Abstract
This retrospective analysis included intent‐to‐treat control patient data from two published, randomised, diabetic foot ulcer (DFU) trials in an effort to differentiate ulcers that are unlikely to heal by 12 weeks despite early healing progress [≥50% percent area reduction (PAR) at 4 weeks]. Predicted and actual wound area trajectories in DFUs that achieved early healing progress were analysed from weeks 5 to 12 and compared for ulcers that did and did not heal at 12 weeks. In 120 patients who achieved ≥50% PAR by week 4, 62 (52%) failed to heal by 12 weeks. Deviations from the predicted healing course were evident by 6 weeks for non healing ulcers. A 2‐week delay in healing significantly lowered healing rates (P = 0·001). For DFUs with ≥50% PAR at 4 weeks, those achieving ≥90% versus <90% PAR at 8 weeks had a 2·7‐fold higher healing rate at 12 weeks (P = 0·001). A PAR of <90% at 8 weeks provided a negative predictive value for DFU healing at 12 weeks of 82%. For ulcers that fail to progress or worsen from weeks 4 to 6, and those that fail to achieve 90% PAR at 8 weeks, reevaluation of the wound and its treatment is recommended.
Keywords: Diabetic foot ulcer, Healing rates, Percent area reduction, Predicting failure to heal
INTRODUCTION
Delayed healing of diabetic foot ulcers (DFUs) can increase the risk of infection and likelihood of amputation (1) and negatively affect many aspects of daily living such as mobility and quality of life (2). After 4 weeks of standard wound care, few DFUs with <50% percent area reduction (PAR) heal by 12 weeks 3, 4, 5. On the basis of these findings, <50% PAR at 4 weeks is suggested as a clinical monitoring parameter to distinguish DFUs that will not heal within 12 weeks.
Being able to tell early in the treatment of a DFU whether a therapy is effective is pivotal and could affect patient outcomes 6, 7. However, about half of DFUs with early healing progress (at least 50% PAR by 4 weeks) do not heal by 12 weeks 3, 4, 5. It is unclear what changes in healing patterns occur during weeks 5–12 for DFUs that fail to heal by 12 weeks despite early healing progress.
We conducted post hoc analyses to better understand changes in the PAR of DFUs that did not heal despite good early healing progress. Control patient data were obtained from two previously published, randomised, controlled trials involving patients afflicted with non healing DFUs for 6 or more weeks 8, 9. DFUs with a minimum of 50% PAR by week 4 were analysed and the proportion of healed DFUs, healing patterns and median PAR from weeks 5 to 12 were determined. In addition, DFU healing at 12 weeks was evaluated based on interim progress at weeks 6, 7 and 8. The predictive value of applying a novel secondary prognostic marker at 8 weeks of standard care was evaluated.
RESEARCH DESIGN AND METHODS
Control subjects were enrolled for at least 12 weeks and received standard wound care consisting of DFU debridement, saline‐moistened gauze dressings to fill the ulcer, dry gauze, adhesive fixation sheets, therapeutic footwear and off‐weight bearing instructions. Ulcer area was determined using computer planimetry (determined from a bilayer acetate tracing, no depth measurement was taken) and photographs of the ulcer were taken as visual record of the wound site before and after treatment. Inclusion and exclusion criteria were previously published and are similar for each trial. Institutional review board approval was granted at each study site and the trials were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from participants in both trials.
Data were extracted from two databases containing patient identification numbers and wound planimetry measurements that were gathered by the clinical investigators. From these original data, PAR was calculated as the difference in ulcer area from baseline, divided by the baseline area and multiplied by 100. Only control group patient data were analysed and no investigative treatment group patient data were included. Only DFUs that had achieved at least 50% PAR from baseline to week 4, had not healed by 4 weeks, and had documented wound area measurements taken during weeks 5–12 were analysed in this study.
For the purposes of this analysis, non normally distributed variables are summarised by medians and interquartile ranges. Confidence intervals were calculated for proportions based on the binomial distribution (exact method). Curve fitting was conducted via non linear least squares regression. Differences in the proportions for healing at week 12 were evaluated using a 2 × 2 Fisher exact test. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for the dichotomous classifier of 90% PAR at weeks 6, 7 and 8. Exploratory hypothesis testing was conducted at α = 0·05. Statistical tests were executed using Minitab (version 15.1.20.0; Minitab, Inc., State College, PA) and MATLAB (version 7.6.0 R2008a; The Mathworks, Natick, MA).
RESULTS
Data from 120 patients with DFUs that achieved ≥50% PAR from weeks 0 to 4 were analysed. In all, 58 (48%) patients' DFUs had healed at 12 weeks; 62 (52%) patients had DFUs that failed to heal by 12 weeks. Wound area measurements taken at week 0 were similar for patients who healed and did not heal (median values 1·33 cm2 versus 1·26 cm2, respectively). Median ulcer areas from weeks 0 to 4 for healed and non healed ulcer groups were fitted to an exponential 12‐week curve. Ulcer area as measured for DFUs that successfully healed at 12 weeks closely followed a predicted healing curve during the entire course of the treatment period (Figure 1A). Non healed DFUs, on the other hand, deviated from the predicted healing curve soon after 4 weeks (Figure 1B) and appeared to stop healing at a relatively small size (0·2–0·4 cm2). For ulcers that did not reduce in size during the 2‐week period following week 4, healing at 12 weeks was less than half that for the ulcers that showed some reduction in size during this period (26·2% versus 60·3% healed, respectively; P = 0·001). If ulcer size did not reduce for a third week (weeks 4–7) healing rates were less than one third of that for patients whose wounds showed some progress during the same period (19·5% versus 63·3% healed, respectively; P < 0·001).
Figure 1.
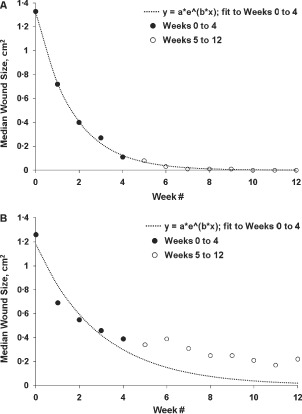
Scatter plots of median ulcer area versus time from diabetic foot ulcers that healed and did not heal by 12 weeks. Healed at 12 weeks (n = 58) (A). Non healed at 12 weeks (n = 62) (B). Fitted curves are exponential functions with negative constants (a t = a 0 *e−b*t). Fitting and analysis conducted via non linear least squares regression.
Comparison of the median PAR value by week was conducted to determine at what time point a differentiation in healing trajectories could be seen for the populations that healed versus those that did not heal. A separation between the interquartile bands for the healed and non healed wounds was observed at 90% PAR at 8 weeks (Figure 2). Further analyses were conducted to compare the predictive value of 90% PAR as a dichotomous classifier at treatment weeks up to and including week 8 in order to identify the earliest possible time point at which a reliable prognosis for healing could be made. The greatest diagnostic efficiency (sensitivity multiplied by specificity) for the 90% PAR threshold was observed at week 8 (Table 1). Using 90% PAR at 8 weeks as a prognostic marker of healing at 12 weeks provided a sensitivity of 66%, a specificity of 71% and a negative predictive value of 82%. Across patients who achieved ≥50% PAR at week 4, a subsequent achievement of ≥90% PAR at week 8 was associated with a 2·7‐fold higher healing rate (P = 0·001) at week 12 versus patients who achieved <90% PAR at week 8 (Figure 3). The number of patients included in the current dataset and their healing rates based on each of the prognostic categories mentioned above were tracked (Figure 4). The prognostic markers (50% PAR at 4 weeks and 90% PAR at 8 weeks) divided the patient populations into groups that were approximately equal in number.
Figure 2.
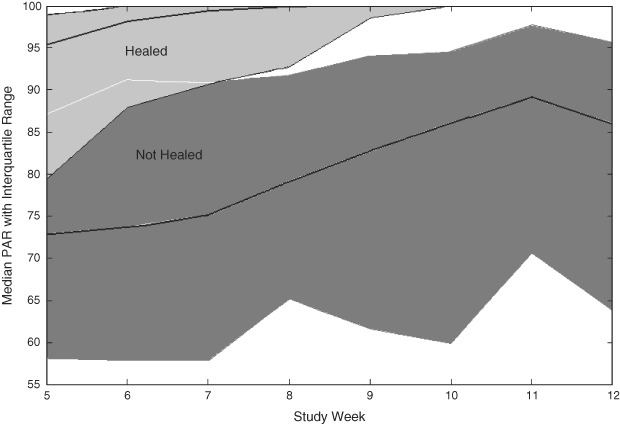
Percent area reduction (PAR) for diabetic foot ulcers (DFUs) that achieved ≥50% PAR at week 4 stratified by healed and non healed DFUs by week 12 (n = 120). Median PAR from week 5 presented as line plots of PAR (1st and 3rd quartile indicated in shaded areas) stratified by healed and non healed DFUs at week 12.
Table 1.
Predictive value of 90% change in wound area on healing outcomes for diabetic foot ulcers by treatment week
| 90% PAR | Sensitivity, % | Specificity, % | NPV, % | PPV, % |
|---|---|---|---|---|
| Week 6 | 59 | 74 | 73 | 60 |
| Week 7 | 58 | 73 | 78 | 51 |
| Week 8 | 66 | 71 | 82 | 51 |
PAR, percent area reduction; NPV, negative predictive value; PPV, positive predictive value.
Figure 3.
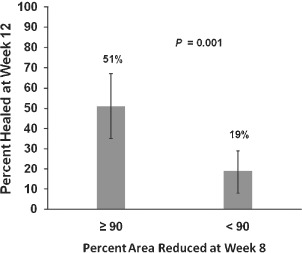
Healing rate at week 12 stratified by patients who achieved ≥90% (n = 19) and <90% (n = 10) percent area reduction (PAR) at week 8. After achieving ≥50% closure at week 4, a total of 51% of patients had diabetic foot ulcers (DFUs) that healed by week 12 after achieving ≥90% PAR at week 8 compared with 19% of patients with DFUs that achieved <90% PAR at week 8 (P = 0·001). Error bars represent 95% confidence intervals for the proportions.
Figure 4.
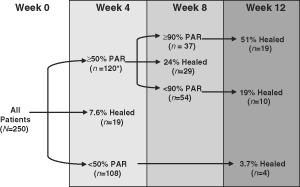
Number of patients and percent of healed wounds reported for patients who qualified for the various prognostic groups. PAR, percent area reduction. *Total does not add to 250 patients because data for 3 patients are not reported after week 4.
DISCUSSION
Ulcer healing is a highly complex and integrated process. These post hoc analyses show that a delay or worsening in healing can arise in a majority of patients despite the presentation of encouraging results in the early treatment period. The ability to predict whether a DFU is following versus not following a healing trajectory could dramatically alter patient outcomes by alerting the clinician to reassess the wound condition and consider an alternate treatment regimen (4). Thus, it is important to develop monitoring tools to identify patients with DFUs who may have early healing progress but who later fail to heal.
Studies have assessed the ability of initial DFU healing rates to predict healing failure. Data support the use of <50% PAR at 4 weeks as a negative predictor of healing at 12 weeks 3, 4, 5, 10. We found that DFU healing trajectories at weeks 5–12 were different for DFUs that went on to heal at 12 weeks versus those that did not heal, despite the fact that all the wounds studied had achieved ≥50% PAR by week 4. Wounds that did not heal demonstrated a slowing in the area reduced soon after 4 weeks. Furthermore, failure to reduce the ulcer area for 2 weeks or more resulted in substantially lower healing rates at 12 weeks. On the basis of these observations, we propose that diligence be observed in monitoring wound healing progress during the period following 4 weeks, regardless of the degree of success achieved up to this point. If a 2‐week delay in wound size reduction is observed, the wound should be reassessed for potential pathophysiological causes for the delay. In addition, a secondary assessment point of 90% at 8 weeks may differentiate between wounds that will and will not heal. Healing at 12 weeks was substantially greater for patients with ≥50% wound closure at 4 weeks who went on to achieve a second milestone of ≥90% PAR at 8 weeks compared with patients who achieved <90% PAR at 8 weeks. Sensitivity and specificity analyses showed that failure to achieve 90% PAR at 8 weeks is 82% predictive of identifying a non healing DFU, while achieving at least 90% PAR at 8 weeks is 51% predictive of identifying a healing DFU. The negative predictive power of the 8‐week/90% PAR assessment is similar to that reported by others for the negative prognostic measure of <50% PAR at 4 weeks 3, 11.
Using interim wound area assessment as an indicator of DFU healing tends to provide greater power for predicting non healing wounds than healing wounds. This is because data used to develop these tools comes from populations where the overall rate of healing is low. It may be possible to examine a higher PAR cutoff in order to derive a greater positive predictive value; however, the benefit of using such a measure would apply to a minority of cases and would diminish the ability to predict wounds that are less likely to heal. The primary goal of this analysis was to identify patients who are likely to not heal despite achieving a good early response at 4 weeks. The authors believe that this can be achieved by applying diligent monitoring of wound healing progress during the 5‐ to 8‐week period. Should healing progress stop for 2 or more weeks, or if the wound fails to achieve 90% PAR at 8 weeks, it is recommended that the wound be reassessed for any evidence of complications (sub‐clinical infection, poor vascular flow, non migrating wound edge, etc.) and that advanced treatment options be considered in order to expedite the healing progress. In cases where patients achieve both milestones of ≥50% PAR at 4 weeks and ≥90% PAR at 8 weeks, it would seem reasonable to continue the treatment but closely monitor the wound for any signs of a delay in healing throughout the entirety of the treatment course.
When DFU healing is delayed, complications such as prolonged wound exposure that may lead to infection (12) and an increased risk of amputation (13), add to patient morbidity and mortality and are associated with high health care costs 14, 15. Cost‐containment strategies used by insurance carriers may only consider unit costs of treatments and disregard the overall costs associated with long‐term care of DFUs that fail to heal despite standard of care (16).
It is not known why some DFUs with ≥50% PAR at 4 weeks fail to heal within 12 weeks, while other DFUs continue to progressively heal over this time frame. Delays in DFU healing could occur if patients developed impaired renal or cardiac function. Delays may also be linked to other diabetes‐related factors (17) that were not monitored in the clinical trials 8, 9. In addition, sub‐clinical changes at the cellular level could also contribute to delays in wound healing. The morphologies of cells surrounding the non ulcerated and ulcerated areas of the wound margin are unique and may explain the differing responses of these cells to wounding and healing stimuli (18).
The present analysis has its limitations. It is a retrospective look at a combined subgroup of patients enrolled in separate clinical studies, raising the possibility of bias in the data. Also, because the population studied was participating in clinical trials that selected patients with non infected DFUs that did not penetrate to the bone or tendon, there was no evidence of significant negative risk factors that indicated an immediate need for therapy outside of standard wound care. While the data presented may provide guidance to clinicians treating these types of DFUs, our findings require validation in a large cohort of patients with these demographic characteristics.
In conclusion, close monitoring of DFU healing and reporting of wound area measurements is essential. A stalling in wound healing for 2 weeks or more is indicative of failure to heal, regardless of early positive healing progress. For DFUs that achieve ≥50% PAR by 4 weeks, failure to progress or show worsening at 6 weeks, or achieve <90% PAR at 8 weeks may differentiate DFUs that are unlikely to heal at week 12. These assessments complement the recommended 4‐week/50% predictor and may be beneficial in the identification of changes that are impacting DFU closure rates despite early positive wound healing.
REFERENCES
- 1. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513–21. [DOI] [PubMed] [Google Scholar]
- 2. Goodridge D, Trepman E, Sloan J, Guse L, Strain LA, McIntyre J, Embil JM. Quality of life of adults with unhealed and healed diabetic foot ulcers. Foot Ankle Int 2006;27:274–80. [DOI] [PubMed] [Google Scholar]
- 3. Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 4. Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med 2004;351:48–55. [DOI] [PubMed] [Google Scholar]
- 5. Snyder R, Cardinal M, Dauphinée DM. A post‐hoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage 2010;56:44–50. [PubMed] [Google Scholar]
- 6. Donohue K, Falanga V. Healing rate as a prognostic indicator of complete healing: a reappraisal. Wounds 2003;15. URL www.woundsresearch.com/article/1376 [accessed on 3 March 2010]. [Google Scholar]
- 7. Kurd SK, Hoffstad OJ, Bilker WB, Margolis DJ. Evaluation of the use of prognostic information for the care of individuals with venous leg ulcers or diabetic neuropathic foot ulcers. Wound Repair Regen 2009;17:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pollak RA, Edington H, Jensen JL, Kroeker RO, Gentzkow GD. A human dermal replacement for the treatment of diabetic foot ulcers. Wounds 1997;9:175–83. [Google Scholar]
- 9. Marston WA, Hanft J, Norwood P, Pollack R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers. Diabetes Care 2003;26:1701–5. [DOI] [PubMed] [Google Scholar]
- 10. Coerper S, Beckert S, Küper MA, Jekov M, Königsranier A. Fifty percent area reduction after 4 weeks of treatment is a reliable indicator for healing – analysis of a single‐center cohort of 704 diabetic patients. J Diabetes Complicat 2009;23:49–53. [DOI] [PubMed] [Google Scholar]
- 11. Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003;26:1696–1700. [DOI] [PubMed] [Google Scholar]
- 12. Yates C, May K, Hale T, Allard B, Rowlings N, Freeman A, Harrison J, McCann J, Wraight P. Wound chronicity, inpatient care, and chronic kidney disease predispose to MRSA infection in diabetic foot ulcers. Diabetes Care 2009;32:1907–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–93. [DOI] [PubMed] [Google Scholar]
- 14. Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev 2000;16 Suppl 1:S75–S83. [DOI] [PubMed] [Google Scholar]
- 15. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–87. [DOI] [PubMed] [Google Scholar]
- 16. Mulder G, Armstrong D, Seaman S. Standard, appropriate, and advanced care and medical‐legal considerations: part one – diabetic foot ulcerations (A). Wounds 2003;15. URL www.woundsresearch.com/article/1526 [accessed on 3 March 2010]. [Google Scholar]
- 17. Edmonds M. Diabetic foot ulcers. Practical treatment recommendations. Drugs 2006;66:913–29. [DOI] [PubMed] [Google Scholar]
- 18. Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, Golinko M, Roseberg H, Tomic‐Canic M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med 2007;13:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


