Abstract
Most chronic wounds are colonised with different microorganisms, especially problematic bacteria like methicillin‐resistant Staphylococcus aureus (MRSA), which represent an increasing therapeutic challenge in the modern wound therapy regimen. Therefore, it is essential to specify the bacteria in wounds for an individual‐specific treatment. In most patients, an exemplary bacterial swab is taken from the centre of the wound surface. This so‐called Levine technique is propagated currently as the gold standard. The aim of our clinical investigation was to compare the results of different swab techniques to the new established Essen Rotary. In this monocentric prospective investigation, 50 patients with chronic leg ulcers were examined consecutively. The results of our clinical study show that bacteria are heterogeneously spread on wound surfaces. The analysis of the semiquantitative measured results showed that the Essen Rotary could detect significant more bacteria with a total amount of 111 bacteria (P = 0·049) compared to usual swab techniques. Considerably, only the Essen Rotary identified five compared to three MRSA‐patients detected by other techniques. The Essen Rotary is an efficient, economic and uncomplicated modification of bacteriological swab techniques which detects significant more bacteria compared to other conventional swab techniques. Therefore, the Essen Rotary may become the new gold standard in routinely taken bacteriological swabs especially for MRSA screenings in patients with chronic leg ulcers.
Keywords: Essen Rotary; leg ulcer; Levine technique; MRSA; swab
Introduction
Enhanced knowledge about the bacterial colonisation of chronic wounds can improve treatment outcomes by reducing the risk of wound infection and sepsis, rate of bacterial spreading particularly with regard to methicillin‐resistant Staphylococcus aureus (MRSA) in hospital facilities and may have a positive influence on the wound treatment periods (1). Therapists have to differentiate between harmless wound contamination, where the presence of bacteria will not lead to any host reactions; the wound colonisation, where bacteria initiate or multiply a host reaction; and the so‐called critical colonisation, which may cause a delay in wound healing and where there is a danger of bacterial burden being switched from colonisation to local infection. The basis for this differentiation must be the clinical evaluation of the patient. Microbiological tests can help, however, in the estimation of these clinical situations. In case of an apparent infection, it is necessary for an individual adapted systemic antibiotic therapy to identify the causative organisms with their specific resistograms. Until now, there was no well‐defined practicable method available which provides reliable results about the bacteria in chronic wounds. However, the so‐called Levine technique is currently propagated by the World Union of Wound Healing Societies as the gold standard for bacterial swabs in patients with chronic wounds (2). According to the Levine technique the bacteriological swab is performed by rotating a swab over a 1 cm2 area of the wound surface with sufficient pressure to extract fluid from within the wound tissue 3, 4, 5.
The current open, monocentric and prospective clinical trial investigated the clinical outcome of these conventional swab techniques in the detection of bacterial burden in patients with chronic leg ulcers by comparing it to a new modified technique, the so‐called Essen Rotary. Our investigation aims to evaluate if the bacteria on the wound surfaces and especially if the verification of MRSA can be detected more reliably by the use of the Essen Rotary in comparison to conventional bacterial swab techniques.
Patients and methods
Patients
Participants (n = 50) were recruited from a dermatologic outpatient clinic in the University Hospital. All patients suffered from a chronic leg ulcer of minimum size of 10 cm2. A wound was defined as chronic if it had lasted for at least 3 months (6). None of the patients had a local or systemic infection, so that there was no need for antibiotic therapy during the investigation. The survey included 27 female patients and 23 male patients. Their ages ranged from 29 to 95 years, and the average was 65·9 years (women: 65·6 years and men: 65·5 years). In 37 cases, the wound was a chronic venous leg ulcer, other 5 cases were in accordance with a mixed arterial and venous genesis. The remaining eight wounds were of different genesis caused by vasculitis, pyoderma gangrenosum or graft‐versus‐host disease.
Bacterial swab
An important criterion for a correct survey of the distribution pattern of the bacterial burden was to perform the bacterial swab immediately after removing the wound dressing to avoid contamination and manipulation of the wound surface. The wound surfaces were divided into five different sectors, each of 1 cm2. Altogether, we took seven swabs from each patient. Four swabs in clockwise direction at 12, 3, 6 and 9 o'clock positions, and one in the centre of the wound. Afterwards, we took one swab according to the Levine technique as a reference and finally one swab by performing the Essen Rotary technique (Figure 1). The Essen Rotary represents a modification of Levine technique by taking the swab with pressure in spiral form from the outside of the wound into the centre (Figure 2).
Figure 1.
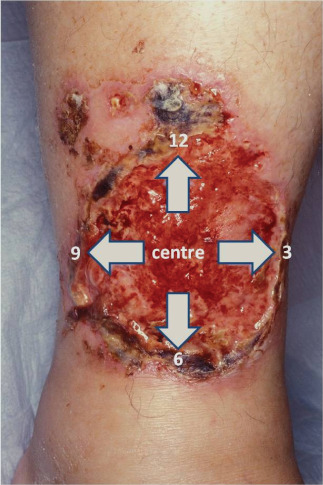
Partition of the wounds.
Figure 2.
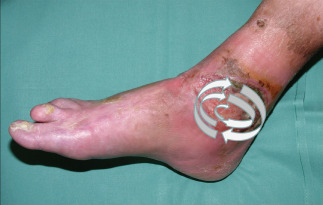
Schematic representation of the Essen Rotary.
Qualitative analysis
Bacteria were processed in the Institute for Microbiology, University of Essen, using qualitative procedures that allowed the identification of organisms per swab using the Vitek 2™ (bioMérieux Sa, Marcy l'Etoile, France) and Siemens WalkAway® 96 si (Siemens Medical Solutions Diagnostic GmbH, Bad Nauheim, Germany) automated analysis systems for bacterial resistance determination. The identification of the bacteria was done using the Advanced Colorimetry™ method. Several studies document that methicillin resistance can be detected reliably using this system 7, 8. The results from the swab cultures using the Levine technique were then compared to the results of the Essen Rotary and the five other sectors under the same terms and conditions.
Statistical analysis
The results from our investigation were compared by performing a paired two‐sample t‐test in which a P‐value of <0·05 was considered significant using SPSS 15.0 software (SPSS, Tokyo, Japan) for personal computers.
Ethical considerations
Our clinical investigations comply with the ethical rules for human experimentation as stated in the 1975 Declaration of Helsinki. This study was approved by the Ethics Committee of the University of Essen‐Duisburg.
Results
We confirmed that the bacterial spectrum was mostly inhomogeneous over the wound surface, which resulted insignificant discrepancies between the bacteria findings in the various areas of the wounds. Quantitatively the most relevant detected bacteria were Staphylococcus aureus in 52% and Pseudomonas aeruginosa in 46% of all investigated wounds. The analysis of the semiquantitative results also showed that we detected 111 bacteria of more significance with the Essen Rotary (P = 0·049) compared to all other techniques (Figure 3, Table 1). For instance, the Levine technique detected 90 bacteria only. A differentiated analysis of the various bacteria species with regard to the so‐called problematic bacteria, which are defined by the German Robert Koch Institute, provides evidence that 63 bacteria (26% more) were detected with the Essen Rotary compared with the conventional swab techniques which were able to detect only 50 problematic bacteria during the clinical investigation (Figure 4) (9). In some wounds, we found a very heterogeneous microenvironment presented by residential skin‐flora and facultative pathogenic bacteria, which could be detected by the Essen Rotary only. The Essen Rotary showed in 10% of all wounds a contamination with MRSA in the sector‐specific swab‐analysis. There was an additional mixed flora in 6% of the MRSA‐positive cases. In 4% of the cases, MRSA was the only detectable bacteria in the chronic wound. The conventional swab in the Levine technique was verified in only 7% of the cases as contamination with MRSA. A mixed flora could be found in 5%. It was of particular clinical importance that two of the five MRSA‐positive patients could be identified only with the Essen Rotary (5, 6, Table 2). In either instance an adequate targeted therapy and special hygienic procedures like isolation would not have taken place. Had the above been provided, consequently bacterial spreading could have been prevented.
Figure 3.
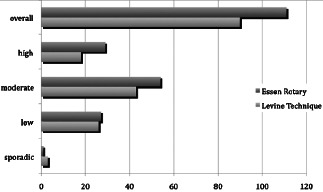
Results of the semiquantitative swab‐analysis in comparison of the conventional technique (Levine) and the Essen Rotary.
Table 1.
Results of the semiquantitative determined bacteriological swabs
| Area | Sporadic | Low | Moderate | High | Overall |
|---|---|---|---|---|---|
| Sector 3 | 6 | 23 | 28 | 21 | 78 |
| Sector 6 | 4 | 12 | 39 | 26 | 81 |
| Sector 9 | 4 | 24 | 50 | 19 | 97 |
| Sector 12 | 2 | 15 | 46 | 17 | 80 |
| Centre | 8 | 17 | 40 | 14 | 79 |
| Essen Rotary | 1 | 27 | 54 | 29 | 111 |
| Levine | 3 | 26 | 43 | 18 | 90 |
| Average | 4 | 20.6 | 42.9 | 20.6 | 88 |
Figure 4.
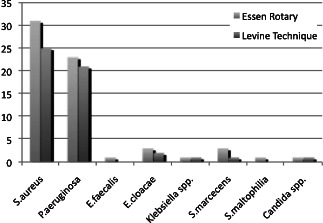
Problem‐bacteria according to bacteria with antibiotic resistances (German Infection Protection Act § 23 Cl. 1, page 1).
Figure 5.
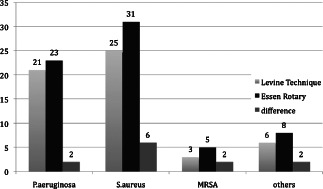
Direct comparison of the absolute results. Essen Rotary vs. conventionally performed swab according to the Levine technique.
Figure 6.
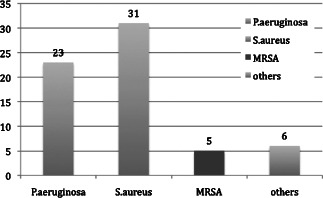
Results of MRSA‐positive wounds detected with the Essen Rotary.
Table 2.
Registration of the problem‐bacteria according to bacteria with antibiotic resistances (German‐Infection‐Protection‐Law, Act § 23 Cl. 1, page 1)
| Essen Rotary | Levine technique | |
|---|---|---|
| Staphylococcus aureus | 31 | 25 |
| Pseudomonas aeruginosa | 23 | 21 |
| Enterococcus faecalis | 1 | 0 |
| Enterobacter cloacae | 3 | 2 |
| Klebsiella spp. | 1 | 1 |
| Serratia marcecens | 3 | 1 |
| Stenotrophomonas maltophilia | 1 | 0 |
| Candida spp. | 1 | 1 |
Discussion
Owing to the world‐wide demographic shift, in Germany an increase in number of persons older than 60 years from currently 26·2% to 38·9% in the year 2050 is predicted. The number of 60‐year‐olds will be about 1 million, twice as high as the number of newborns. Those older than 80 years in Germany will increase from about 4 million to 10 million by 2050 (10). The incidence for suffering from a chronic leg ulcer increases up to 4% in those over 80 years each year, based on several studies (4). Therefore, it is absolutely necessary to optimise therapeutic and preventive measures for those patients. An important part of an optimised therapeutic treatment regimen is the knowledge about the bacterial burden in chronic wounds. Besides infections, even a clinical unproblematic bacterial colonisation can be very important for the treatment strategies especially if so‐called problematic bacteria like MRSA are verified. The detection of MRSA in wounds causes numerous practical consequences for the patient and the medical institution because the patient should be treated isolated from other patients 1, 5, 7, 11. This approach not only causes higher costs for the medical institution but also leads to a considerable psychological stress on patients. In the treatment of these patients, it is important to note that therapeutic decisions have to be based on clinical findings. If no clinical signs for a relevant infection can be found but rather an unproblematic colonisation, a local antiseptic therapy is adequate and the use of systemic antibiotics can be avoided completely 1, 12.
Currently, there exist different options to detect bacteria in patients with chronic wounds 13, 14. In daily routine, the performance of a swab is dependent on the person executing it. Several clinical investigations show that chronic wounds are not homogeneously colonised such that different swab techniques and swab areas may lead to absolutely different results. The analyses techniques used daily can be divided into at least three different methods (15). These are, according to their clinical relevance, the conventional swab in the Levine technique, the wound biopsy and a flush technique.
A comparative study of the informational value of the three different swab methods with regard to the identification of bacteria in a group of 83 patients with chronic wounds led to an infection rate of 36% for the Levine technique with an accuracy of P = 0·8. Levine et al. showed the use of a bacteriological swab in a collective of burn patients and that the bacterial count is linearly related to biopsy quantification of viable bacteria in the underlying tissue. He showed that the visualisation of bacteria indicates that at least 106 or more bacteria per swab are present after performing a Gram‐stained swab (3). It was also demonstrated that at a minimum threshold of 37 000 organisms per swab a sensitivity of 90% and a specificity of 57% could be achieved for the diagnostic value in the identification of infected chronic wounds with the Levine technique (14). He described his method as a simple technique which requires no surgical manipulation of the wound.
In another study which was performed in 1969 in 41 patients with wounds of different aetiology, a sensitivity of 100% and a specificity of 93·5% for the bacteriological survey findings could be detected by the use of wound biopsies (16). Therefore, the biopsy of wound tissue was seen in the last decades as the gold standard for clinical investigations. Owing to the high expenditure of time, the sole practicability by medical personnel, high costs compared to the bacteriological swabs, infringement of the healing tissue and the pain associated with a biopsy, this method should be reserved for specific clinical issues and especially for scientific investigations. In addition, it has already been proven scientifically that a biopsy has no significant higher outcome for the identification of clinically relevant bacterial infections in patients with chronic wounds compared to the quantitative bacteriological swabs (17).
Another specific diagnostic procedure, such as the proof of bacterial colonisation by the use of a special flush technique, is currently used only scientifically. In this case, the superficial bacterial flora is dissolved from the wound bed with a special rinse solution and subsequently analysed microbiologically. One advantage of this technique is the relatively safe detection of contamination or colonisation with MRSA on chronic wounds through the mobilisation of the pathogen bacteria. This procedure has not been standardised yet and cannot be used in daily practice for the routine examination of the microbial spectrum of a chronic wound because no similar results can be achieved. This method was presented several times at different wound congresses but scientific data in patients with chronic wounds are so far not yet available.
None of these methods was investigated adequately in greater collectives with wound patients and have been standardised so far, so that their results are not comparable or sufficient enough for current clinical diagnostic requirements. Quality and informational value of the bacteriological analyses differ similarly, for instance regarding the detection of MRSA. The most widely used swab technique currently is the Levine technique. The advantage of this method compared to the wound biopsy is that it is a non‐invasive procedure which is quite practicable, is mostly painless for the patient and can be performed without high technical expenditure. This swab technique is suitable for the semiquantitative evaluation of the aerobic bacterial burden of the wound surface. The disadvantage of this method is the fact that it will only be representative if the swab is taken exactly over the infected area. Furthermore, it is possible to get false‐negative results if too less wound fluid is collected. If the wound is too scabbed, necrotic or massively fibrinous clogged, a prior wound cleansing is necessary to receive a representative result. According to the current S3 guideline of the German Association for Phlebology (DGP) for diagnosis and therapy of chronic venous leg ulcers, a routinely performed bacterial analysis is not necessary for a wound with normal healing tendency and no signs of a clinically relevant infection. This procedure should be considered critical, particularly with regard to the identification of patients who are MRSA positive and have to be treated in isolation. Only few MRSA‐positive patients with chronic leg ulcers have clinically relevant signs for an underlying infection. An unproblematic bacterial colonisation does not obligate disturbance of the wound healing, so that these patients would not be completely identified and the medical staff can become a vector for these problematic bacteria (11). Therefore, we would suggest that a bacterial swab from the wound should be performed at least within the first consultation. If the patients have no clinical signs for a wound infection, the result of the bacterial swab has no direct influence on the further practical wound treatment. Exceptions result from detection of so‐called problem‐bacteria like MRSA and the ability of different bacteria to form a biofilm. This biofilm can complicate therapy immensely. Biofilms consist of biopolymers that include polysaccharides, proteins, lipids and nucleic acids. These components give the biofilm a firm consistency and bind to water. The different bacterial populations hosted in a biofilm are surrounded by a matrix composed of exopolysaccharides. This leads to a protection of the bacteria in the biofilm from the immune system of the host, local and systemic antibiotics and various other environmental influences. Particularly, with regard to biofilms of MRSA, researchers have drawn considerable interest to find out new approaches for its treatment over the past decade. As an example, the enzyme‐linked treatment may help to detach cells from a mature biofilm and return them to a planktonic state. The combination of matrix‐degrading agents, small molecules or targeting quorum sensing for biofilm treatments in combination with antibiotics is showing promise as an approach for eliminating established biofilms in different models for infections (18). These biofilms can be found on many wound surfaces, too. It has been suggested that biofilm maturation can lead to detachment of larger amounts of bacteria. These could be responsible for chronic and systemic infections or even sepsis based on a local infection starting from a chronic wound (12). In the current literature, it is still controversially discussed whether and how a biofilm can be objectified microbiologically, for example by using a novel multiplex polymerase chain reaction method, the Biofilm Ring Test (BFTR®, BioFilm Control SAS, Saint‐Beauzire, France) or crystal violet staining method 19, 20. Although the clinical relevance of biofilms can be very important and may affect the results of this study, biofilms were within our investigation and not examined separately.
Conclusion
The results of our clinical investigation show that bacteria can be very heterogeneously spread on the surfaces of chronic leg ulcers. Significant more bacteria can be detected with the new established Essen Rotary compared to other conventional swab techniques. This easy procedure takes only an additional few seconds to perform. Especially, for larger wounds where various bacterial species may be present in different areas it is important to investigate large areas of the wound surface to have representative data of the bacteria. In clinical practice, this approach is particularly useful for screening problematic bacteria such as MRSA.
The Essen Rotary is an efficient, economic and uncomplicated modification of a conventional bacteriological swab technique, which is able to detect almost the whole spectrum of aerobic bacteria of the wound surface in patients having a chronic leg ulcer compared to the Levine technique. Therefore, we would like to propose that the Essen Rotary may represent a new gold standard in routinely taken bacteriological swabs, especially for MRSA screenings in patients with chronic leg ulcers.
References
- 1. Dissemond J. Methicillin resistenter Staphylococcus aureus (MRSA): Diagnostik, klinische Relevanz und Therapie. J Dtsch Dermatol Ges 2009;7:544–53. 19302228 [Google Scholar]
- 2. WUWHS Expert Working Group . Wound infection in clinical practice. An international consensus. Int Wound J 2010;5(Suppl. 3):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levine NS, Lindberg RB, Mason AD, Pruitt BA. The quantitative swab culture and smears: a quick, simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma 1976;16:89–94. [PubMed] [Google Scholar]
- 4. Dissemond J. Ulcus cruris – Grundlagen, Diagnostik und Therapie, 3rd edn. Bremen: UNI‐MED Verlag, 2009. [Google Scholar]
- 5. Körber A, Schmid EN, Buer J, Klode J, Schadendorf D, Dissemond J. Bacterial colonisation of chronic leg ulcers: Current results compared to data 5 years ago in a specialised dermatologic department. J Eur Acad Dermatol Venereol 2010;24:904–9. [DOI] [PubMed] [Google Scholar]
- 6. Dissemond J. Wann ist eine Wunde chronisch? Hautarzt 2006;57:55. [DOI] [PubMed] [Google Scholar]
- 7. Ligozzi M, Bernini C, Bonora M, De Fatima M, Zuliani J, Fontana R. Evaluation of the VITEK‐2 system for identification and antimicrobial susceptibility testing of medically relevant Gram positive cocci. J Clin Microbiol 2002;40:1681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanders CC, Peyret M, Moland ES, Cavalieri SJ, Shubert C, Thomson KS, Boeufgras JM, Sanders WE Jr. Potential impact of the VITEK 2 system and the advanced expert system on the clinical laboratory of a University‐Based hospital. J Clin Microbiol 2001;39:2379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. RKI. Bundesgesundheitsbl Gesundheitsforsch. Gesundheitsschutz 2000;43:887–9. [Google Scholar]
- 10. Statistisches Bundesamt . 2006. URL http://www.bpb.de/files/H8YZFH.pdf [accessed on 7 November 2006].
- 11. Linde H, Lehn N. Methicillin‐resistenter Staphylococcus aureus (MRSA) – Therapie und Hygienemaßnahmen. Dtsch Med Wochenschr 2005;130:586–8. [DOI] [PubMed] [Google Scholar]
- 12. Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol 2008; 322:207–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dissemond J, Schmid EN, Esser S, Witthoff M, Goos M. Untersuchungen zur bakteriellen Kolonisation chronischer Wunden in einer universitären dermatologischen Wundambulanz unter besonderer Berücksichtigung von ORSA. Hautarzt 2004;55:280–8. [DOI] [PubMed] [Google Scholar]
- 14. Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair Regen 2006;14:548–55. [DOI] [PubMed] [Google Scholar]
- 15. Zemsauer N. Vergleich von Methoden zur beschleunigten mikrobiologischen Sepsisdiagnostik. Klinisches Institut für Hygiene und Medizinische Mikrobiologie AKH Wien, 11.2007.
- 16. Robson MC, Heggers JP. Bacterial quantification of open wounds. Mil Med 1969;134:19–24. [PubMed] [Google Scholar]
- 17. Bill TJ, Ratliff CR, Donovan AM, Knox LK, Morgan RF, Rodeheaver GT. Quantitative swab culture versus tissue biopsy: a comparison in chronic wounds. Ostomy Wound Manage 2001;47:34–7. [PubMed] [Google Scholar]
- 18. Kiedrowski MR, Horswill AR. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci 2011;1241:104–21. [DOI] [PubMed] [Google Scholar]
- 19. Iorio NL, Azevedo MB, Frazão VH, Barcellos AG, Barros EM, Pereira EM, de Mattos CS, dos Santos KR. Methicillin‐resistant Staphylococcus epidermidis carrying biofilm formation genes: detection of clinical isolates by multiplex PCR. Int Microbiol 2011;14:13–7. [DOI] [PubMed] [Google Scholar]
- 20. Liesse Iyamba JM, Seil M, Devleeschouwer M, Takaisi Kikuni NB, Dehaye JP. Study of the formation of a biofilm by clinical strains of Staphylococcus aureus . Biofouling 2011;27:811–21. [DOI] [PubMed] [Google Scholar]


