Abstract
Tissue oedema plays an important role in the pathology of chronic and traumatic wounds. Negative pressure wound therapy (NPWT) is thought to contribute to active oedema reduction, yet few studies have showed this effect. In this study, high frequency diagnostic ultrasound at 20 MHz with an axial resolution of 60 µm was used to assess the effect of NPWT at – 80 mmHg on pressure ulcers and the surrounding tissue. Wounds were monitored in four patients over a 3‐month period during which changes in oedema and wound bed thickness (granulation tissue) were measured non‐invasively. The results showed a rapid reduction of periwound tissue oedema in all patients with levels falling by a mean of 43% after 4 days of therapy. A 20% increase in the thickness of the wound bed was observed after 7 days due to new granulation tissue formation. Ultrasound scans through the in situ gauze NPWT filler also revealed the existence of macrodeformation in the tissue produced by the negative pressure. These preliminary studies suggest that non‐invasive assessment using high frequency diagnostic ultrasound could be a valuable tool in clinical studies of NPWT.
Keywords: Granulation tissue, High frequency diagnostic ultrasound, Macrodeformation, Negative pressure wound therapy, Tissue oedema
Introduction
Numerous papers have been written about negative pressure wound therapy (NPWT) and it is regarded as an important tool for managing chronic, subacute and acute wounds 1, 2, 3. NPWT consists of a wound filler substance attached by a tube to a negative pressure pump. The wound and its associated filling are then covered with an adhesive drape to form an airtight seal over the wound. Earlier debate as to what the optimum pressure value is and whether the wound packing substance needs to be made from a specific material, has given way to consensus that a range of materials and pressures are likely to be needed to meet the full range of clinical needs 4, 5, 6. There appears to be no single mechanism of action which can explain the effect NPWT has on wounds. Multiple simultaneous activities have been identified such as changes in the patterns of blood flow, drawing the wound edges together, the removal of wound exudate and microdeformations of the wound surface, which together achieve the observed clinical effects 7, 8, 9.
It is also widely believed that the accumulation of tissue oedema or third space fluid plays a fundamental role in the pathogenesis of chronic wounds and acute injuries (10). Extravascular fluid may restrict capillary blood flow and expose cells and tissues to inflammatory substances released during tissue injury or in response to infection. NPWT is known to extract large quantities of wound exudate, particularly in the days following application and the implication is that this assists in the removal of tissue fluids. Given the importance of tissue oedema, there have been surprisingly few published studies that examine the reduction of oedema during NPWT, although Kamaloz et al. showed improved blood flow following NPWT versus controls in a study of bilateral burn wounds to the hands using indocyanine green video angiography which was ascribed to reduction of tissue oedema (11).
High frequency diagnostic ultrasound (HFDU) is a non‐invasive method, which allows the clinician to get a high‐resolution image of the wound and surrounding tissue 12, 13, 14, 15. This simple procedure can often be used without removing the dressing. The technique allows the clinician to see the wound bed beneath the surface, giving the opportunity to see changes occurring in the wound before they become clinically evident, such as an early warning as to whether the wound is deteriorating or improving (16). HDFU images lend themselves to quantification and can be considered as taking a tissue biopsy without subjecting the patient to an invasive procedure. In the present pilot scale study, we have used HFDU to measure the level of tissue oedema and accumulation of new tissue and to show tissue distortion during NPWT of pressure ulcers.
Materials and methods
Patients
Four women within the age range 60–87 contributed to this pilot study. The patients were bed‐bound nursing home residents who had suffered pressure ulceration despite local preventative measures to combat a range of predisposing co‐morbidities. The wound durations were from 9 months to 1 year. The wounds were all grade IV pressure ulcers (three sacral and one hip), with mild to moderate levels of exudation. Prior to treatment with NPWT, they had received a range of moist wound healing therapies but were making insufficient progress before the clinical decision to commence NPWT. All patients gave written informed consent to non‐invasive assessment with the ultrasound device. Unfortunately, one patient died before the end of the study from causes unrelated to the wound or NPWT.
Negative pressure wound therapy
The NPWT device used was the V1STA (Smith and Nephew, Largo, FL). The system was set at a negative pressure of – 80 mmHg. The wound filler was saline‐moistened antimicrobial gauze (Kerlix‐AMD; Tyco, Gosport, UK). The device was applied as described by Chariker et al.17, 18. NPWT was delivered using continuous pressure over a period of 3 months. Wounds were inspected and dressings changed at 2–3 day intervals.
High frequency diagnostic ultrasound
Ultrasound scanning
The scanner used in this study was the Episcan (Longport International Ltd, Reading, UK). The device was operated at a frequency of 20 MHz as previously described 12, 13, 14, 15, 16. This frequency gives an axial resolution of 60 µm. Although some experience is required to operate the instrument expertly, ultrasound is widely used across many medical disciplines. Scans were acquired from three regions on each patient as shown in Figure 1A. Region 1 was the centre of the wound, region 2 was over the periwound tissue and region 3 was over the uninjured adjacent skin. All patients were scanned at the beginning of the study to establish a baseline profile of their wound and surrounding periwound tissue (Figure 1B and C). A scan adjacent to the wound site was taken to establish the condition of the patient's normal skin as shown in Figure 1D. Several scans were taken of each area to get a representative value of the tissue. The images were then saved to the scanner's hard drive for later analysis. In order to standardise measurements, the instrument assesses pixel values in a defined cross‐sectional area which is saved and the same area is used for each subsequent measurement. The patients were scanned at the initial consultation, then at 2, 4, 7 and 14 days and then 1, 2 and 3 months following the commencement of NPWT, the same regions being scanned on each visit. Two aspects of the ultrasound scans were analysed: pixel distribution and wound bed thickness. Wound area measurements were not collected.
Figure 1.
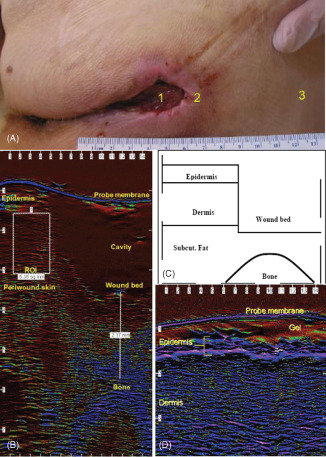
Assessment of negative pressure wound therapy (NPWT)‐treated pressure ulcers by high frequency diagnostic ultrasound (HFDU). (A) Indication of regions assessed: 1, pressure ulcer; 2, periwound skin; 3, healthy unaffected skin. (B) HFDU image through pressure ulcer region 1 and region 2. The rectangle region of interest (ROI) upper left indicates an area of 5·35 mm2 in which tissue oedema can be quantified. The vertical line lower right indicates a measurement from bone to the base of the pressure ulcer cavity (2·17 mm). (C) Indication of anatomical areas in B. (D) HFDU image of uninjured skin indicating the epidermis. The horizontal line labelled probe membrane is the tip of the ultrasound probe which is coupled to the tissue with high water content gel.
Pixel distribution
Each scan was analysed using a form of pixel distribution analysis whereby pixels below certain intensity (red/black) are classed as low echogenic pixels (LEPs). The ratio of LEPs to total pixel (TP) count has been shown by Gniadecka et al. to reflect changes in dermal water content 19, 20. Using this technique, it was possible to get a quantitative assessment of the level of oedema present in the tissues immediately surrounding the wound.
Wound bed thickness
Using ultrasound it is possible to image the skin, wound and underlying bone as shown in Figure 1B and C. The thickness of tissue overlying the bone at time 0 was measured in each patient and then at each assessment following the start of NPWT. By doing this, it was possible to get a quantitative assessment of the rate at which new tissue was being laid down in the wound bed. In the interpretation of high frequency ultrasound images of tissue, areas that are dark red/black are high in fluid (Figure 1B) whereas areas which are blue tend to be occupied by more fibrous and less hydrated structures. Normal uninjured skin has a greater proportion of blue reflections to red/black, as shown in Figure 1D.
Results
Longitudinal study of pixel distribution
NPWT was applied to the wounds using a gauze‐based Chariker‐Jeter system and was changed every second or third day. Ultrasound measurements could be taken with dressings in place because the sound waves travel well through the moist gauze and film. Figure 2 shows a sample of ultrasound scans of the wound edge at various time points after treatment commenced. On each scan, the region of interest where the pixel analysis was carried out is indicated by the inset rectangle. The region of interest was placed in the same location on each scan, i.e. in the tissue adjacent to the wound cavity just below the epidermis. The scans show that initially the edge of the wound is saturated with oedema, which is shown in the scans as a red/black colour. After only 2 days of NPWT, the nature of the tissue surrounding the wound cavity changed dramatically in that it lost a high degree of the oedema that was present at time 0. By 7 days, the fluid had decreased even more and by 3 months the tissue at the wound edge has a predominance of blue pixels, which are indicative of a fibrous tissue comparable to that seen in normal skin (see Figure 1D).
Figure 2.
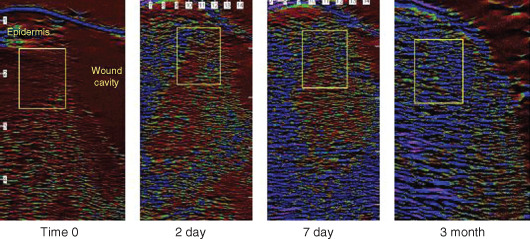
Changes in levels of oedema in periwound tissue of pressure ulcers during gauze‐based negative pressure wound therapy (NPWT) at−80 mmHg. The region of interest where the pixel analysis was carried out is indicated by the boxed area on each image. The region of interest was placed in the same location on each scan for time 0, day 2, day 7 and 3 months.
Figure 3 shows a quantitative assessment looking at the mean (±SEM) LEP:TP ratio against time of NPWT therapy. This analysis allows the combination of data from the four patients. The ratio decreases towards the normal skin ratio soon after the start of the treatment. By 4 days, the ratio has fallen by an average of 43%. By 3 months, the mean ratio in the tissues of the two remaining patients was 73% below that seen at time 0, moving to levels seen in normal uninjured tissue.
Figure 3.
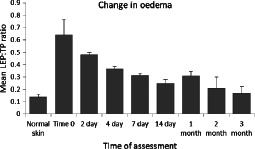
Analysis of changes in levels of oedema in periwound tissue of pressure ulcers during gauze‐based negative pressure wound therapy (NPWT) at−80 mmHg. Histogram of mean (±SEM) Low echogenic pixel:total pixel (LEP:TP) ratio against time of NPWT (n = 4: normal skin, time 0, days 2, 4, 7; n = 3: day 14, 1 month; n = 2: 2 months, 3 months).
Wound bed thickness
The ability of ultrasound to penetrate wound tissue with a dressing in place also allows for a non‐invasive assessment of the accumulation of tissue over the bone in full thickness pressure ulcers. Figure 4 shows a sample of ultrasound scans of the wound bed at various time points after treatment commenced. The increase in wound bed depth is visible and also the change in the relative distribution of red to blue pixels can be seen as the wound base granulation tissue matures. Figure 5 shows the mean % change in wound bed thickness over time. From day 2 after the commencement of treatment the wound bed thickness starts to increase. By 7 days, the tissue thickness is approximately 20% greater than at time 0. After 1 month, the tissue is approximately 60% thicker rising to over 100% by the 2‐month time point.
Figure 4.
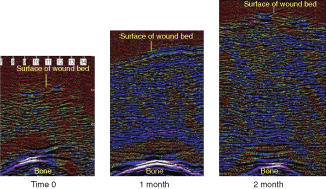
Change in wound bed tissue thickness in pressure ulcers during gauze‐based negative pressure wound therapy (NPWT) at−80 mmHg. Using the underlying bone as a fixed reference point ultrasound scans show the generation of new wound bed tissue building over the bone to fill the pressure ulcer cavity. Note reduction in oedema as colour changes from red/black to blue.
Figure 5.
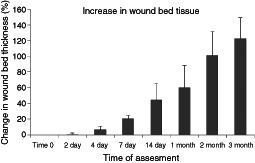
Quantification of change in wound bed tissue thickness in pressure ulcers during gauze‐based negative pressure wound therapy (NPWT) at−80 mmHg using non‐invasive high frequency diagnostic ultrasound (HFDU). Histogram of mean (±SEM) % change in the thickness of tissue covering bone in pressure ulcers versus time of NPWT (n = 4: normal skin, time 0, days 2, 4, 7; n = 3: day 14, 1 month; n = 2: 2 months, 3 months). The mean tissue thickness at time 0 was 3.77 mm.
Tissue distortion due to NPWT
Figure 6 illustrates an effect which was noted when the NPWT device was activated to deliver a negative pressure of – 80 mmHg. As the negative pressure was applied, it could be seen that the skin underneath the adhesive film was being pulled or distorted producing a rippling effect (Figure 6A). Normally it would not be possible to verify whether this surface distortion of the film dressing is reflected in the deeper tissues. However, using ultrasound it was possible to scan through the dressing in situ to see if there were effects of this force on the underlying tissue. Figure 6B shows that a rippling effect can be noted in the intact periwound skin extending through the epidermis and underlying dermis. Figure 6C shows that this rippling effect or deformation can also be seen within the granulation tissue beneath the wound dressing.
Figure 6.
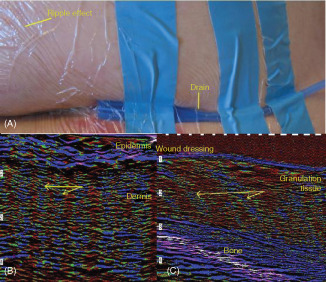
Tissue deformation monitored with high frequency diagnostic ultrasound (HFDU) scans through gauze and film dressing as−80 mmHg negative pressure wound therapy (NPWT) is applied. (A) Surface deformation effect on film; note linear effects and concentric distortions. (We subsequently found that wrapping gauze around the end of the silicone drain improves the reliability of the NPWT technique when using drains.) Tissue deformation monitored within (B) periwound skin and (C) wound bed granulation tissue. The plane of the ultrasound is perpendicular to the surface striations on the film.
Discussion
Many of the advances in our understanding of the mechanisms of action of NPWT have been derived from animal studies with experimental acute wounds 1, 7, 21, 22. However, few animal models have the capability to examine NPWT in the presence of significant tissue oedema which is frequently encountered in the clinical situations where it is deployed. Studies aiming at mechanistic investigations are sometimes difficult to perform unless suitable parameters can be measured which do not disturb the wound or harm the patient. In this pilot study, we explored the use of HFDU to non‐invasively conduct a quantitative assessment of the reduction in oedema.
The results from the pixel distribution analysis showed that the levels of oedema seen initially in the tissues surrounding the ulcers decreased rapidly after the application of NPWT. By 4 days, after the start of treatment, the level of tissue fluid had fallen by a mean of 43% and by 14 days tissue fluid content was near to the level seen in uninjured tissue in the same patients. The removal of excess fluid from periwound tissue is believed to be important as its presence can impede healing. Stagnant fluid in the wound area can contain a range of inhibitory substances such as pro‐inflammatory mediators; therefore, its removal would be expected to have a positive effect on healing (10). Tissue oedema is also expected to impede capillary blood flow and it would be interesting to determine if there were blood flow changes in the periwound tissue as oedema levels fell in such patients. The importance of fluid removal by NPWT has been described in the treatment of burns where rapid removal of excess fluid is thought to reduce the degree of damage caused in the intermediate zone of ischemia in Jackson's injury model 11, 23.
HFDU was also used to chart the accumulation of new tissue at the base of the wound by non‐invasively measuring the depth of tissue over the fixed bony prominences which had originally given rise to the ulcers. Differences were readily detectable by 7 days. This measurement is different from the more commonly reported reduction of area or volume and might in future complement these measurements, particularly in studies of pressure ulcers.
It is now widely accepted that at least part of the effect of NPWT derives from the mechanical induction of tissue deformation (9). Microdeformations in the surface tissue are able to induce cellular proliferation and angiogenesis and have been the subject of several reports 24, 25, 26. In contrast, very few studies have addressed the larger scale macrodeformation which may contribute to changes in blood flow and the phenomenon is termed ‘reversed tissue expansion’27, 28. In this study, deep deformations or ripples were observed in both the periwound and wound bed tissue on application of – 80 mmHg. Further investigation of macromechanical deformation during NPWT with HFDU could be valuable.
In conclusion, this pilot investigation has shown that HFDU is a simple method that is able to non‐invasively quantify reduction in oedema and increases in tissue accumulation during NPWT. It was also able to show the macrodeformation of wound tissue. Of course understanding how different filler materials or different levels of negative pressure affect oedema reduction, tissue accumulation or macrodeformation, would be interesting subjects for further study.
Acknowledgements
The study was funded by Smith and Nephew. RM is an employee of Smith and Nephew.
References
- 1. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 2. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76, discussion 577. [PubMed] [Google Scholar]
- 3. Mouës CM, van Toorenenbergen AW, Heule F, Hop WC, Hovius SER. The role of topical negative pressure in wound repair: expression of biochemical markers in wound fluid during wound healing. Wound Repair Regen 2008;16:488–94. [DOI] [PubMed] [Google Scholar]
- 4. Dunn R, Hurd T, Chadwick P, Cote J, Cockwill J, Mole T, Smith J. Factors associated with positive outcomes in 131 patients treated with gauze‐based negative pressure wound therapy. Int J Surg 2012;9:258–62. [DOI] [PubMed] [Google Scholar]
- 5. Dorafshar AH, Franczyk M, Gottlieb LJ, Wroblewski KE, Lohman RF. A prospective randomized trial comparing subatmospheric wound therapy with a sealed gauze dressing and the standard vacuum‐assisted closure device. Ann Plast Surg 2012, DOI: 10.1097/SAP.0b01 3e318221286c ;69:79–84. [DOI] [PubMed] [Google Scholar]
- 6. Birke‐Sorensen H, Malmsjo M, Rome P, Hudson D, Krug E, Berg L, Bruhin A, Caravaggi C, Chariker M, Depoorter M, Dowsett C, Dunn R, Duteille F, Ferreira F, Francos Martínez JM, Grudzien G, Ichioka S, Ingemansson R, Jeffery S, Lee C, Vig S, Runkel N, International Expert Panel on Negative Pressure Wound Therapy [NPWT‐EP], Martin R, Smith J. Evidence‐based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer) ‐ steps towards an international consensus. J Plast, Reconstr Aesthet Surg 2011;64:S1–16. [DOI] [PubMed] [Google Scholar]
- 7. Malmsjö M, Ingemansson R, Martin R, Huddleston E. Negative‐pressure wound therapy using gauze or open‐cell polyurethane foam: similar early effects on pressure transduction and tissue contraction in an experimental porcine wound model. Wound Repair Regen 2009;17:200–5. [DOI] [PubMed] [Google Scholar]
- 8. Borgquist O, Ingemansson R, Malmsjö M. The influence of low and high pressure levels during negative‐pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg 2011;127:551–9. [DOI] [PubMed] [Google Scholar]
- 9. Orgill DP, Bayer LR. Update on negative‐pressure wound therapy. Plast Reconstr Surg 2011;127 Suppl:S105–15. [DOI] [PubMed] [Google Scholar]
- 10. Webb LX, Dedmond B, Schlatterer D, Laverty D. The contaminated high‐energy open fracture: a protocol to prevent and treat inflammatory mediator storm‐induced soft‐tissue compartment syndrome (IMSICS). J Am Acad Orthop Surg 2006;14(10 Spec No):S82–6. [DOI] [PubMed] [Google Scholar]
- 11. Kamolz L‐P, Andel H, Haslik W, Winter W, Meissl G, Frey M. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns 2004;30:253–8. [DOI] [PubMed] [Google Scholar]
- 12. Chen L, Dyson M, Rymer J, Bolton PA, Young SR. The use of high‐frequency diagnostic ultrasound to investigate the effect of hormone replacement therapy on skin thickness. Skin Res Technol 2001;7:95–7. [DOI] [PubMed] [Google Scholar]
- 13. Mirpuri NG, Dyson M, Rymer J, Bolton PA, Young SR. High‐frequency ultrasound imaging of the skin during normal and hypertensive pregnancies. Skin Res Technol 2001;7:65–9. [DOI] [PubMed] [Google Scholar]
- 14. Kerr A, Young S, Hampton S. Has packing sinus wounds become a ritualistic practice? Br J Nurs 2006;15:S27–30. [DOI] [PubMed] [Google Scholar]
- 15. Young SR, Bolton PA, Downie J. Use of high‐frequency ultrasound in the assessment of injectable dermal fillers. Skin Res Technol 2008;14:320–3. [DOI] [PubMed] [Google Scholar]
- 16. Quintavalle PR, Lyder CH, Mertz PJ, Phillips‐Jones C, Dyson M. Use of high‐resolution, high‐frequency diagnostic ultrasound to investigate the pathogenesis of pressure ulcer development. Adv Skin Wound Care 2006;19:498–505. [DOI] [PubMed] [Google Scholar]
- 17. Chariker M, Jeter K, Tintle T. Effective management of incisional and cutaneous fistulae with closed suction wound drainage. Contemp Surg 1989;34:59–63. [Google Scholar]
- 18. Campbell PE, Smith GS, Smith JM. Retrospective clinical evaluation of gauze‐based negative pressure wound therapy. Int Wound J 2008;5:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gniadecka M. Localization of dermal edema in lipodermatosclerosis, lymphedema, and cardiac insufficiency. High‐frequency ultrasound examination of intradermal echogenicity. J Am Acad Dermatol 1996;35:37–41. [DOI] [PubMed] [Google Scholar]
- 20. Gniadecka M, Quistorff B. Assessment of dermal water by high‐frequency ultrasound: comparative studies with nuclear magnetic resonance. Br J Dermatol 1996;135:218–24. [PubMed] [Google Scholar]
- 21. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. [DOI] [PubMed] [Google Scholar]
- 22. Malmsjö M, Ingemansson R, Martin R, Huddleston E. Wound edge microvascular blood flow: effects of negative pressure wound therapy using gauze or polyurethane foam. Ann Plast Surg 2009;63:676–81. [DOI] [PubMed] [Google Scholar]
- 23. Jackson DM. The diagnosis of the depth of burning. Br J Surg 1953;40:588–96. [DOI] [PubMed] [Google Scholar]
- 24. Saxena V, Hwang C‐W, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 25. Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses M, Orgill DP. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006;56:418–22. [DOI] [PubMed] [Google Scholar]
- 26. Wilkes R, Zhao Y, Kieswetter K, Haridas B. Effects of dressing type on 3D tissue microdeformations during negative pressure wound therapy: a computational study. J Biomech Eng 2009;131:031012. [DOI] [PubMed] [Google Scholar]
- 27. Borgquist O, Gustafsson L, Ingemansson R, Malmsjö M. Micro‐ and macromechanical effects on the wound bed of negative pressure wound therapy using gauze and foam. Ann Plast Surg 2010;64:789–93. [DOI] [PubMed] [Google Scholar]
- 28. Banwell P, Téot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Tissue Viab 2006;16:16–24. [DOI] [PubMed] [Google Scholar]


