Abstract
Topical wound‐healing potential of autologous bone marrow‐derived nucleated cells along with placental extract was evaluated in comparison with buffy coat of autologous blood on full‐thickness cutaneous wounds in the thoracolumbar region of 15 clinically healthy New Zealand rabbits. Three wounds of 2 × 2 cm, one on the right side of the body and two on the left side of the midline were created on the dorsal lumbar region of each rabbit under xylazine–ketamine anaesthesia. The wounds of each animal were randomly assigned to one of the three treatments: topical application of autologous bone marrow‐derived cells with placental extract (group I), application of buffy coat in the autologous plasma with placental extract (group II) and autologous plasma with placental extract as control (group III). Wounds were observed for 30 days macroscopically and for granulation tissue formation, histomorphological and histochemical evaluation. Time of appearance of granulation tissues and filling of wound beds were faster in group I followed by group II and group III animals, respectively. Histomorphological findings exhibited an earlier disappearance of inflammatory reaction, better epithelialisation, significantly maximum neovascularisation, fibroplasias and collagenation in group I followed by group II and group III animals, respectively. Histochemical findings also depicted maximum number of robust, thick, interwoven type of collagen fibres, stout, highly tortuous and interwoven network of elastin fibres and numerous mesh war form of reticulin fibres within the dermal component were present in group I when compared with group II and III animals. Experiment conclude that single application of autologous bone marrow‐nucleated cells with placental extract topically could be a novel option for faster healing in complicated non healing wounds both in human beings and animals.
Keywords: Animal model, Autologous bone marrow, Placental extract, Wound healing
INTRODUCTION
Wound healing is a complex and well‐orchestrated process involving multiple biological pathways and signalling. Various kinds of intra‐ and intercellular mechanisms encompassing the immune system, blood coagulation cascade and pro‐inflammatory signalling are intricately associated with the healing and repair process 1, 2.
Wounds are dynamic and treatment for healing may need to be tailored during the sequence of the healing through different phases. Surgeons often face difficulties in treating traumatic wounds that are infected or too large to close immediately or both. The routine ways of open wound management include irrigation, mechanical and chemical debridement, use of antiseptics and antimicrobials, adherent and non adherent dressings, and miscellaneous topical applications 3, 4. Second‐intention wound healing occurs by wound contraction, reepithelialisation and creation of granulation tissue which develops within 5–7 days from underlying connective tissue to cover a wound 5, 6. Even if a topical application of different drugs and medicines or agents has shown to favour healing (7), the subject is still controversial (8). However, various types of treatment protocols for healing of open wounds concluded that topical medicaments do not affect wound healing or inhibit rather activate it 9, 10. Despite appreciable advances have been reported in this arena, yet there is a need to select ideal materials thoughtfully and on scientific basis for optimal healing environment.
Bone marrow‐derived mesenchymal stromal cells are multipotential or totipotent stem cells which are capable of differentiating into numerous cell types and thus their application was indicated in varieties of reconstructive and restorative surgeries for rapid healing. Bone marrow‐derived stem cells possess distinctive ability to renew themselves by mitotic division as well as they can differentiate into wide spectrum of cells as it is seen during embryonic development (11). In adults, stem cells and progenitor cells are involved in different repair processes and also maintain normal turnover of regenerative organs like skin. Because of their plasticity to differentiate, and the fact that bone marrow cells are not lineage restricted, therefore, these cells can easily be adjusted when transplanted on foreign tissues. Bone marrow‐derived cells (BMDC) are also capable of producing various cytokines and growth factors which also act as triggering factor to accentuate the healing process (12) and may take part in active inflammation for the repair and healing of chronic non healed wounds (13). Recent studies have indicated the implication of BMDC in tissue repair or regeneration of many tissues including skin 14, 15, 16, 17. Application of human placental extract is proved to be a good option for topical use in case of healing (18). The aim of the present study was therefore to evaluate the topical wound‐healing potential of autologous bone marrow‐derived cells and buffy coat of autologous blood in combination with placental extract in cutaneous wound.
MATERIALS AND METHODS
Wound‐healing model
Fifteen clinically healthy New Zealand rabbits of either sex, weighing between 2 and 3 kg and between 3 and 9 months of age were divided randomly into three groups (n = 5 for each group; Table 1). All the animals were maintained under similar husbandry environment. Three experimental wounds were outlined in each rabbit using clean transparency sheet template and by permanent marker. Three square full‐thicknesses of 2 × 2 cm of skin including subcutaneous tissue were excised with surgical blade, two in one side about 2 cm apart and one in opposite side of dorsal midline of the body. Haemorrhage, if any, was controlled by digital gauze pressure. All the wounds were cleaned/irrigated and treated on the same day of wound creation as per treatment protocol (Table 1). Animals of each group were evaluated for a total of 15 wounds on days 1, 2, 3, 7, 14, 21 and 30. Broad spectrum antibiotic Cefotaxime sodium (Injection Taxim, Alkem laboratories Ltd. Himachal Pradesh, India) at 10 mg/kg body weight twice daily for five consecutive days and analgesic meloxicam (Injection Melonex, Intas Pharmaceuticals Ltd. Vadodhara, India) at 0·2 mg/kg intramuscularly were administered to each animal for three consecutive days from the day of operation. Bone marrow aspiration was carried out aseptically under xylazine hydrochloride (Injection Xylaxin, Indian Immunologicals Ltd., India) at 5 mg/kg body weight intramuscularly and ketamine hydrochloride (Injection Ketmin‐50, Themis Medicare ltd., Haridwar, India) at 35 mg/kg body weight (19). The Institutional Animal Ethics Committee of West Bengal University of Animal and Fishery Sciences, India, approved the study.
Table 1.
Different experimental groups and their respective treatment
| Group | No. of animals | Treatment |
|---|---|---|
| Group I | 5 | Bone marrow in the autologous plasma with placental extract ointment |
| Group II | 5 | Buffy coat in the autologous plasma with placental extract ointment |
| Group III | 5 | Autologous plasma with placental extract ointment (control) |
Bone marrow aspiration, separation and count of bone marrow‐nucleated cells
Under general anaesthesia with xylazine and ketamine, the antero‐medial aspect of each proximal tibia was prepared aseptically. Aspiration of bone marrow from each rabbit was carried out as per the method described by Crow and Walshaw (20). After excising the skin and muscle, a 20‐gauge needle was inserted into the proximal medullary cavity of tibia after drilling the bone with a micro‐motor dental drill. For each animal, 10‐ml disposable syringe containing 5000 IU of heparin was used, and negative pressure was applied forcefully pulling back on syringe plunger. Approximately 3 ml of bone marrow aspirate was collected from each tibia. Bone marrow‐nucleated cells (BMNCs) were collected from each aspirate by volume reduction centrifuge ‘buffy coat' protocol as per the method described by Kasten et al. (21). The mononuclear cells were isolated from each bone marrow aspirant using density gradient centrifugation. After collection (aspirating) of fluid from each bone marrow (0·5–1 ml), dilution was made with phosphate buffered saline (PBS) (pH 7·2) to make the volume up to 3 ml. The diluted fluid was layered onto Hisep® (1 part of Hisep and 3 parts of diluted fluid) and centrifuged for 30 min at 400 g. Following centrifugation, the mononuclear cell‐rich interface layer (buffy coat) was collected using separate micropipettes, transferred into coded clean sterile test tubes and washed thrice with PBS. Viable cells were enumerated by trypan blue dye exclusion method using Neubauer's counting chamber and 2 × 106 cells/ml was adjusted in sterile PBS for instillation (1 ml) into the experimental wound sites.
Evaluation of wound healing
Wounds were evaluated on the basis of clinical, macroscopical, histopathological and histochemical examinations of the biopsy specimens.
Clinical examination and macroscopic evaluation of the wound healing
Clinical parameters like rectal temperature, heart and respiration rate and general condition of the rabbits as well as of the wounds were recorded on days 0, 1, 2 and 3 after the surgery.
Evaluation of exudate
Quantity of exudate, type of exudate and peripheral swelling of the wounds were evaluated/recorded subsequently and were graded as follows:
| Grading | Quantity of exudate | Type of exudate | Peripheral swelling |
|---|---|---|---|
| 1 | Apparently dry wound | Dry cast | No swelling |
| 2 | Wound is moist but no oozing on pressing | Slight | Slight |
| 3 | Wound is moist and there is slight oozing on pressing | Fibrinious | Moderate |
| 4 | Exudate is evident and slight pressing leads to excessive exudation | Purulent | Marked |
Evaluation of granulation tissue
Time of appearance of granulation tissue was recorded as the first day when the granulation tissues were observed first. Evaluation of granulation tissue (22) and colour of granulation tissues were recorded/scored as follows:
| Grading | Granulation tissue | Colour of granulation tissue |
|---|---|---|
| 1 | Absent | Pale yellow |
| 2 | Depressed below the skin edge | Pale red |
| 3 | Proliferated to the level of skin edges | Pink |
| 4 | Elevated above skin edges | |
| 6 | Elevated above skin edges, projecting over the advancing border of epithelium |
Gross morphology
Clinical assessment of healing processes were recorded during the course of wound dressing after observing the general appearance of the wounds, granulation tissue, colour of the wound sites and discharge if any as well as odour from the wound areas. Any other changes like evidence of abnormal granulation tissue were also keenly recorded.
Collection of tissue samples and processing for histomorphological and histochemical study
For histo‐morphological and histo‐chemical studies, tissue samples were collected from the margins of the wounds from each animal on days 7, 14, 21 and 30 of post‐operation without affecting the healing process carefully. All the autopsy samples were preserved in 10% neutral buffer formalin. Each preserved tissue samples were processed following standard protocol and tissue sections of 5‐µm thickness were prepared from each sample in quadruplet, one for histo‐morphology and the other three for histo‐chemistry. Histo‐morphological sections were subjected to routine Haematoxylin and Eosin (H & E) stain and histo‐chemical sections were stained for collagen with Masson's Trichrome stain (23), reticulin with Gridley's modification of the silver impregnation method (24) and elastin with Weigert's Resorcin‐Fuschin method (25).
Evaluation of collagen, elastin and reticulin formation was also graded as follows: absent – 0; small thin amount – 1; thin to diffused thick – 2; diffuse thick – 3; wavy – 4.
Statistical method
Independent samples' equality of mean test was performed for all the parameters for each day because of two different treated groups by student ‘t’ and level of significance of test was either 5% or 1%. If significance of probability of rejection is more than 0·05, we concluded no significant difference between the two treated groups. Statistical Package for Social Scientists (SPSS‐16, Windows version 7) was used for statistical analyses of the data.
RESULTS
Clinical observation
The recorded values of rectal temperature as well as respiratory rate and heart rate in the different treatment groups were ranged from 38·88 ± 0·18 to 39·44 ± 0·16°C, 26·12 ± 2·66 to 44·22 ± 5·06 per minute and 95·82 ± 7·21 to 110·82 ± 11·55 per minute, respectively. All the values were within the physiological limit and devoid of any significant differences throughout the period of observation.
Macroscopic evaluation of wound healing
In most of the animals, observations revealed few wounds remained moist initially with slight oozing on pressure. By third day after operation, most of the wounds became dry and after day 7 all the wounds dried completely. Initially slight exudation in wounds were observed in all the groups which decreased gradually; however, day 3 onwards exudation remained absent in all the groups (Table 2). There was no significant (P > 0·05) difference in the type of exudates among the different groups. Initially in few rabbits, slight‐to‐moderate swelling was observed at the periphery; however, it subsided gradually. Finally, on day 7 no wounds were visible except few peripheral swelling. None of the animals of any treated groups exhibited the sign of infection and all the wounds healed satisfactorily. Significantly less amount of granulation tissues appeared in the wounds of group II and III animals when compared with group I (Table 3). The granulation tissues of the wounds were mainly composed of fibroblasts, collagen fibres and remained with small new blood vessels. It has also been observed that these tissues were wider and more compact in group I animals followed by group II and group III animals, respectively. The overall assessment of wound healing among the three groups suggested better healing in group I followed by group II and group III (Figure 1).
Table 2.
Gradation for type of exudates in rabbit's excisional wounds in different treatment groups at different day's interval *
| Treatment | Post‐wounding days | ||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 7 | Day 14 | Day 21 | Day 30 | |
| Group I | 1·48 ± 0·11 | 1·27 ± 0·13 | 1·13 ± 0·08 | 1·01 ± 0·01 | 1·02 ± 0·02 | 1·01 ± 0·11 | 1·01 ± 0·01 |
| Group II | 1·51 ± 0·11 | 1·24 ± 0·08 | 1·11 ± 0 ·07 | 1·01 ± 0·01 | 1·01 ± 0·00 | 1·01 ± 0·01 | 1·00 ± 0·01 |
| Group III | 1·55 ± 0·11 | 1·20 ± 0·11 | 1·06 ± 0·04 | 1·01 ± 0·01 | 1·01 ± 0·01 | 1·02 ± 0·00 | 1·00 ± 0·01 |
*Values expressed as mean ± SE.
Table 3.
First appearance of granulation tissue in excisional wound of rabbits of different treatment groups at different day's interval *
| Group | Appearance of granulation tissue (days) |
|---|---|
| Group I | 3·21 ± 0·22 † |
| Group II | 3·85 ± 0·42 † |
| Group III | 4·84 ± 0·44 † |
*Values expressed as mean ± SE.
†Values with different superscript differ significantly (P < 0·05).
Figure 1.
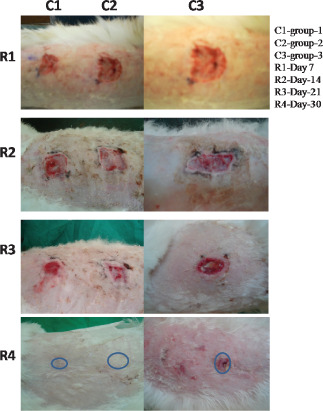
Photomicrographs showing wound of skin at different days of interval.
Histomorphological observations
On day 7 in group I animals, greater areas of vascularisation and cellular infiltration of the epidermis were observed that incites a quick response to healing processes, however, few dead and degenerated tissues were seen and yet to be phagocytosed by macrophages and other inflammatory cells (plasma cells). In dermis layer fibrovascular tissues were found to be proliferated and bulged upwards to reduce the gap of wounds indicated progressive healing in group II animals where proliferating collagen and scanty reticular fibres formed a network to bridge wound gaps. Moderate‐to‐scanty vascularisation indicated primary healing accompanying with cellular network initiated the healing processes. In group III animals, organisation of vascular layer and fibrocollagenous structures were scanty to moderate. Mild edematous changes in chorium (dermis) along with patches of clusters of lymphocytes were evident. Reticular fibre network had in different directions along with collagenous tissue and the tendency of tissue healing was moderate (Figure 2).
Figure 2.
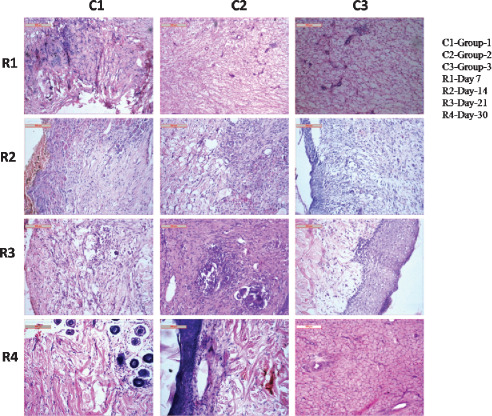
Photomicrographs showing histological disposition of healing tissues of skin at different days of interval.
On day 14, even distribution of bulging capillary bud rims towards the wound margin of the affected area was observed in group I animals. The robust collagen and reticular fibres remained all through the sections. Macrophage populations were comparatively more indicated, enhancing a quick healing process. Fibrovascularisation in the epidermal and dermal layer was quite normal. In group II animals, proliferating collagenous tissue admixture with reticular fibre developed in different directions were observed. This mass was surrounded by highly vascular tissue which initiated the inflammatory and phagocytic response to the healing process. Infiltration by mononuclear and phagocytic cells was moderate to high which also indicated a gradual development of the healing processes. In group III animals, proliferating collagenous tissue admixture with reticular fibres was found to run throughout all the sections, although the vascularity of the affected regions was not so well developed. Macrophages and other mononuclear cells were present moderately throughout the healing sites which indicated slower healing process (Figure 2).
Tissue sections of group I animals had well‐developed collagen architecture with finely woven reticular fibres that indicated a progressive rate of healing as observed on day 21. Cellular accumulations were quite abundant in the healing areas. Vascular beds from the dermal layer exhibited proliferation which indicated a positive support for angiogenesis to the newly formed repairing tissues. Presence of macrophages and other mononuclear cell clusters towards the epidermal layer also indicated a satisfactory rate of healing process. In group II animals, tissue sections of wounds depicted clusters of accumulated macrophages with engulfed cellular debris in some foci. Collagen structure regeneration and reticular fibre formations were quite satisfactory although vascularisation did not inactivate the healing process to much which indicated fair response to the healing process. In group III animals, tissue sections had well‐developed squamous cell layers along with underlying supporting tissues namely fibrocollagenous and reticular fibre. The metaplastic changes in some cases were also evident. Vascularisation was scanty to normal which indicated the occurrence of a comparatively reduced healing process (Figure 2).
On day 30, in group I animals well‐organised cellular healing structures involving a plump of vascular mass with numerous blood vessels were observed. The macrophages and other mononuclear cells were found to be present throughout the sections and the route of hair follicles were quietly immersed in the supporting structures. The inflammatory reactions were very quick to accomplish regeneration processes along with other cellular phenomena. In group II animals, the sections had well‐formed epidermal layer underneath; the presence of a layer of vascular granulation tissues and other inflammatory cell masses was found to bridge the gap of wounds. Fibroblasts had their suitable position to invade the accompanying reticular fibre, encroaching around the blood vasculature pits (vessels) to take their normal nutrition. Wound repairing response in these rabbits was quite satisfactory. In group III animals, the sections depicted healing by loose areolar tissue and epithelium of the epidermis runs in different directions to bridge the gap of the wound. The collagenous tissue and reticular fibres were in static position although some of the spaces were found to be invaded by inflammatory cells and macrophages. Angiogenesis seemed reducing gradually which replaces the other fibrocytes and endothelium for vascular supply (Figure 2).
Histochemical observations
Collagen
On day 7 there was a formation of moderate to high collagen layers; thin structures were quite robust, thick and interwoven type in group I animals, which was graded as 4. In group II animals, moderate to flatter type of collagen was found to run through all the directions of the wounds area. In some places, minimal gaps were created and the structures were less robust than former and were graded as 3. In group III animals, collagen fibres appeared thin and almost captured in entire section. Inflammatory reactions were moderate and were graded as 2 (Figure 3).
Figure 3.
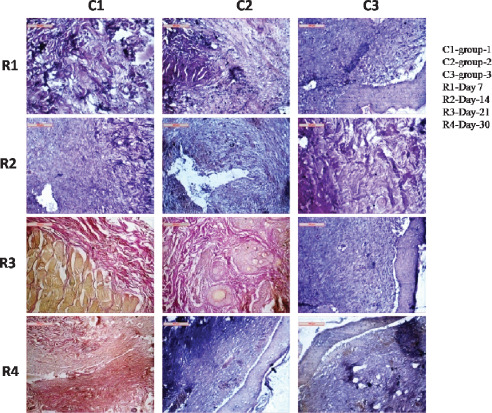
Photomicrographs showing collagen fibre disposition in different groups at different days of interval.
On day 14 in group I animals, collagens remained stout, long and almost run throughout the sections keeping minimal gap inside their fibre space and was graded as 3. In group II animals, collagens seemed to be thin, long, run in wavy pattern. Inter fibre spaces were moderate to low and were graded as 2. In group III animals, collagens had flatter and robust in shape and was graded as 2 (Figure 3).
On day 21 in group I animals, collagen structures appeared robust, wavy and were swollen in nature. Cellular architecture and other inflammatory reactions indicated favourable environment for wound healing and was graded as 4. In group II animals, collagen structures in the section were present in large amount, although their structures were thin, long and comparatively flatter. It was graded as 2. In group III animals, slender collagen fibres were thin in architecture running all throughout the tissue sections along with infiltrated macrophage and was graded as 3 (Figure 3).
On day 30 orientation of collagen fibres had wavy, stout and thick in nature which indicated a satisfactory healing process in group I animals and was graded as 4. In group II animals, collagen fibres remained slender, flatter and less wavy. Inflammatory reaction and angiogenesis were quite satisfactory and was graded as 3. In group III animals, scanty collagen fibres were thin and short. Inter‐collagen fibre gaps were higher and were graded as 2 (Figure 3).
Elastin
Tissue sections of group I animals had mild proliferation of elastin fibres around the hair follicle and dermal matrix as observed on day 7, the area below dermis also exhibited interwoven bundle of elastin along with collagen, however, cellular responses were mild and proliferation of mononuclear cells in the layer of stratum germinativum were also found. In group II animals, there was proliferation of elastin fibres that were thin, numerous and invaded especially in the dermal layer. The dermal appendages were partly encircled by elastin. Mononuclear cell reaction in dermal layer was quite satisfactory. In group III animals, tissue sections depicted proliferation of elastin fibres in dermal and epidermal layers where the fibres were thin and scanty with minimal mononuclear cell infiltration (Figure 4).
Figure 4.
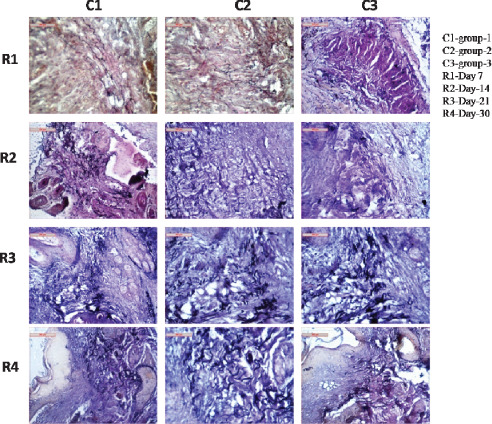
Photomicrographs showing elastin fibre disposition in different groups at different days of interval.
On day 14 the tissue sections exhibited presence of elastin fibres running throughout the wound areas which were stout, highly tortuous and in some places form a bundle focally in group I animals. Other regular structure of hair follicle and glandular structure were surrounded by few elastin fibres. In group II animals, tissue sections had a large network of elastin fibres at the margin of the wounds. The structures of elastin were thin, long, slender and tortuous. Cellular reaction was satisfactory. In group III animals, sparse amount of elastin fibre in dermal layer was observed which were long, straight and running in dermal appendages. Cellular reaction was moderate to satisfactory (Figure 4).
On day 21 in group I animals, tissue sections had fine network of elastin fibre formation throughout all the sections. Hair follicles and other sebaceous glands were cuffed up by elastin fibres indicated a better healing process. Cellular reaction and vascularisation were quite satisfactory. In group II animals, robust and flat elastin fibres were found throughout the sections. The elastin fibres were mainly found in dermal layers around the gland and hair follicles as clumps of fibres indicating progressive healing process. In group III animals, sections exhibited short, stumpy elastin fibres running isolated in different portion of dermal and epidermal stroma and were encircling around glandular structure, and hair follicles were scanty. Cellular reaction and vascularisation were found moderate to high (Figure 4).
Elastin fibre formation had a interwoven network all throughout the sections as observed on day 30 in group I animals. Cellularity and vascularisation in the sections depicted a fairer healing process. Some portion of dermis also had a fewer number of elastin fibres. In group II animals, tissue sections exhibited stumpy, long and presence of abundant slender elastin fibres. Elastin fibres were sparsely interwoven with collagen fibres and remained encircled with hair follicle and glandular structure. In group III animals, elastin fibres remained predominant in dermal layer where their structures were thin, short and less tortuous and numerous. There were minimum encircling of elastin fibres around the hair follicles and glands (Figure 4).
Reticulin
Comparatively, reticulin fibres which appeared in the dermal component of the wounds in group II and III animals on day 7 were less in number than group I animals. In group II, very few reticulin fibres were observed around the healing tissues whereas there was no existence of reticulin fibres in group III animals (Figure 5).
Figure 5.
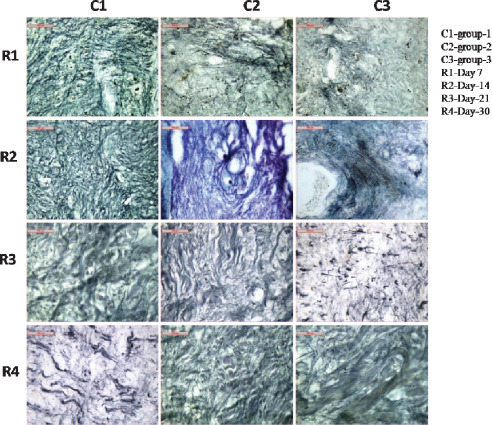
Photomicrographs showing reticulin fibre disposition in different groups at different days of interval.
On day 14 in group I animals, numerous reticulin fibres were found to be present in the healing areas than group II and III animals. In group II animals, moderate number of reticulin fibres was identified, whereas, in group III, very scanty number of reticulin fibres appeared around the hair follicles (Figure 5).
Complete healing process was observed on day 21 in group I animals where reticulin fibres and cellular organisations were found intact and large number of reticulin fibres appeared in the form of a mesh war within the dermal component, which appeared to be the best reticular organisation when compared with other two groups. In group II animals, moderate number of reticulin fibres remained within the dermal component of the healing tissues whereas in group III, few reticulin fibres were found within the healing tissues (Figure 5).
On day 30, excellent fibrous and cellular organisations remained in group I animals. Number of reticulin fibres were numerous and well distributed within the dermal component of the healing tissues. The tissue sections had normal histo‐arehitecture and appeared to be the best when compared with other two groups. In group II animals, moderate reticulin fibres were observed as compared to group III animals (Figure 5).
DISCUSSION
Wound is a surgical problem but can be manipulated by clinical management. However, informations regarding the potential efficacy of topical application of autologous BMNCs along with placental extract in wound healing in animal model are very scanty.
It has been reported that wound surface remain moist initially as fluid oozes into the tissue because of migration of leucocytes and dead tissues leading to inflammatory exudates (26). Moist wound environment allows optimal healing processes by enhancing autolytic debridement through endogenous enzymes that break down necrotic tissue, promoting granulation tissue formation thereby fast epithelialisation. Moist wound surface triggered epithelialisation by the epithelial cells to migrate faster and for a shorter distance for re‐epithelialisation, however, the potential disadvantages for moist wound healing include bacterial colonisation of the wound surface, folliculitis and maceration of the wound border (27). Slight to moderate swelling could be attributed to the normal inflammatory mechanisms of the body in favour of tissue repair to bring the wound edges in apposition during wound healing (28). Present findings also simulate with the observations of Borena et al. (29). Non significant difference of clinical parameters like temperature, heart rate and respiratory rate suggested that the surgical wounds were devoid of from any major infections. Present observation revealed that granulation tissue was also wider and more compact in group I animals followed by group II and group III animals, respectively. This was because of infiltration of fibroblasts and generation of new granulation tissues in group I and group II wounds when compared with group III. Inflammation plays a critical role in fighting infection and inducing the proliferation phase which is essential for healing (30). In wound healing process, there is a marked gradient of oxygen that could be partially accountable for the branching and in‐growth of new blood vessels in the form of capillaries (26). Collagen is the major component of extracellular tissue which provides support and strength for healing processes (31). Granulation tissue is of paramount necessity for the healing of wounds because it is extremely resistant to infection and serves as a barrier against systemic infection, provides a surface over which epithelium is able to migrate and plays a vital role in wound contraction and contains fibroblasts that produce the collagen for healing (26). Higher amount of granulation tissue formation which appeared in group I animals indicated BMDC triggered granulation tissue genesis. This finding also supports the histological observations where early deposition of relatively more granulation tissues with more cellularity appeared in group I animals as compared to group II and group III animals. These observations about the healing process also corroborated with the findings of Badiavas et al. 32, 33. Histopathological evaluations also depicted mode and rate of healing in wounds with more accuracy (34).
The efficiency of collagen formation depends mainly on the synthesis of hydroxyproline, and the collagen formation was recorded to be highest in group I followed by group II and III animals respectively indicated better healing (35). Present observations further substantiated from the histopathological changes of wounds showing maximum accumulation of collagen fibrils and epithelisation. In another study, similar findings were observed using only human placental extract in rat wound healing which might be because of either stimulation of growth factor(s) signals cascade system at the cellular level or itself act as growth factor(s) (36).
More neovascularisation, denser, thicker and better arranged reticulin and elastin fibres were also recorded in the wound sections of group I and II animals than control groups (group III). Pittenger et al. (11) reported that bone BMSCs are multipotential stem cells capable of differentiation into numerous cell types including fibroblasts, bone, cartilage and muscles. In the present study wounds treated with BMNCs in combination with placental extracts had more fibroblasts and formation of collagen, reticulin and elastin fibres than wounds treated with buffy coat of blood and plasma. When tissues are disrupted following injury, collagen is needed to repair the defect and restore anatomic structure and function (37). Moreover, wound healing effects of skin is mediated by the regenerative effects of transforming growth factor, fibroblast growth factor, fibronectin‐like peptide, keratinocytes growth factor, epidermal growth factor, and growth hormone releasing factor 38, 39, 40. Furthermore, cytokine growth factors and extracellular matrix molecules present in placental extracts are also known to activate and be released by dermal multipotent stem cells to promote the proliferation of fibroblasts and epidermal cells 41, 42.
Experiments conclude that autologous BM NCs in combination with placental extract could accelerate healing of the cutaneous incisional wounds in animal model as compared to application of buffy coat of autologous blood with placental extract. Data also revealed that there was an increase in the synthesis of collagen in the wound tissue and a more rapid wound maturation of BMNCs‐placental extract treated wounds. These findings indicate that combine autologous BMNC with placental extract may augment faster healing in healing excisional cutaneous wounds in animal model.
ACKNOWLEDGEMENTS
The authors wish to express their sincere thanks to the Vice Chancellor, West Bengal University of Animal and Fishery Sciences, Kolkata, India, for his generous and kind support to this work. AA has done the experimental work. SKN, PD, DB, SR and UD have conceptualized the work, analyzed the data and gone through the final version of the manuscript.
References
- 1. Nguyen DT, Orgill DP, Murphy GF. The pathophysiologic basis for wound healing and cutaneous regeneration. Biomaterials for treating skin loss. Boca Raton/Cambridge: CRC Press (US) & Woodhead Publishing (UK/Europe), 2009:25–57. [Google Scholar]
- 2. Deodhar AK, Rana RE. Surgical physiology of wound healing: a review. J Postgrad Med 1997;43:52–6. [PubMed] [Google Scholar]
- 3. Lee AH, Swaim SF, Yang ST, Wilken LO. Effects of gentamicin solution and cream on the healing of open wound. Am J Vet Res 1984;45:1487–92. [PubMed] [Google Scholar]
- 4. Yamada KM. Cell surface interaction with extracellular materials. Annu Rev Biochem 1983;52:761–99. [DOI] [PubMed] [Google Scholar]
- 5. Swaim SF, Hinkle SH, Bradley DM. Wound contraction: basic and clinical factors. Compendium 2001;23:20–4. [Google Scholar]
- 6. Layton CE. Nutritional support of the surgical patient. In: Harari J, editor. Surgical complications and wound healing in the small animal practice. Philadelphia: Saunders, 1993:89–125. [Google Scholar]
- 7. Singer AJ, Clark RA. Cutaneous wound healing. New Eng J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 8. Harari J. Surgical complication and wound healing in the small animal practice. Philadelphia: Saunders, 1993:78–9. [Google Scholar]
- 9. Lee AH, Swaim SF, McGuire JA, Hughes KS. Effects of non‐adherent dressing materials on the healing of open wound in dogs. J Am Vet Med Assoc 1987;190:416–22. [PubMed] [Google Scholar]
- 10. Sardari K, Mirshahi A, Maleki M, Aslani MR, Barjasteh MN. Effects of topical Allicin on second intention wound healing in dogs (histological aspects). Comp Clin Pathol 2006;15:98–102. [Google Scholar]
- 11. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- 12. Yongbo L, Deborah S, Dulchavsky PA, Gao X, Kwon D, Chopp M, Dulchavsky S, Gautam SC. Wound repair by bone marrow stromal cells through growth factor production. J Surg Res 2006;136:336–41. [DOI] [PubMed] [Google Scholar]
- 13. Neagos D, Mitran V, Chiraku G, Ciurab R, Lanku C, Cimpean A, Iordachescu D. Skin wound healing in a free floating fibroblast populated collagen lattice model. Rom J Biophys 2006;16:157–68. [Google Scholar]
- 14. Kataoka K, Medina RJ, Kageyama T, Miyazaki M, Yoshino T, Makino T, Huh NH. Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol 2003;163:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow‐derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin‐substitute wound healing. Br J Dermatol 2005;153:29–36. [DOI] [PubMed] [Google Scholar]
- 17. Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, You S, Deng H, Murad F, Zhao RC. Engrafted bone marrow‐derived flk‐(11) mesenchymal stem cells regenerate skin tissue. Tissue Eng 2005;11:110–9. [DOI] [PubMed] [Google Scholar]
- 18. Tiwary SK, Shukla D, Tripathi AK, Agrawal S, Singh MK, Shukla VKJ. Effect of placental‐extract gel and cream on non‐healing wounds. J Wound Care 2006;15:325–8. [DOI] [PubMed] [Google Scholar]
- 19. Kahn CM. Merck veterinary manual, 9th edn. Whitehouse Station, NJ, USA: Merck and Co., Inc, 2005:149–50. [Google Scholar]
- 20. Crow SE, Walshaw SQ. Manual of clinical procedures in the dog, cat and rabbit. Philadelphia, PA 19106, USA: Lippincott Williams and Wilkins, 1997:169–278. [Google Scholar]
- 21. Kasten P, Luginbuhl R, Feebuer K, Suda AJ, Egermann M, Gasser B, Beyen I. Instant mesenchymal stem cell therapy: how can we do it? Characterization and concentration of mesenchymal stem cells in vitro. Orthop Surg, European cells Mater 2007;14:39. [DOI] [PubMed] [Google Scholar]
- 22. Bigbie RB, Schumacker J, Swaim SF, Purohit KC, Wright JC. Effect of amnion and yeast cell derivate on second intention healing in horses. Am J Vet Res 1991;52:1376. [PubMed] [Google Scholar]
- 23. Masson P. Trichrome staining and their preliminary techniques. J Tech Methods 1929;12:75–90. [Google Scholar]
- 24. Gridley MF. A modification of the silver impregnation method of staining reticulin fibers. Am J Clin Pathol 1951;21:897–9. [DOI] [PubMed] [Google Scholar]
- 25. Mallony FB. Pathological techniques. New York: Saunders, 1961:152. [Google Scholar]
- 26. Swaim SF, Henderson RA. Small animal wound management. Philadelphia, London: Lea and Fabiger, 1990:1–33. [Google Scholar]
- 27. Fossum TW, Hedlund CS, Hulse DA, Johnson AL, Seim HB, Willard MD, Carroll GL. Surgery of integumentary system. In: Manual of small animal surgery, 2nd edn. St Louis, MO, USA: Mosby, 2007:132–228. [Google Scholar]
- 28. Kumar A. Veterinary surgical techniques. New Delhi: Vikas Publishing House, 1996:151–65. [Google Scholar]
- 29. Borena B, Pawde AM, Amarpal, Aithal HP, Kinjavdekar P, Singh R, Kumar D. Evaluation of healing potential of autologous bone marrow‐derived nucleated cells on incisional wounds in dogs. Indian J Vet Surg 2009;30:85–9. [Google Scholar]
- 30. Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 2004;36:1031–7 [DOI] [PubMed] [Google Scholar]
- 31. Singh SDJ, Krishna V, Manikani KL, Manjunatha BK, Vidya SM, Monohara YN. Wound healing activity of the leaf extracts and de‐oxyelephantopin isolated from Elephantopus scaber Linn . Indian J Pharmacol 2005;37:237–42. [Google Scholar]
- 32. Badiavas EV, Abedi M, Butmare J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 2003;196:245–50. [DOI] [PubMed] [Google Scholar]
- 33. Badiavas EV, Falanga V. Treatment of chronic wound with bone marrow derived cells. Arch Dermatol 2003:139:510–6. [DOI] [PubMed] [Google Scholar]
- 34. Abaramo F, Martins P, Lloyd DH, Auxilia ST, Noli C, Leotta R, Pfeiffer D. An evaluation of methods for the assessments of healing of open wounds in the dog. Vet Dermatol 2004:15:13. 14989700 [Google Scholar]
- 35. Nelson DL, Cox MM. Lehninger principles of biochemistry, 3rd edn. New York: Macmillan Worth Publishers, 2000. [Google Scholar]
- 36. Biswas TK, Auddy B, Bhattacharya NP, Bhattacharya S, Mukherjee B. Wound healing activity of human placental extracts in rat. Acta Pharmacol Sin 2001;22:1113–6. [PubMed] [Google Scholar]
- 37. Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, Robson MC. Definition and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994;130:489. [PubMed] [Google Scholar]
- 38. Wu CH, Chang GY, Chang WC, Hsu CT, Chen RS. Wound healing effects of porcine placental extracts on rats with thermal injury. Br J Dermatol 2003;148:236–45. [DOI] [PubMed] [Google Scholar]
- 39. Chakraborty PD, Bhattacharyya D. Isolation of fibronectin type III like peptide from human placental extract used as wound healer. J Chromatogr B Analyt Technol Biomed Life Sci 2005;818:67–73. [DOI] [PubMed] [Google Scholar]
- 40. Farmer C, Gaudreau P. Presence of a bioactive and immunoreactive growth‐hormone‐releasing‐factor‐like substance in porcine placenta. Biol Neonate 1997;72:363–9. [DOI] [PubMed] [Google Scholar]
- 41. Chunmeng S, Tianmin C, Yongping S, Xinze R, Yue M, Jifu Q, Shufen L, Hui X, Chengji L. Effects of dermal multipotent cell transplantation on skin wound healing. J Surg Res 2004;121:13–9. [DOI] [PubMed] [Google Scholar]
- 42. Narine K, De Wever O, Van Valckenborgh D, Francois K, Bracke M, DeSmet S, Mareel M, Van Nooten G. Growth factor modulation of fibroblast proliferation, differentiation, and invasion: implications for tissue valve engineering. Tissue Eng 2006;12:2707–16. [DOI] [PubMed] [Google Scholar]


