Abstract
An observational study was carried out at the Plastic and Reconstructive Surgery Unit of the University of Pavia – Salvatore Maugeri Research and Care Institute, Pavia, Italy, to assess the clinical and histological long‐term outcomes of autologous skin grafting of fresh surgical wounds following previous repair with a hyaluronic acid three‐dimensional scaffold (Hyalomatrix®). Eleven fresh wounds from surgical release of retracted scars were enrolled in this study. A stable skin‐like tissue cover was observed in all of the treated wounds in an average 1 month's time; at the end of this study, after an average of 12 months' time, all of the reconstructed areas were pliable and stable, although an average retraction rate of 51·62% was showed. Histological observation and immunohistochemical analysis displayed integration of the graft within the surrounding tissues. A regenerated dermis with an extracellular matrix rich in type I collagen and elastic fibres and with reduced type III collagen rate was observed. The epidermis and dermoepidermal junction featured a normal appearance with well‐structured dermal papillae, too. Although the histological features would suggest regeneration of a skin‐like tissue, with a good dermis and no signs of scarring, the clinical problem of secondary contracture is still unsolved.
Keywords: Bioengineered tissue, Hyaluronic acid, Retraction, Scar, Skin regeneration
Introduction
Bioengineered dermal substitutes have been gaining a large and stable consensus in the treatment of difficult‐to‐heal skin and soft tissue wounds over the last two decades.
Hyalomatrix® PA (HM, Fidia Advanced Biopolymers, Abano Terme, Padua, Italy) is a three‐dimensional acellular matrix‐based compound made of a benzyl ester of hyaluronic acid (HYAFF 11; Fidia Advanced Biopolymers) layer and a transparent silicone membrane. It has been widely used since 2001 in burn care for full or deep partial thickness wound temporary repair. Alternative indications have been difficult‐to‐heal wounds, post‐traumatic or complicated surgical wounds as a good option for temporary coverage 1, 2, 3.
The aim of this study was the assessment of the clinical and histological long‐term outcome of autologous skin grafting of fresh surgical wounds following previous cover with a hyaluronic acid three‐dimensional scaffold (Hyalomatrix®).
Materials and methods
Patients
Six consecutive patients, all females, aged between 10 and 60 years (average 42 years), in fair general clinical conditions, without any actual or potential wound healing disorder, were enrolled in this study. All of the patients were suffering from inveterate disabling scar retraction, with clinical contraindication for any conventional surgical revision, as skin expansion or flap transfer. In four patients the scars followed severe burn trauma; in one patient the scar followed harvesting of a myo‐cutaneous flap (platysma); in one patient scar retraction complicated a thin skin graft inlay. An overall of 11 individual scars were enrolled in the trial, as in two patients multiple anatomical scarred areas were treated. All of the areas were identified by Arabic numerals (Table 1). All of the treated scars were located on the flexor sides of mobile segments of the body: four cervical, three axillar, three popliteal and one infragluteal. Patient recruitment started in April 2007 and ended in June 2008. Follow‐up concluded in October 2009. An informed consent was obtained from each patient. The treated areas were photographed with the same digital camera, at a standard focal distance and under the same light exposure conditions, at four time intervals: preoperatively, intra‐operatively, 1 month post‐operatively and 1 year post‐operatively. Wound linear measurements were carried out by processing digital clinical pictures with IMAGE J Program (National Institute of Health, Bethesda, MD) (4).
Table 1.
Synopsis of the areas enrolled in the trial
| Patient | Area number | Anatomical site |
|---|---|---|
| AV | 1 | Left axilla, anterior pillar |
| RR | 2 | Right axilla, posterior pillar |
| 3 | Upper right lateral cervical area | |
| 4 | Lower right lateral cervical area | |
| 5 | Right axilla, anterior pillar | |
| SS | 6 | Left lateral cervical area |
| RS | 7 | Left popliteal fossa |
| 8 | Left infragluteal area | |
| 9 | Right popliteal fossa | |
| OC | 10 | Left popliteal fossa |
| AM | 11 | Right lateral cervical area |
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Surgical procedure
The general scheme of the trial is depicted in Table 2.
Table 2.
General scheme of the trial
| Study time | Clinical time | Activity | Description |
|---|---|---|---|
| T0 | 0 | Enrolment | Scar clinical photography |
| T1 | 7–28 days from T0 | Surgery | Scar removal |
| Surrounding tissues release | |||
| Scar biopsy | |||
| Wound bed clinical photography Hyalomatrix® inlay | |||
| T2 | Every 3 days from T1 | Post‐operative observation | Dressing care |
| T3 | 13–27 days from T1 | Surgery | Silicone layer removal |
| Regenerated derma‐like biopsy | |||
| Wound bed photography | |||
| Split skin graft | |||
| T4 | Within 2 months from T1 | Post‐operative and primary conclusive observation | Dressing care |
| Clinical post‐operative photography | |||
| T5 | 10–13 months from T1 | Conclusive follow‐up | Reconstructed area photography |
| Grafted skin biopsy (three cases only) |
All surgical procedures were carried out under general anaesthesia. The retracting scars were surgically removed up to the deep fascia and the surrounding tissues were meticulously released (T1). Samples for histology were harvested from the scars at this time.
The soft tissue defect resulting from scar removal and surrounding soft tissue release was measured and photographed. The defect was immediately covered with Hyalomatrix® fixed with metallic staples. The silicone sheet was evenly fenestrated to allow any leakage of serum and a petrolatum gauze plus non‐woven fabrics dressing was tied over. Perioperative antibiotic prophylaxis was routinely administrated (Amoxicillin 2 g + Clavulanic acid 200 mg intravenous intra‐operatively and Amoxicillin 875 mg + Clavulanic acid 125 mg per os twice a day for 1 week after surgery).
The patients were instructed to keep the operated areas as still as possible. At day 3, the tie‐over dressings were removed to check the wounds. A simple inspection of the silicone layer was carried out at this stage (T2) and, if appropriate, serum draining from the margins and from the holes in the silicone sheet was gently removed with sterile saline solution. The tie‐over dressing was replaced by a tight dressing until day 6 when the staples were removed.
After an average of 19 days (range 13–27 days), according to clinical evidence of regenerated tissue growth, a second surgical procedure provided stable wound repair with an autologous skin graft (T3). At this stage, the silicone sheet was removed to expose the wound bed. Any clinically detectable granulation tissue was carefully removed in order to expose the underlying regenerated pink derma‐like tissue. Samples for histology were harvested from the regenerated derma‐like tissue. The wound bed was eventually grafted with an autologous split skin graft. Post‐operative clinical follow‐up was the same as in any skin graft procedure (T4).
In the two teen‐aged patients, two split skin grafts in four failed and the procedure had to be repeated after an average time of 2 weeks. At the time of those secondary skin grafting, a greater amount of granulation tissue had to be removed from the wound bed in order to expose the regenerated derma‐like tissue layer.
About 1 year after healing the treated areas were inspected, measured and photographed (T5). In three cases it was possible to harvest samples for histology at this time.
Wound contraction of the treated areas was assessed comparing the measures taken at the different follow‐up times and processed by IMAGE J Program.
Histological evaluations
To asses the morphology of the skin before and after Hyalomatrix® inlay and split skin grafting, biopsies were conventionally harvested from the centre of the wounds or the grafted areas.
Each specimen underwent two different histological processes: a conventional haematoxylin/eosin plus orcein staining and an immunohistochemical frozen section. In the conventional staining the tissues were fixed in formalin, embedded in paraffin and stained with haematoxylin/eosin and orcein. In the immunohistochemical process, the specimens were snap frozen in liquid nitrogen and treated with anticollagen type I, III and VII antibodies (all from Sigma; Sigma‐Aldrich Inc., St. Louis, MO). The 5 µm thick cryosection was mounted on poly‐l lysine coated slides, fixed in 10% paraformaldehyde for 10 minutes and immunostained for type I collagen. Any unspecific binding was blocked by the addition of serum from the same animal species as the secondary antibodies. The slides were washed twice in Tris‐buffered saline (TBS), and then 100 µl of the appropriately diluted antibodies were placed on each tissue section and incubated in a moist chamber for 30 minutes. After this primary incubation, the slides were washed three times in TBS and further incubated with 100 µl of secondary antibodies (Vector, Burlingame, CA). Subsequently, 100 µl of avidin–biotin complex (Vectastain, ABC‐AP, CA; Vector) were placed on each section and incubated for 30 minutes. After washing, fast red (Sigma) was used as developer chromagen and nuclear counterstaining was performed with haematoxylin (Sigma). All specimens were mounted with Glycergel mounting medium (Dako Denmark A/S, Glostrup, Denmark). Sections without the primary antibodies (negative controls) and tissue sections of lymphoid tissue (positive controls) served as experimental controls.
Statistical analysis
The median and the interquartile range shrinkage rate derived from IMAGE J Program analysis was calculated for the whole sample at T3 versus T1. The correlation between the shrinkage rate at T3 versus T1 and the age group (10–18 and 30–60) and the anatomical site (axilla, neck and lower limb), respectively, was calculated, too.
Results
Clinical assessment
All of the treated areas healed with a stable skin‐like tissue cover in an average 1 month's time; at an average of 12 months' time, all of the reconstructed areas were still pliable and stable, but displayed a 51·62% retraction rate (1, 2, 3). The dimensions of the treated areas derived from IMAGE J Program analysis at the different times of the trial are reported in Table 3. The wound retraction trend showed a peak between T3 and T4.
Figure 1.
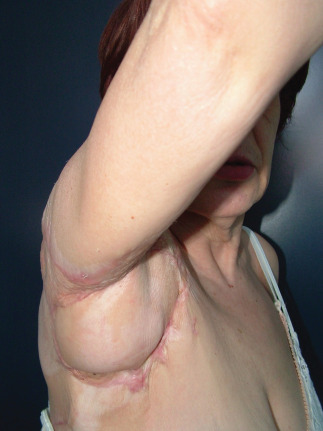
Right axillar posterior pillar scar retraction pre‐operative view.
Figure 2.
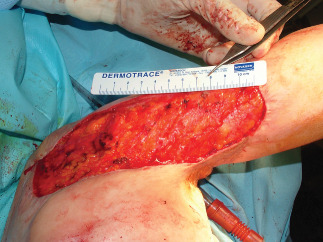
Scar release intra‐operative view.
Figure 3.
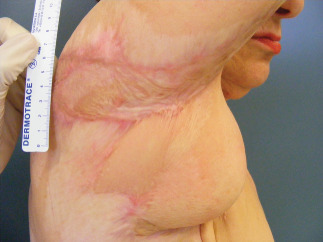
One year post‐operative view after scar release and serial grafting with Hyalomatrix® and split skin graft.
Table 3.
Dimensions of the treated areas at different times of the trial *
| Area | T1 | T3 | T4 | T5 |
|---|---|---|---|---|
| 1 | 30·4 | 15·2 | 13·7 | |
| 2 | 76·7 | 55·1 | 32·8 | |
| 3 | 13·9 | 7·4 | ||
| 4 | 8·7 | 5·2 | ||
| 5 | 68 | 55 | 24 | 21·5 |
| 6 | 13·2 | 11·4 | 9·7 | 5·5 |
| 7 | 25·3 | 24·9 | 12·7 | |
| 8 | 38·1 | 23 | 17·6 | 15·7 |
| 9 | 20·5 | 20·1 | 11·9 | 9·7 |
| 10 | 11·7 | 11·6 | 5 | |
| 11 | 10·7 | 8·3 | 3·2 |
*Measures from clinical pictures IMAGE J Program analysis are in cm2.
Statistical analysis
The statistical analysis failed to show any significant correlation between the retraction rate and neither the patient age or the anatomical site (Table 4).
Table 4.
Median and IQR shrinkage rate at T4 versus T1
| Variable | Subgroup | Cases | Median (IQR)% T4 versus T1 |
|---|---|---|---|
| Overall | All patients | 10 | 51·62 (43·6–59·34) |
| Age | 10–18 years | 3 | 50 (48·1–54·02) |
| 30–60 years | 7 | 53·24 (39·01–65·8) | |
| Site | Axilla | 3 | 50 (42·65–60·92) |
| Neck | 4 | 56·5 (47·4–63·2) | |
| Lower limb | 3 | 46·19 (44·46–52·12) |
IQR, interquartile range.
Histology
All of the scar biopsies presented the typical histological aspect with a multilayered epidermis and little or no evidence of dermal–epidermal ridges (Figure 4A) and spare elastin fibres in the dermis (Figure 4B). Immunostaining showed a high content of type III collagen fibres and elastin (Figure 4C).
Figure 4.
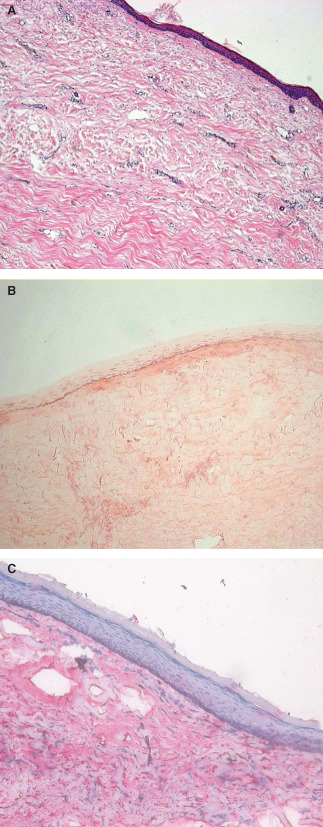
Scar biopsy. (A) Haematoxylin–eosin staining for tissue morphology. (B) Orcein staining for elastic fibres. (C) Immunostaining for type III collagen and haematoxylin counterstaining.
Twenty days after scar removal and Hyalomatrix® application, the regenerated superficial dermis appeared well organised. Biomaterial remnants were found deeply in the dermis (Figure 5A), surrounded by type I collagen fibres (Figure 5B) and, in some cases, covered by granulation tissue. At this time an autologous split skin graft was applied.
Figure 5.
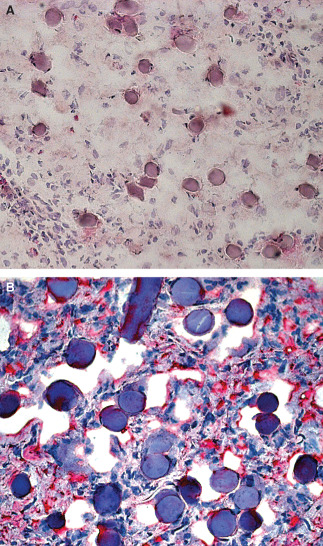
Regenerated dermis 20 days after Hyalomatrix® application. (A) 200× magnification haematoxylin–eosin staining. (B) 400× magnification type I collagen immunostaining; biomaterial remnants are still present in the dermis.
At 1 year's time a biopsy could be harvested in 3 wounds out of 11. Such specimens showed a much better skin‐like architecture than cutaneous scars, with a fully stratified and keratinised epidermis interdigitating with a dermis whose cellular density was remarkably reduced (Figure 6A). Immunohistochemical analysis confirmed full integration of the graft within the surrounding tissues with type VII collagen underlying the epidermis (Figure 6B). The extracellular matrix deposition and organisation were of high quality, too, with a higher elastic fibres type I collagen rate and a lower type III collagen, when compared with the scar tissue (Figure 6C–E).
Figure 6.
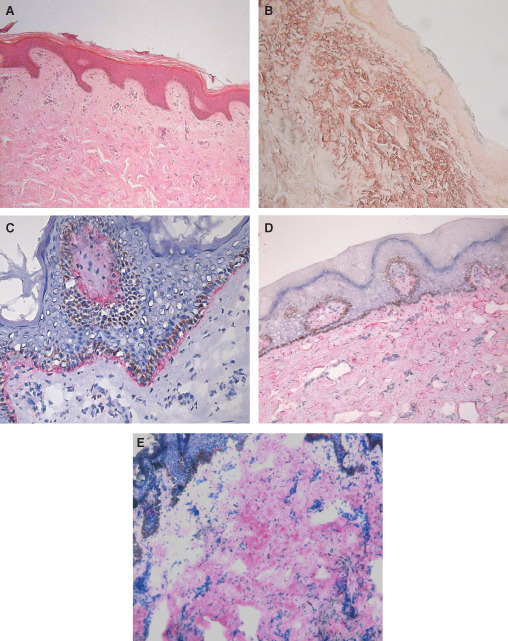
One year after autologous skin graft biopsy. (A) 100× magnification haematoxylin–eosin staining for tissue morphology. (B) 100× magnification orcein staining for elastic fibres and (C) 400× magnification type VII collagen immunostaining show a good integration within the surrounding tissues. (D) 100× magnification immunostaining for type I collagen. (E) 100× magnification immunostaining for type III collagen.
Discussion
The definition of the role of Hyaluronic acid is a milestone in the knowledge of wound healing process (5).
The semi‐synthetic insoluble polymer derived by the hyaluronic acid esterification with benzyl alcohol (HYAFF) has been in use in tissue engineering, regenerative medicine and clinical practice since 1999 6, 7.
Hyalomatrix®, a three‐dimensional acellular matrix‐based compound made by a layer of a hyaluronic acid benzyl ester (HYAFF 11) and a transparent silicone membrane, proved to be a useful skin substitute in different clinical situations 8, 9, 10.
Our study on the sequential combination of Hyalomatrix® with an autologous thin skin graft aimed two goals: first, the long‐term histological pattern in a human experimental wound model, not affected by any bias from poor wound healing or poor general clinical conditions; second, the eventual contracture rate.
Our histological observations show that Hyalomatrix® promotes the growth of a connective tissue histochemically similar to normal dermis within the first 3 weeks of application. Actually, at this stage the histological analysis shows biomaterial remnants surrounded by well‐organised type I collagen fibres. One year after autologous thin skin grafting, well‐defined dermal papillae can be appreciated and the presence of type VII collagen shows a good morphological and functional interaction between the regenerated dermis and the overlying epidermis. In the long‐term biopsies, the high prevalence of type I collagen fibres versus type III collagen fibres and the presence of rare elastic fibres in the regenerated dermis are both suggestive for a tissue more similar to normal skin than to scar.
The clinical data match with the histological ones, too: 1 year after autologous split skin graft cover of Hyalomatrix®‐induced dermis the regenerated skin looks soft and pliable, stable and trauma resistant.
Nevertheless, the severe contracture rate of the regenerated skin remains an unsolved issue.
Failure in showing any statistical significant correlation between the shrinkage rate and the clinical parameters (age and anatomical site) is related to the short numbers of our sample featuring subtle inter‐record differences.
Actually, the IMAGE J Program, used to measure scar retraction, could provide only an approximation of the actual datum. Nevertheless, this method added an objective score to a clinically appreciable evidence and therefore the margin for error in featuring a three‐dimensional reality into a bi‐dimensional picture could be considered non essential in such a case.
Scar retraction is the most disabling complication of any wound healing process. The gold standard in the treatment of scar retraction is surgical excision of the scar followed by flap reconstruction. Unfortunately, flap surgery is not always possible, particularly when adequate flap donor sites are not available for replacement of large areas of skin scarring or in patients in poor general clinical conditions where major surgery is contraindicated.
The alternative use of split skin grafts is an easy technique that can provide large amount of healthy skin after scar removal. Nevertheless, the long‐term result for a skin graft is generally poor, due to secondary contraction. The relative thickness of the dermis seems to play a crucial role in preventing contracture formation (11), therefore full thickness skin grafts would represent a fair alternative to the thin ones, but their use is limited to small areas.
The quality of the recipient bed is also of great importance for the eventual outcome of a split skin graft. Many studies already showed the ability of a dermal equivalent, such as collagen membranes and acellular dermal matrix, in preventing the massive skin scarring and related poor elasticity in wounds repaired with skin grafts 12, 13, 14.
In our sample the scarred areas to be treated were too large for any available local or distant flap reconstruction. Neither a full thickness skin graft was not an appropriate solution in all of the cases both for the large requirements of healthy skin and for the related shortage of donor sites.
Within the large dermal equivalent family, the hyaluronic acid‐based biomaterials seem to work as scaffolds for fibroblast and keratinocyte proliferation 15, 16.
To date, the role of hyaluronic acid in the mechanism of collagen contraction is still being investigated. Recent in vitro experimental data show that the hyaluronan may have opposite effects on collagen gel contraction, according to its molecular weight and to exogenous versus endogenous origin (17). Another recent experimental animal study seems to show that the addition of hyaluronic acid after transplantation of porcine acellular dermal matrix may boost the expression of type I and III collagen, thus reducing the eventual contraction of skin grafts (18).
In our experience Hyalomatrix® proved to be a good and reliable dermal substitute, promoting the regeneration of a skin‐like tissue, clinically and histologically better than scarred skin although still unable to control the eventual collagen contraction.
Acknowledgements
The authors wish to acknowledge the liberal ‘Mario Ballestra’ memorial grant to the Humana Forma non profit socially useful organisation which partially support this study. The authors wish to thank Alberto Malovini, PhD, Department of Computer Engineering and Systems Science, Salvatore Maugeri Research and Care Institute, University of Pavia, Pavia, Italy, for his help with the statistical analysis. The authors have no financial relationships that might pose a conflict of interest with the products mentioned in this article.
References
- 1. Gravante G, Delogu D, Giordan N, Morano G, Montone A, Esposito G. The use of Hyalomatrix PA in the treatment of deep partial‐thickness burns. J Burn Care Res 2007;28:269–74. [DOI] [PubMed] [Google Scholar]
- 2. Vaiude P, Anthony ET, Soranzo C, Myers SR. Hyalomatrix®– a novel dermal pre‐treatment. J Plast Reconstr Aesthet Surg 2007;60:S14. [Google Scholar]
- 3. Gravante G, Sorge R, Merone A, Tamisani AM, Di Lonardo A, Scalise A, Doneddu G, Melandri D, Stracuzzi G, Onesti MG, Cerulli P, Pinn R, Esposito G. Hyalomatrix PA in burn care practice: results from a national retrospective survey, 2005 to 2006. Ann Plast Surg 2010;64:69–79. [DOI] [PubMed] [Google Scholar]
- 4. Collins TJ. ImageJ for microscopy. Biotechniques 2007;43(1 Suppl):25–30. [DOI] [PubMed] [Google Scholar]
- 5. Chen WYJ, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen 1999;7:79–89. [DOI] [PubMed] [Google Scholar]
- 6. Myers SR, Partha VN, Soranzo C, Price RD, Navsaria HA. Hyalomatrix: a temporary epidermal barrier, hyaluronan delivery, and neodermis induction system for keratinocyte stem cell therapy. Tissue Eng 2007;13:2733–41. [DOI] [PubMed] [Google Scholar]
- 7. Vindigni V, Cortivo R, Iacobellis L, Abatangelo G, Zavan B. Hyaluronan benzyl ester as a scaffold for tissue engineering. Int J Mol Sci 2009;10: 2972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vindigni V, Bassetto F, Abatangelo S, Pandis L, Lancerotto L, Zavan B, Brocco E, Abatangelo G. Temporary coverage of a forehead defect following tumor resection with a hyaluronic acid biological dressing: a case report. Ostomy Wound Manage 2011;57:56–60. [PubMed] [Google Scholar]
- 9. Scuderi N, Anniboletti T, Carlesimo B, Onesti MG. Clinical application of autologous three‐cellular cultured skin substitutes based on esterified hyaluronic acid scaffold: our experience. In Vivo 2009;23:991–1003. [PubMed] [Google Scholar]
- 10. Perrot P, Dellière V, Brancati A, Duteille F. Hyalomatrix PA® in skin substitutes. About 10 cases. Ann Chir Plast Esthet 2011;56:7–11. [DOI] [PubMed] [Google Scholar]
- 11. Corps BV. The effect of graft thickness, donor site and graft bed on graft shrinkage in the hooded rat. Br J Plast Surg 1969;22:125–33. [DOI] [PubMed] [Google Scholar]
- 12. Cuono CB, Langdon R, Birchall N, Barttelbort S, McGuire J. Composite autologous‐allogenic skin replacement: development and clinical application. Plast Reconstr Surg 1987;80:626–37. [DOI] [PubMed] [Google Scholar]
- 13. Langdon RC, Cuono CB, Birchall N, Madri JA, Kuklinska E, McGuire J, Moellmann GE. Reconstitution of structure and cell function in human skin grafts derived from cryopreserved allogenic dermis and autologous cultured keratinocytes. J Invest Dermatol 1988;91:478–85. [DOI] [PubMed] [Google Scholar]
- 14. Leffler M, Horch RE, Dragu A, Bach AD. The use of the artificial dermis (Integra®) in combination with vacuum assisted closure for reconstruction of an extensive burn scar – a case report. J Plast Reconstr Aesthet Surg 2010;63:e32–5. [DOI] [PubMed] [Google Scholar]
- 15. Brun P, Cortivo R, Zavan B, Vecchiato N, Abatangelo G. In vitro reconstructed tissues on hyaluronan‐based temporary scaffolds. J Mater Sci Mater Med 1999;10:683–8. [DOI] [PubMed] [Google Scholar]
- 16. Price RD, Das‐Gupta V, Leigh IM, Navsaria HA. A comparison of tissue‐engineered hyaluronic acid dermal matrices in a human wound model. Tissue Eng 2006;12:2985–95. [DOI] [PubMed] [Google Scholar]
- 17. Allison DD, Braun KR, Wight TN, Grande‐Allen KJ. Differential effects of exogenous and endogenous hyaluronan on contraction and strength of collagen gels. Acta Biomater 2009;5:1019–26. [DOI] [PubMed] [Google Scholar]
- 18. Zhao JY, Chai JK, Song HF, Sun TJ, Li DJ, Liu LY, Gao QW, Liang LM. Effects on collagen I and III after transplantation of porcine acellular dermal matrix with hyaluronic acid. Zhonghua Yi Xue Za Zhi 2011;91:1276–80. [PubMed] [Google Scholar]


