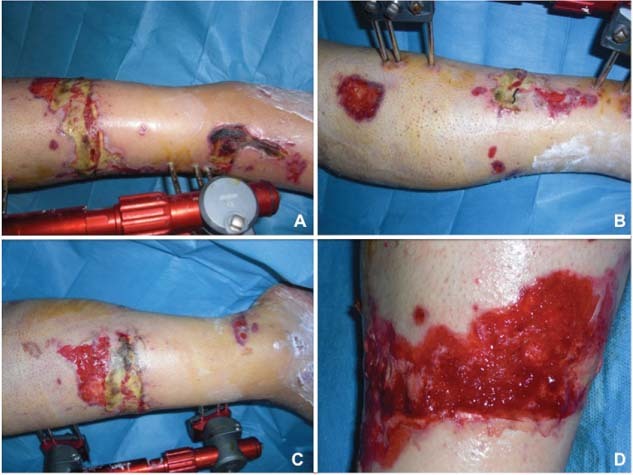
Preoperative view of lower limb wound at 30 days after trauma. (A) Anterior view, (B) lateral view, (C) medial view and (D) specific area of the wound.
The wound examination was based on: wound area (cm3), wound bed, wound margins and surrounding skin. Swab culture was performed to evaluate any microbiological infections and to find the appropriate antibiotic therapy. Echo–colour‐Doppler was performed in the lower limbs in order to achieve a complete evaluation of local vascular circulation. All procedures were performed in a completely asepsis ambience with epidural and/or loco‐regional anaesthesia. The surgical step included the curettage of damaged areas and the dermal substitute application in combination with a split‐thickness auto graft in one‐step procedure (Figure 2).
Figure 2.
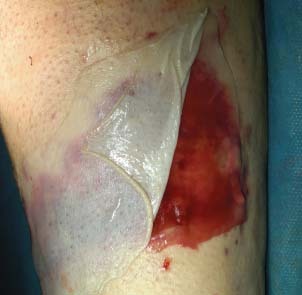
Intra‐operative view. Application of MatriDerm® on wound bed.
MatriDerm®
MatriDerm® is a single‐use three‐dimensional matrix composed of native, structurally intact collagen fibrils and elastin for supporting dermal regeneration. The collagen is obtained from bovine dermis and contains the dermal collagen types I, III and V. The elastin is obtained from bovine nuchal ligament by hydrolysis. MatriDerm® serves as a scaffold in the skin reconstitution and modulates scar tissue formation. Moreover, MatriDerm® has an excellent haemostatic property and thus reduces the risk of split‐skin sub‐graft haematoma. The non use of chemical cross‐linking of the collagen results in a matrix, which is especially biocompatible (5). MatriDerm®, applied using a single‐stage, is immediately covered with split skin through the 1‐mm‐thick matrix by diffusion. MatriDerm® is supplied in sterile double‐bagged packs, and these may only be opened under sterile conditions. Before the use, MatriDerm® must be rehydrated in ample physiological saline solution, and to avoid trapped pockets of air (air pockets can hinder the diffusion and thus jeopardise the attached graft), MatriDerm® should be laid on the surface of the water and not immersed. The matrix is ready for use as soon as the appearance of the entire surface has changed from white to translucent (5).
Skin grafting
Classic skin grafting was performed with a dermatome using a thin split‐thickness depth, meshing all grafts (1:2 ratio) and fixing to the wounds by 3/0 nylon sutures. A moulage compressive dressing with hyaluronic acid gauze was used to cover the surgical wound.
To apply MatriDerm®, it should be cut to the exact size of the wound. We applied the matrix by hands, and it is crucial that MatriDerm® is in complete contact with the whole area of the wound bed, allowing to adhere to it. Air bubbles between the wound bed and the matrix should therefore be carefully removed by smoothing them out the margins of the matrix. A split‐thickness cutaneous was harvested from an uninvolved area (the inguinal region whenever possible) using a Zimmer dermatome (Zimmer, IN). The split skin is grafted into the wound area directly on the top of MatriDerm®; an additional attachment of MatriDerm® with the split skin is achieved by sutures. A slight pricking is recommended to avoid the formation of seromas (5). This treatment was compared with the conventional treatment: a split‐thickness autograft. One week after the operation, the percentage of auto graft survival was observed. Postoperative follow‐up consisted of four visits during the first month – one for each week – and two additional visits at months 3 and 6. During this visits, to test the effectiveness of the dermal substitute and to compare it with the current gold standard, skin grafting, we evaluated the time for complete epithelisation (both treated area and biopsy site), aesthetic and functional quality of the epithelisation (colour, joint contractures) as primary endpoints of the study. The aesthetic appearance of the epithelisation was evaluated with the help of one plastic surgeon unaware of the procedure, according to the Manchester Scar Scale (MSS) (6). MSS was used to assess the colour of the scars defined as perfect, slight, obvious or gross mismatch compared with the surrounding skin; to assess the appearance of the skin in matte or shiny; to assess the contours from flush with surrounding skin, slightly proud/indented, hypertrophic to keloid; to assess the texture from normal, just palpable, firm to hard; and to assess the margins, whether distinct or not (7). We did not include the items associated with the size and number of the scars because the wounds were all single and from 2 to 10 cm. We did not associate any other information in the Manchester scar proforma such as race or ethnic background and symptoms of the wounds because the groups of patients were homogeneous for these characteristics.
Mann–Whitney U test was performed for the five items of the MSS and combined with the results of patient's self‐estimation scale and for the re‐epithelialisation percentage to test the significance between the two groups.
RESULTS
The percentage of re‐epithelialisation was significantly different after 3 months from the treatment between the two groups (P < 0·001), as clearly shown in Figure 3. After the first treatment, we observed a mean re‐epithelialisation time of 15 days. In 13 patients treated with skin grafting combined with MatriDerm®, we observed complete re‐epithelialisation and 80–90% epithelialisation in 17 patients. Minimal or absence of skin grafting contracture was observed in these patients.
Figure 3.
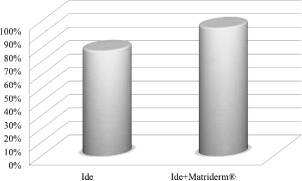
Mean and standard deviation of the re‐epithelialisation percentage for the two groups.
The percentage of living tissue was evaluated to be 95% of the wounds in patients treated with MatriDerm® combined with skin grafting, whereas it was almost 75–80% in the wounds treated with skin grafting alone. In almost all these patients treated with skin grafting alone, we observed skin grafting contracture.
All the scores associated with the items of MSS resulted significantly different between the two groups, especially in colour, appearance and contour, as shown in Table 1. Almost all areas treated with skin graft appeared grossly mismatching or obviously mismatching in colour, slightly proud/indented or hypertrophic in contour and shiny in appearance. Wounds treated with MatriDerm® had a slight mismatch, flush with surrounding skin or slightly proud/indented contour and matte appearance. None of the 60 patients reported intra‐operative or postoperative adverse effects.
Table 1.
Manchester Scar Scale assessment combined with patient's self‐estimation *
| Manchester Scar Scale (mean ± SD) | Skin graft | Skin graft + MatriDerm® | P |
|---|---|---|---|
| Self‐estimation | 4·7 ± 4·3 | 1·5 ± 0·5 | 0.032 |
| Colour | 2·8 ± 1·2 | 1·2 ± 0·8 | <0.001 |
| Contour | 1·9 ± 0·8 | 1·1 ± 0·5 | 0.005 |
| Texture | 3·0 ± 1·5 | 1·6 ± 1·2 | 0.012 |
| Appearance | 1·8 ± 0·4 | 1·1 ± 0·4 | <0.001 |
| Margins | 1·3 ± 0·5 | 1·0 ± 0·0 | 0.038 |
*Mean and standard deviation were reported for the two treatments and a Mann–Whitney U test was performed for each item of the scale to test the significance of the differences (P).
The self‐estimation of the patients resulted highly variable in the group treated only with skin grafting; however also this item leads to a significant difference between the two group scores. Dermal matrix and skin grafting integrated successfully in most of the treated sites. The resultant biointegration enabled staged, definitive and durable soft tissue coverage. Results with MatriDerm® are presented in 4, 5.
Figure 4.
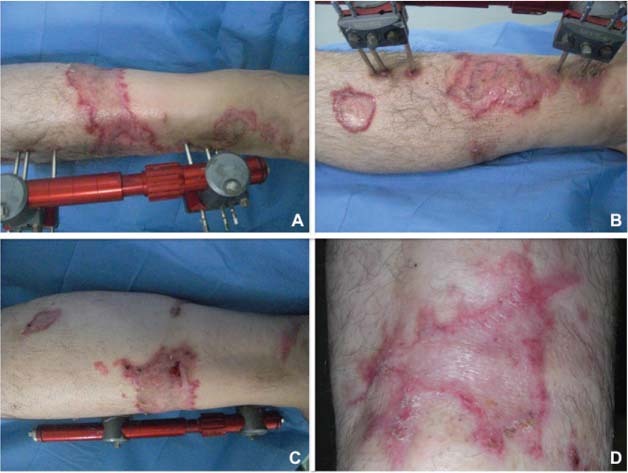
Postoperative view at 30 days of follow‐up. (A) Anterior view, (B) lateral view, (C) medial view and (D) specific area of the wound.
Figure 5.
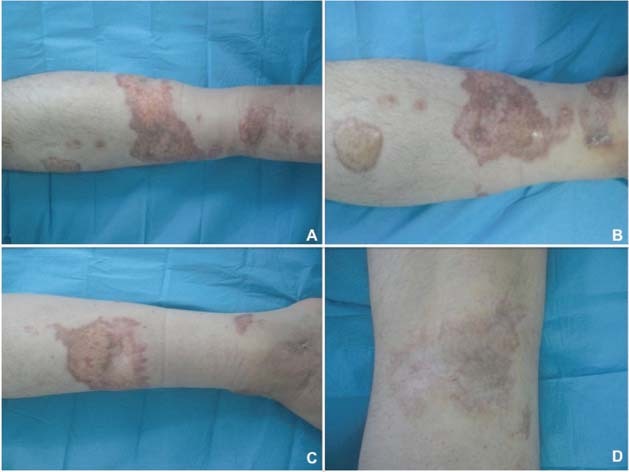
Postoperative view at 1 year of follow‐up. (A) Anterior view, (B) lateral view, (C) medial view and (D) specific area of the wound.
DISCUSSION
Wound healing is an evolutionarily conserved complex multi‐cellular process that aims at restoring skin barrier. This process involves coordinated efforts of several cell types including keratinocytes, fibroblasts, endothelial cells, macrophages and platelets. The migration, infiltration, proliferation and differentiation of these cells will culminate in an inflammatory response, the formation of new tissue and ultimately wound closure. This complex process is executed and regulated by an equally complex signalling network involving numerous growth factors, cytokines and chemokines 8, 9.
In 2000, the use of a collagen–elastin matrix for the treatment of burn wounds in a one‐stage grafting model was described by Van Zuijlen et al. (10). This is a highly porous, 1‐mm‐thick membrane, fully biodegradable, three dimensional, composed of native bovine collagen fibre I, II and V and coated with elastin hydrolysate derived from bovine ligamentum nuchae in a concentration of three weight‐to‐weight ratio, and the matrices were treated with gamma‐irradiation (1000 Gy) and stored at room temperature. This has been available in Europe since 2004 (MatriDerm®; Dr Suwelack, Skin and Health Care AG, Billerbeck, Germany).
The aim of our study was the comparison between the traditional skin autologous graft combined to dermal matrix and skin autologous graft procedure to obtain the restoration and regeneration of post‐traumatic wounds. The split‐thickness autograft is used for large ulcers under general, spinal or extensive local anaesthesia in large leg ulcers, and graft failure may occur because of build up of exudates underneath the graft. Those grafts are usually successful but on the other hand they may contract after harvesting and they need a large donor site. In most cases the donor site may heal slowly and cause a lot of pain. In each patient, the dermal substitute, MatriDerm®, showed positive effects into accelerating the improvement of the quality and the functionality of skin reconstruction.
Dermal substitutes may act as a barrier for vascular ingrowths and hamper diffusion of nutrients by increasing the distance between wound bed and the graft, for this reason it was postulated that survival of the overlying skin graft could be at risk after dermal substitution. Furthermore, the skin elasticity parameters of wound area treated with MatriDerm® were significantly improved in less time when sheet auto grafts were used (11).
CONCLUSIONS
Our study demonstrates the role of MatriDerm® and skin autologous graft in tissue regeneration and wound closure with a significant reduction in healing time with faster mean re‐epithelialisation time at 15 days, with differences still present after 3 months. On the basis of our clinical practice, we consider MatriDerm® and skin autologous graft as the key element to improve functional and aesthetic outcomes. This association guarantees a temporary barrier with multiple functions: haemostatic, reduction of contracture wound, infection, maintenance of skin elasticity and dermal architecture and better appearance of the scar. MatriDerm® can also be used to cover tendons and bone. Furthermore, the minimally invasive technique is well accepted by patients with a noteworthy improvement of quality of life along with cost reduction due to the fewer number of medications. In conclusion, our results show that the integration of different disciplines such as cell therapy, bioengineering and biomaterials sciences is an effective support to the surgical procedure.
ACKNOWLEDGEMENTS
None of the Authors has any financial interest in any products and in any other devices or drugs mentioned in this article. No fund was received for the research reported in the article.
REFERENCES
- 1. Wetzing T, Gebhardt C, Simon JC. New Indication for artificial collagen‐elastin matrices? Covering tendons. Dermatology 2009;219:272–73. [DOI] [PubMed] [Google Scholar]
- 2. Yannas IV, Burke JF. Design of an artificial skin I. Basic design principles. J Biomed Mater Res 1980;14:65–81. [DOI] [PubMed] [Google Scholar]
- 3. Golinski PA, Zöller N, Kippenberger S, Menke H, Bereiter‐ Hahn J, Bernd A. Development of an engraftable skin equivalent based on MatriDerm® with human keratinocytes and fibroblasts. Handchir Mikrochir Plast Chir 2009;41:327–32. [DOI] [PubMed] [Google Scholar]
- 4. Haslik W, Kamolz LP, Nathschläger G, Andel H, Meissl G, Frey M. First experience with the collagen‐elastin matrix MatriDerm® as a dermal substitute in severe burn injuries of the hand. Burns 2007;33:346–8. [DOI] [PubMed] [Google Scholar]
- 5. Cervelli V, Lucarini L, Cerretani C, Spallone D, Palla L, Brinci L, De Angelis B. The use of MatriDerm® and autologous skin grafting in the treatment of diabetic ulcers: a case report. Int Wound J 2010;7:291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bayat A, McGrouther DA, Ferguson MWJ. Skin scarring. BMJ 2003;326:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roques C, Teot L. A critical analysis of measurements used to assess and manage scars. Int J Low Extrem Wounds 2007;6:249–53. [DOI] [PubMed] [Google Scholar]
- 8. Eming SA, Yarmush ML, Morgan JR. Enhanced function of cultured epithelium by genetic modification: cell‐based synthesis and delivery of growth factors. Biotechnol Bioeng 1996;52:15–23. [DOI] [PubMed] [Google Scholar]
- 9. Myers S, Navsaria H, Sanders R, Green C, Leigh I. Transplantation of keratinoctes in the treatment of wounds. Am J Surg 1995;170:75–83. [DOI] [PubMed] [Google Scholar]
- 10. Van Zuijlen PP, van Trier AJ, Vloemans JF, Groenevelt F, Kreis RW, Middelkoop E. Graft survival and effectiveness of dermal substitution in burns and reconstructive surgery in a one stage grafting model. Plast Reconstr Surg 2000;106:615–23. [DOI] [PubMed] [Google Scholar]
- 11. Ryssel H, Gazyakan E, Germann G, Öhlbauer M. The use of MatriDerm® in early excision and simultaneous autologous skin grafting in burns – a pilot study. Burns 2008;34:93–7. [DOI] [PubMed] [Google Scholar]


