Abstract
Negative pressure wound therapy (NPWT) is in widespread use and its role in wound care is expanding worldwide. It is estimated that 300 million acute wounds are treated globally each year. Currently, sporadic data exist to support NPWT in acutely contaminated wounds. Despite lack of data, use of negative pressure wound therapy in such cases is increasing across the globe. We retrospectively reviewed 86 consecutive patients, totalling 97 contaminated wounds. All wounds were Class IV based on US Center for Disease Control criteria. Sepsis criteria were present in 78/86 (91%) of patients. All patients were managed with NPWT. Wound type, degree of tissue destruction, presence of infection, wound dimension, timing of initial NPWT, type and timing of wound closure and patient comorbidities were recorded. Outcome endpoints included durability of wound closure and death. Wound location was 41/97 (42%) in the torso; 56/97 (58%) at the extremities. Tissue necrosis was present in 84/97 (87%) of wounds. Infection was present in 86/97 (89%) of wounds. Average wound size was 619 cm2 when square surface area measured; 786 cm3 when volume measurements taken. Mean time to wound closure was 17 days, median 10 days and mode 6 days. Durability of wound closure 73/79 (92%). Deaths were noted in 6/86 (7%) of patients. No deaths appeared related to NPWT. Contemporary NPWT related acute wound care is expanding empirically, in quantity and scope across the globe. However, several areas of concern are known regarding this contemporary use of NPWT in acute wounds. Thus, it is important to assess the safety and efficacy of such expanded empiric NPWT practice. Based on our findings with NPWT in the largest known patient cohort of this type, NPWT appears safe and effective in managing acute, contaminated wounds including patients meeting sepsis criteria. These findings provide evidence‐based support for current worldwide empiric NPWT‐related acute wound care.
Keywords: Contaminated, Negative pressure wound therapy
INTRODUCTION
Negative pressure wound therapy (NPWT) came into widespread use in 1997 after the publication of a subatmospheric wound care technique (1). Foam or gauze is placed into the wound which is then covered by a plastic drape and the system set to suction via tubing. The Vacuum Assisted Closure (V.A.C.®) device [Kinetic Concepts Inc. (KCI), San Antonio, TX], is the most widely available commercial product, though at least nine proprietary products exist worldwide2, 3.
NPWT in wound care is in wide clinical use. Over 338 million V.A.C.® therapy days are reported worldwide during a 2‐year period, 2009–2011, (c/o Steve Jackson/KCI, telephone conversation 7/1/2011). Yet the exact mechanisms of action of NPWT in wound healing are yet to be fully elucidated (3). Evidence to date supports four main processes: decreasing oedema results in lessened tissue pressure and improved capillary perfusion; increasing vascularity enhances oxygenation and nutrient delivery; removing wound area inflammatory proteases and cytokines causes favourable alterations in the biochemical cascade; and mechanical wound deformation causes tissue strain and resultant cellular proliferation 2, 3, 4, 5.
To date, nine randomised control trials (RCT) of NPWT in wound care exist (n = 429); however, the methodological quality of these studies is questioned because of varied outcome definitions and endpoints 3, 6, 7, 8, 9, 10, 11, 12, 13, 14. Several studies note surrogate wound closure variables of decreased wound dimension and/or presence of granulation tissue, rather than complete wound closure itself 8, 9, 10. The US Food and Drug Administration (FDA) notes the latter as ‘the most clinically meaningful’ wound endpoint and states that ‘the clinical benefit of incremental wound size changes has not been established’(15). Of note, only 3/7 of the RCT studies reported a significantly faster time to complete wound closure, development of granulation tissue or reduction in wound surface area 8, 9, 11. Hence, evidence‐based conclusions relating to wound closure endpoints are lacking regarding the clear benefit of NPWT compared to contemporary non NPWT wound care.
Despite the lack of evidence‐based data relating to wound closure, NPWT continues to grow into an integral part of current empiric wound care practice. Total revenues for KCI were 700 million dollars (US) in 2004, increasing to 2·02 billion dollars (US) in 2010 3, 16. The amplified use is probably because of the documented (but non wound closure related) benefits of NPWT. These include ease of use, patient comfort via decreased dressing changes, control of wounds with increased secretions, and facilitated outpatient care 2, 6, 17, 18. In addition, the empiric use of NPWT in complex wounds is increasingly reported as related to military conflicts (e.g. wars in Iraq and Afghanistan), terrorist attacks (e.g. 2005 London Underground Transport Network bombing) and natural disasters (e.g. 2005 Southeast Asia tsunami) 19, 20, 21, 22, 23. Furthermore, a specific benefit of NPWT in transporting patients with complex acute traumatic war wounds is reported and undergoing formal investigation by the US military (22).
Not surprisingly, with the increased use of NPWT in the industrialised world, acceptance of this technique in ‘least developed countries' (as defined by the United Nations based on socio‐economic criteria) is occurring, as well 7, 24. However, lack of resources for sterile supplies and lack of comprehensive wound management in these settings raises reasonable concern about the growing empiric use of NPWT in such acute wound care.
With a lack of clearly defined outcome data for NPWT in acute wound care, but with a rapidly expanding, but empiric global practice occurring, the aim of this study was to assess the safety and efficacy of NPWT, used in a consecutive series of patients with acute, contaminated wounds. Importantly, the patient cohort in this study mirrors currently expanded, worldwide, empiric NPWT acute wound care. Specifically, the wounds in this patient cohort were all contaminated and of large dimension, and thus analogous to wounds from war injuries, terrorism, natural disasters and the environment of ‘least developed countries'.
Of note, the vast majority of patients in this cohort meeting sepsis criteria define the acuity of these wounds; and it corroborates the patient cohort being evaluated. Extrapolating the results of this study can guide assessments of the safety and efficacy of empirically expanding worldwide practices of NPWT in acute wound care.
METHODS
Study population
Acute wounds created ≤24 hours prior to, or subsequent to the time of hospital admission, and requiring surgical intervention. Wound aetiologies were traumatic, infectious, or post surgical (Table 1). Presence of wound contamination was required (e.g. tissue necrosis and/or infection). Thus, all wounds were Class IV based on US Center for Disease Control (CDC) criteria (25). ‘Open abdomen’ wounds, that is, defects below the level of the midline fascia were excluded.
Table 1.
Patient and wound cohort characteristics with outcomes
Databases
A single institution, single surgeon's records were used for data collection. This methodology limited variables in wound care management. Inpatient and outpatient records were reviewed.
Wound care strategy
Indications for surgery followed practice standards of care. History, physical examination, laboratory and radiographic data were utilised in decision making. Evidence or concern for tissue necrosis, presence or concern for undrained and infected fluid (and not amenable/appropriate to lesser invasive approaches) and presence or concern for spreading/necrotising soft tissue infection prompted surgical intervention.
Surgical care was provided in the operating room under general anaesthesia or monitored anaesthesia care (MAC). Intraoperative care involved sharp, blunt and/or hydromechanical (Versajet™; Smith and Nephew, St. Petersburg, FL) resection of all grossly necrotic tissue. Complete evacuation of infected fluid was done. Intra‐operative pulse irrigation (InterPulse Powered Lavage System; Stryker, Kalamazoo, MI) was used in all cases except for one case involving the epidural space. Intra‐operative cultures (swab and/or tissue) were taken in cases where there was concern for deep space infection, spreading soft tissue infection, or resistant organisms.
Negative pressure wound therapy via the V.A.C.® system was used in all wounds except the few cases where cross‐covering physicians were involved (7/97 wounds). A gauze‐based negative pressure wound therapy was applied in these select cases, with a V.A.C.® instituted, or wound closure done thereafter.
All patients with concern for infection were treated with antibiotics. Specialists in infectious disease were utilised where resistant microbial organisms and/or osteomyelitis were involved.
The above wound care strategies were effected in similar fashion in each case. Care was provided predominantly by a single surgeon Board‐certified in General Surgery and Surgical Critical Care. Cross coverage was provided by a limited number of partnering surgeons.
Study design
This was a retrospective chart review of a consecutive series of patients between August 2004 and April 2011. A single institution and single surgeon's data set were involved. This methodology limited variables in wound care management. Study criteria were applied in assessing contiguous wound cases. Informed consent for surgery and the use of negative pressure wound therapy was obtained in all cases. Informed consent was obtained from patients or surrogates (if patient capacity was an issue). The study design was reviewed and accepted by the Christiana Care Health System's Institutional Review Board.
RESULTS
Wound location
Wounds involving the extremities, defined as distal to the axilla or inguinal crease, were present in 41/97 (42%). Wounds involving the torso, head and neck were 56/97 (58%). (Because of contiguity, two wounds involved a combination of extremity and torso locations.)
Degree of tissue destruction and/or infection
Tissue necrosis was present in 84/97 (87%) of wounds. Depth of necrosis within skin only was present in 0/84 wounds; skin and subcutaneous tissue in 13/84 wounds; skin, subcutaneous tissue and fascia in 38/84 wounds; skin, subcutaneous tissue, fascia and muscle in 21/84 wounds; and skin, subcutaneous tissue, fascia, muscle and bone in 8/84 wounds. Necrosis within muscle only (i.e. myositis) was present in 3/84 wounds.
Traumatic amputations from an inciting event were involved in 5/41 wounds. Subsequent amputation because of irrecoverable tissue loss and/or infection occurred in 3/41 extremity wounds. (Of these 1/3 did not have NPWT applied at initial presentation but rather after the amputation was effected on hospital day 4; and 2/3, based on concurring opinions at the time of initial evaluation (pre‐NPWT), were anticipated to need future amputation. Thus, precipitation of amputation from the use of NPWT seems unlikely). Refractory osteomyelitis was present in 1/41 wounds, but the patient declined a recommended amputation and was lost in follow‐up. Loss of function of the lower extremity was noted in 1/41 wounds, and amputation recommended, but the patient refused and was also lost in follow‐up. Overall 10/41 (24%) of extremity wounds involved an amputation or advisement thereof.
Infection was present in 86/97 (89%) of wounds. This was confirmed by wound purulence and/or cultures in 83/86 (97%). In order to standardise and compare initial wound assessments, cultures were reported during the narrow time interval noted (rather than at any time in the patient's hospitalisation). In 2/86 patients, clinical criteria for wound infection were present despite negative initial cultures. However, subsequent cultures were positive, corroborating initial clinical concern. In 1/86 cases, the patient did not have positive cultures, but he improved with evacuation of a clinically suspected infected haematoma and the use of broad‐spectrum systemic antibiotics.
Wound aetiology
Trauma was the aetiology in 25/97 (26%) of wounds; infection in 69/97 (71%) of wounds; and decubiti in 3/97 (3%) of wounds. Of those related to a primary infectious cause, 6/69 were post surgical, 7/69 were Fournier's gangrene, and 8/69 pyomyositis. An uncontrolled enteric fistula (present prior to the use of NPWT) was the aetiology in 1/97 cases. Bowel perforations led to 4/97 wounds.
Wound classification
All wounds, 97/97 (100%), met CDC Class IV criteria due to the presence of contamination: infection and/or tissue necrosis (25). Classification of the type of contamination was infection in 86/97 (89%) of wounds, (with or without associated necrosis); and tissue necrosis in 11/97 (11%), (without evidence of infection). All wounds with infection were treated with systemic antibiotics. All wounds with tissue necrosis required sharp/excisional debridement in the operating room.
Presence of systemic inflammatory response syndrome (SIRS)/degree of sepsis
Based on established international criteria: 4/86 (5%) of patients met SIRS criteria; 24/86 (28%) met sepsis criteria; 19/86 (22%) met severe sepsis criteria; and 31/86 (36%) met septic shock criteria 26, 27, 28. The cause of SIRS/sepsis was because of the acute wound aetiology (trauma or infection) in all cases (Figure 1).
Figure 1.
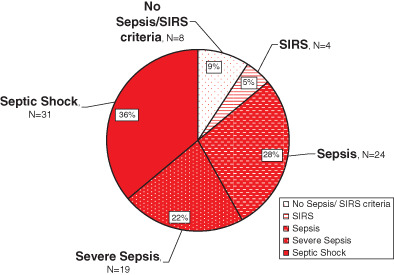
Patient characteristics re: systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock.
Wound size
The average surface area was 619 cm2 where length and width was measured. The average wound volume was 786 cm3 when length, width and depth measurements were taken. Note, because of the three‐dimensional characteristics of these wounds, including crevices, tunnelling and flaps, exact wound dimensions either cm2 or cm3 could not be obtained in all cases (Table 2).
Table 2.
Patient/wound characteristics *
| Patients (n = 86) | Number(%) | Wounds (n = 97) | Number (%) |
|---|---|---|---|
| Demographics | Aetiology | ||
| Male | 50 (58) | Infection | 69 (71) |
| Female | 36 (42) | Trauma | 25 (26) |
| Age, mean (SD), years | 52 (17) | Decubiti | 3 (3) |
| Risk factors | Classification of contamination | ||
| Cardiovascular | 48 (56) | Infection (with or without necrosis) | 86 (89) |
| Diabetes | 34 (40) | Necrosis (without infection) | 11 (11) |
| Obesity | 53 (62) | ||
| Tobacco | 13 (15) | Location | |
| Upper extremity | 8 (8) | ||
| Degree of sepsis | Torso | 41 (42) | |
| Sepsis | 24 (28) | Lower extremity | 48 (50) |
| Severe sepsis | 19 (22) | Combined areas | 2 (2) |
| Septic shock | 31 (36) | ||
| SIRS | 4 (5) | Size | |
| Ø sepsis/SIRS criteria | 8 (9) | Mean | 617 cm2(53 wounds) |
| 786 cm3(43 wounds) | |||
| 45 cm linear (1 wound) | |||
| Upper extremity | |||
| Mean (SD ± 1) | 637 cm2(608–666) | ||
| 1095 cm3(279–1911) | |||
| Torso | |||
| Mean (SD ± 1) | 754 cm2(502–1005) | ||
| 830 cm3(636–1024) | |||
| Lower extremity | |||
| Mean (SD ± 1) | 550 cm2(351–748) | ||
| 583 cm3(497–690) |
SD, standard deviation; SIRS, systemic inflammatory response syndrome.
*Note that, because of the three‐dimensional characteristics of these wounds, including crevices, tunnelling and flaps, exact wound dimensions of either cm2 or cm3 could not be obtained in all cases.
Timing of NPWT
The timing of initial placement of the NPWT was at the first surgical intervention (T‐0) in 81/97 wounds; at the second surgical intervention (T‐1) in 9/97 wounds; at the third surgical intervention (T‐2) in 6/97 wounds; and at the fourth surgical intervention (T‐3) in 1/97 wounds.
Timing for placement of the NPWT, if done other than (T‐0), was because of partnering physicians providing the initial surgical intervention and not electing to institute NPWT at that time. With wound contamination, and/or infection present in re‐evaluation, these cases met inclusion criteria for this study. Timing of NPWT was then noted.
The V.A.C.® polyurethane system was used in 96/97 wounds, and the polyvinyl sponge in 1/97 wounds. Polyvinyl sponges were used when there was exposed bowel. Gauze‐type NPWT was used in 7/97 wounds (elected by partnering physicians at the time of their surgical intervention). In 6/7 of these cases, foam‐based NPWT was used at the subsequent surgical intervention. The remaining patient had subsequent delayed primary closure.
Dressing change status post initial placement
Timing of NPWT dressing changes was determined based on current clinical standards combined with individual patient and wound assessments 6, 17, 28. Because of the presence of tissue necrosis and/or infection in the initial stages of these cases, the NPWT system was changed more frequently during the early phase of wound care. The average time to NPWT change post initial placement was 2·9 days.
Purulence
Purulence was present in 62/97 (64%) of wounds. This was noted as a separate wound parameter irrespective of any cultures that were taken. Purulence was defined by typical appearance, and recorded as present if there was at least enough volume to be collected by syringe.
Wound culture
Wounds were cultured if there was concern for infection based on clinical evaluation; hence, not all wounds were cultured (e.g. traumatic wounds at initial intervention). In order to corroborate the acuity of these wounds, to clarify their features at initial evaluation, and to standardise wound assessments, cultures were recorded at the initial time of surgery and placement of NPWT (time interval defined as cultures ≤48 hours prior to or ≤24 hours after the initial surgery and use of NPWT). Cultures were positive in 62/69 (90%) of wounds (Figure 2).
Figure 2.
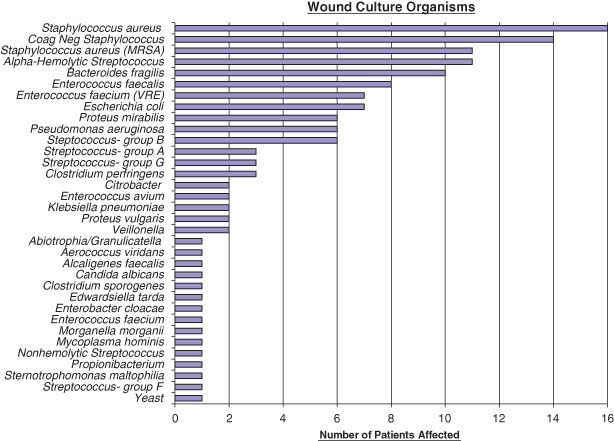
Wound culture organisms and number of patients affected (Corynebacterium also found in 14 wounds; but not felt to be a causative organism).
Type and timing of wound closure
Wounds were closed with delayed primary suturing, split thickness skin grafts and/or secondary intention healing. The average time to closure was 17·1 days, median 10 days, and mode 6 days.
Wounds excluded from the time‐to‐closure calculation were as follows: 4/97 because of patient death prior to wound closure; 2/97 because of comorbidities delaying wound closure (e.g. presence of malignancy and cerebrovascular accident); and 7/97 secondary intention ‘closures’, (i.e. due to lack of accepted published criteria for defining wound ‘closure’ in secondary intention healing) 3, 15. Additional exclusions included 2/97 wounds lost in follow up and 1/97 wound wherein the patient refused split‐thickness skin graft.
Comorbidities
Patient comorbidities were recorded based on a physiologic systems approach. Comorbidities were defined as a system abnormality that predated the current hospital admission. Obesity, if present, was noted as well and body mass index recorded.
Note, specific acute organ system abnormalities were not recorded in the Supporting information (unless contributing to a patient's death), as their presence was represented in the diagnosis of severe sepsis and/or septic shock.
Outcome
Wound related complications
Wound complications were defined as either a delayed primary suture closure that required wound reopening, or a partial take of a split‐thickness skin graft. Wound complications were present in 6/81 (8%) of cases. (Reference the above for the excluded wounds).
Deaths
Patient deaths were noted in 6/86 (7%) and as follows: 2/6 as a result of continued patient deteriorations from established comorbidities (e.g. fulminant calciphylaxis and refractory myelodysplastic syndrome); 2/6 related to irrecoverable septic shock (shock state present at the time of admission and prior to wound intervention); 1/6 deaths occurred on hospital day 22 as a result of a pulmonary embolus and myocardial infarction; 1/6 deaths occurred on hospital day 57 as a result of cardiopulmonary complications from chronic obstructive pulmonary disease and coronary artery disease. No deaths appeared directly related to NPWT.
DISCUSSION
Negative pressure wound therapy is in widespread use and its role in wound care worldwide is rapidly expanding 7, 16, 17, 19, 20, 21, 22, 23, 28, 29, 30, 31 FDA indications for NPWT are ‘chronic, acute, traumatic, subacute and dehisced wounds, partial‐thickness burns, ulcers (such as diabetic or pressure), flaps and grafts' (32). However, several areas of concern arise in the contemporary use of NPWT in acute wounds. First, acute wounds often have tissue necrosis. Empiric practice of NPWT includes such cases, though published contraindications include wounds with necrosis/eschar 29, 30, 33. Second, acute wounds are often of infectious aetiology; yet, current published literature does not confirm decreasing bacterial loads in wounds managed with NPWT 9, 34, 35, 36. Third, case reports exist for infection/sepsis occurring with the use of NPWT in acute wounds 7, 21, 37. Fourth, acute wounds caused from war, terrorism and natural disasters are often caused by significant force, resulting in large wounds; however, there is limited published literature of NPWT in such cases 19, 20, 21, 22, 23. Lastly, NPWT in the Third World is expanding, proposed as being of ‘great value in treating severe wounds in underdeveloped countries' (7). However, lack of resources for comprehensive acute wound care, lack of sterile supplies, loss to follow‐up and lack of published data in this setting are noteworthy.
Our study aimed to assess many of the above concerns. The wounds depicted in this series were all contaminated, and the vast majority infected. Many of the traumatic extremity wounds were created by such force that either amputation had occurred, or was required subsequently. Wounds of infectious aetiology were typically complex, polymicrobial, necrotising soft tissue processes. Average wound dimensions were large. Thus, the wound cohort in this study mirrors current empiric, global acute wound NPWT practice.
Limitations to our study include the lack of a control group; yet, publications to date reflect the inherent difficulty of prospective, randomised trials in wound care 3, 6, 7, 8, 9, 10, 11, 12, 13, 14. Though other observational study designs might have been used to compare wound care practices, our aim was not to document wound care superiority with NPWT. Rather, it was to review and assess the safety and efficacy of current empiric, expanding NPWT‐related acute wound care practice, including our own. Separately, from a patient focused care perspective, there were practical needs to apply NPWT as opposed to non NPWT care in this patient cohort. For example, use of gauze‐based wet‐to‐dry dressing changes would be comparatively painful; and frequent gauze dressing changes would have required higher levels of care because of increased needs of sedatives/analgesics and monitoring 38, 39. In this regard, the large wound sizes and deep wound spaces of this patient cohort, mean wound sizes 617 cm2 and 786 cm3, were of particular concern. (Of note, a recent publication addressing pain level in gauze‐based wound care compared to NPWT did not find less pain with NPWT. Yet that patient cohort was significantly different in having much smaller wound sizes: median size 4 cm in that cohort compared to 400 cm in this cohort). Another potential limitation relates to the heterogeneity of wound locations (albeit limited to the torso and extremities) and aetiology (traumatic and infectious). Yet with our stated aim it was important to include the heterogeneity described as it mirrors current empiric global NPWT‐related acute wound care practice. Lastly, difficulty in quantifying fluid losses from large, open (wet‐to‐dry gauze‐packed) wounds predisposes patients to renal insufficiency/failure, whereas NPWT accurately accounts for fluid losses. (Published acute wound care literature does not address pre‐renal azotaemia. Yet, published cases have denoted much smaller wound sizes than our cohort, and so fluid shifts and pre‐renal azotaemia expectedly of much less concern) 8, 9, 10, 12, 13, 40. From this perspective, our study may in fact reflect a previously undescribed benefit of NPWT in large wounds.
Published data to date do not show clear outcome benefits for NPWT compared to contemporary non NPWT related wound care 3, 6. However, published and accepted non outcome related benefits of NPWT validate its use 2, 6, 17. Contemporary NPWT related acute wound care is expanding empirically, in quantity and scope across the globe. Yet, several areas of concern are known regarding this contemporary expanded use of NPWT in acute wounds. Thus, it is important to assess the safety and efficacy of such expanded empiric, global NPWT practice. Based on our findings with NPWT in the largest known patient cohort of this type, NPWT appears safe and effective in managing acute, contaminated wounds including patients meeting sepsis criteria.
ACKNOWLEDGEMENTS
Sincere thanks go to Linda M. Donahue for her literary guidance in reviewing this manuscript. Authors declare there are no conflicts of interest; this paper has not been previously presented or published; and the authors have received no funding from any source.
REFERENCES
- 1. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 2. Negative pressure wound therapy . Oregon: The Health Resources Commission: Office of Oregon Health Policy and Research, 2010 [WWW document]. URL http://www.oregon.gov/OHA/OHPR/HRC/docs/HRC.Reports/HRC_NPWT_Final_SC_Draft.pdf?ga=t [accessed on 12 August 2011].
- 3. Gregor S, Maegele M, Sauerland S, Krahn JF, Peinemann F, Lange S. Negative pressure wound therapy: a vacuum of evidence? Arch Surg 2008;143:189–96. [DOI] [PubMed] [Google Scholar]
- 4. Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanisms of action of vacuum assisted closure: more to learn. Surgery 2009;146:40–51. [DOI] [PubMed] [Google Scholar]
- 5. Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum‐assisted closure device. Plast Reconstr Surg 2008;122:786–97. [DOI] [PubMed] [Google Scholar]
- 6. Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum‐assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006;118:390–7. [DOI] [PubMed] [Google Scholar]
- 7. Perez D, Bramkamp M, Exe C, von Ruden C, Ziegler A. Modern wound care for the poor: a randomized clinical trial comparing the vacuum system with conventional saline‐soaked gauze dressings. Am J Surg 2010;199:14–20. [DOI] [PubMed] [Google Scholar]
- 8. Joseph E, Hamori C, Bergam S, Roaf E, Swann N, Anastasi G. A prospective randomized trial of vacuum‐assisted closure versus standard therapy of chronic nonhealing wounds. Wounds 2000;12:60–7. [Google Scholar]
- 9. Moues CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004;12:11–7. [DOI] [PubMed] [Google Scholar]
- 10. Wanner MB, Schwarzl F, Strub B, Zaech GA, Pierer G. Vacuum‐assisted wound closure for cheaper and more comfortable healing of pressure sores: a prospective study. Scand J Plast Reconstr Surg Hand Surg 2003;37:28–33. [DOI] [PubMed] [Google Scholar]
- 11. Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative‐pressure wound dressings for diabetic foot wounds. Ann Vasc Surg 2003;17:645–9. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong DG, Lavery LA, for the Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 13. Ford CN, Reinhard ER, Yeh D, Syrek D, De Las Morenas A, Bergman SB, Williams S, Hamori CA. Interim analysis of a prospective, randomized trial of vacuum‐assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg 2002;49:55–61. [DOI] [PubMed] [Google Scholar]
- 14. Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004;114:917–22. [DOI] [PubMed] [Google Scholar]
- 15. FDA Wound Healing Clinical Focus Group. Guidance for industry: chronic cutaneous ulcer and burn wounds‐developing products for treatment. Wound Repair Regen 2001;9:258–68. [DOI] [PubMed] [Google Scholar]
- 16. Kinetic Concepts Inc . (KCI) annual report [WWW document]. URL http://quicktake.morningstar.com/StockNet/SECDocuments.aspx?symbol=KCI [accessed on 8 August 2011].
- 17. Argenta LC, Morykwas MJ, Marks MW, DeFranzo AJ, Molnar JA, David LR. Vacuum‐assisted closure: state of clinic art. Plast Reconstr Surg 2006;117:127S–42S. [DOI] [PubMed] [Google Scholar]
- 18. Herscovici D Jr, Sanders RW, Scaduto JM, Infante A, DiPasquale T. Vacuum‐assisted wound closure (VAC therapy) for the management of patients with high‐energy soft tissue injuries. J Orthop Trauma 2003;17:683–8. [DOI] [PubMed] [Google Scholar]
- 19. Leininger BE, Rasmussen TE, Smith DL, Jenkins DH, Coppola C. Experience with wound VAC and delayed primary closure of contaminated soft tissue injuries in Iraq. J Trauma 2006;61:1207–11. [DOI] [PubMed] [Google Scholar]
- 20. Maegele M, Gregor S, Steinhausen E, Bouillon B, Heiss MM, Perbix W, Wappler F, Rixen D, Geisen J, Berger‐Schreck B, Schwarz R. The long‐distance tertiary air transfer and care of tsunami victims: injury pattern and microbiological and psychological aspects. Crit Care Med 2005;33:1136–40. [DOI] [PubMed] [Google Scholar]
- 21. Marsh DJ, Abu‐Sitta G, Patel H. The role of vacuum‐assisted wound closure in blast injury. Plast Reconstr Surg 2007;119:1978–9. [DOI] [PubMed] [Google Scholar]
- 22. Powell ET IV. The role of negative pressure wound therapy with reticulated open cell foam in the treatment of war wounds. J Orthop Trauma 2008;22:S138–41. [DOI] [PubMed] [Google Scholar]
- 23. Rispoli DM, Horne BR, Kryzak TJ, Richardson MW. Description of a technique for vacuum‐assisted deep drains in the management of cavitary defects and deep infections in devastating military and civilian trauma. J Trauma 2010;68:1247–52. [DOI] [PubMed] [Google Scholar]
- 24. United Nations Publications, Department of Public Information . Yearbook of the United Nations 2004: A more secure world – our shared responsibilities [WWW document]. URL www.un.org/secureworld/report3.pdf [accessed on 8 August 2011].
- 25. Guideline to prevention of surgical site infection . Centers for Disease Control and Prevention, 1999 [WWW document]. URL http://www.cdc.gov/hicpac/SSI/table7‐8‐9‐10‐SSI.html [accessed on 8 August 2011].
- 26. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ, ACCP/SCCM Consensus Conference Committee. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine 1992. Chest 2009;136:e28. [DOI] [PubMed] [Google Scholar]
- 27. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/ SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530–8. [DOI] [PubMed] [Google Scholar]
- 28. Firman G. The sequential organ failure assessment (SOFA) score 2009 [WWW document]. URL http://www.medicalcriteria.com/site/index.php?option=com_content&view=article&id=266%3Autisofa&catid=47%3Acritical‐care&Itemid=80&lang=en [accessed on 11 August 2011].
- 29. Gabriel A, Shores J, Bernstein B, Leon JD, Kamepalli R, Wolvos T, Baharestani MM, Gupta S. A clinical review of infected wound treatment with vacuum assisted closure (V.A.C.) therapy: experience and case studies. Int Wound J 2009;6(2 Suppl):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Attinger CE, Janis JE, Steinberg J, Schwartz J, Al‐Attar A, Couch K. Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound‐healing adjuvants. Plast Reconstr Surg 2006;117:72S–109S. [DOI] [PubMed] [Google Scholar]
- 31. Preston G. An overview of topical negative pressure therapy in wound care. Nurs Stand 2008;23:62–4. [DOI] [PubMed] [Google Scholar]
- 32. US Department of Health & Human Services . Agency for Healthcare Research and Quality. Technology assessment: negative pressure wound therapy devices [WWW document]. URL http://www.ahrq.gov/clinic/ta/negpresswtd/npwtd02.htm [accessed on 23 August 2011].
- 33. Capobianco CM, Zgonis T. An overview of negative pressure wound therapy for the lower extremity. Clin Podiatr Med Surg 2009;26:619–31. [DOI] [PubMed] [Google Scholar]
- 34. Assadian O, Assadian A, Stadler M, Diab‐Elschahawi M, Kramer A. Bacterial growth kinetic without the influence of the immune system using vacuum‐assisted closure dressing with and without negative pressure in an in vitro wound model. Int Wound J 2010;7:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boone D, Braitman E, Gentics C, Afthinos J, Latif J, Sordillo E, Todd G, Lantis JC. Bacterial burden and wound outcomes as influenced by negative pressure wound therapy. Wounds 2010;22:32–7. [PubMed] [Google Scholar]
- 36. Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg 2004;52:276–9. [DOI] [PubMed] [Google Scholar]
- 37. Gwan‐Nulla DN, Casal RS. Toxic shock syndrome associated with the use of the vacuum‐assisted closure device. Ann Plast Surg 2001;47:552–4. [DOI] [PubMed] [Google Scholar]
- 38. Stansby G, Wealleans V, Wilson L, Morrow D, Gooday C, Dhatariya K. Clinical experience of a new NPWT system in diabetic foot ulcers and post‐amputation wounds. J Wound Care 2010;19:496. [DOI] [PubMed] [Google Scholar]
- 39. Kaplan M, Daly D, Stemkowski S. Early intervention of negative pressure wound therapy using Vacuum‐Assisted Closure in trauma patients: impact on hospital length of stay and cost. Adv Skin Wound Care 2009;22:128–32. [DOI] [PubMed] [Google Scholar]
- 40. Ubbink DT, Vermeulen H, Goossens A, Kelner RB, Schreuder SM, Lubbers MJ. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg 2008;143:950–5. [DOI] [PubMed] [Google Scholar]