Abstract
Negative pressure wound therapy (NPWT) is becoming routine for the preparation of wounds prior to grafting for wound closure. We have been using both foam‐ and gauze‐based NPWT to prepare wounds for closure prior to skin grafting and have obtained similar proportions of closed wounds; 7/7 for wounds treated with gauze‐based NPWT and 11/11 for wounds treated with foam‐based NPWT. In our follow‐up consultations we observed that skin grafts on the foam‐treated patients were less pliable than those on the gauze‐treated patients. To assess what the mechanism of this effect might be, we compared the specific details of the treatments of both 11 foam and 7 gauze patients, including depth, location, patients' age and co‐morbidity; biopsies of granulation and scar tissue were taken and stained with haematoxylin–eosin and by Masson's trichrome staining and conducted ultrasound analysis of the closed wounds, to see if there were features which explained those effects. All foam patients were treated at −125 mm Hg for an average of 25·9 days before skin grafts were applied. All gauze patients were treated at −80 mm Hg for an average of 24·7 days before skin grafts were applied. Biopsies of granulation tissue prior to skin grafting from five foam and four gauze‐based NPWT patients did not reveal any obvious histological differences between the treatments. Ultrasound analysis of the skin‐grafted wounds showed an average depth of scar tissue of 18 mm in the wound beds of the foam‐treated wounds and 7 mm in the gauze‐treated ones. Biopsies taken on the scar tissue after treatment with the gauze showed a minor tissue thickness and disorganisation and less sclerotic components. The findings of this preliminary analysis suggest that foam‐based NPWT may induce a thicker layer of scar tissue beneath skin grafts than gauze‐based NPWT which might explain a reduced pliability of the reconstructed bed. At present it is unclear which mechanism might be responsible for the difference in pressure (−125 versus −80 mm Hg), either the length of the time taken to reconstruct the wound bed or the intrinsic nature of the foam or gauze on the tissue surface. Prospective studies are necessary to investigate whether these preliminary observations are confirmed and to investigate what the mechanism might be.
Keywords: Chariker–Jeter method, Foam, Gauze, Negative pressure wound therapy, V.A.C. therapy
INTRODUCTION
Negative pressure wound therapy (NPWT) is widely used in managing and accelerating wound healing. In our department, so far, two different fillers have been used to transfer negative pressure onto the wound: the foam (V.A.C. KCI, San Antonio, TX) introduced by Argenta and Morykwas in 1997 and the gauze (V1STA™ Blue Sky Medical/SMITH&NEPHEW, London, UK) available from 2007. Despite the quick introduction of this device into clinical practice, the mechanism by which this method stimulates wound healing has not been fully defined.
To apply negative pressure on the surface of the wound, polyurethane (PU) or polyvinylalcohol foam and polyhexamethylene biguanide (PHMB) pre‐impregnated gauze are available. These fillers are introduced in the wound and fixed with the use of an adhesive dressing. A negative pressure generator is used to achieve suction and drainage. The optimal pressure range to obtain a good clinical result for the foam is between 80 and −125 mm Hg (1) and for the gauze it is between −40 and −80 mm Hg (2). The aim of our study was not to compare the two different levels of NPWT applied to the wound, but to compare the maximum clinical effect obtained with the foam and that obtained with the gauze, as stated in the literature 1, 2.
NPWT is becoming routine for the preparation of wounds prior to grafting for wound closure. We have been using both foam‐ and gauze‐based NPWT to prepare wounds for closure prior to skin grafting and have obtained similar proportions of closed wounds; 7/7 for wounds treated with gauze‐based NPWT and 11/11 for wounds treated with foam‐based NPWT. In our follow‐up consultations we observed that skin grafts on the foam‐treated patients were less pliable than those on the gauze‐treated patients.
The aim of our work is to evaluate whether the foam (V.A.C. KCI) or the gauze (V1STA™ Blue Sky Medical/SMITH&NEPHEW) can induce different types of granulation or scar tissue and whether these data can suggest different clinical applications.
For this purpose, from May 2008 through September 2010, we evaluated 22 Caucasian patients admitted to our department and treated them with gauze‐based NPWT, out of whom 7 were selected after passing the inclusion/exclusion criteria. We compared them with 11 patients selected from our long‐term case histories of foam‐treated patients. No differences were found when comparing the two different fillers regarding wound healing. However, we noticed some macroscopic differences on the wound bed. Therefore, we led a histological and immunohistochemical evaluation after taking biopsies of the wound beds.
From 6 to 15 months after healing, we did an ultrasonographic (US) study with the aim of appraising the pattern of the newly reconstructed tissue followed by an echocontrastography to assess the revascularisation. A histological and immunohistochemical evaluation was performed later on the biopsies taken from the scar tissue.
In this article, we will discuss our preliminary clinical, histological, immunohistochemical and ultrasonographical findings.
MATERIALS AND METHODS
The study was conducted in the Department of Reconstructive and Aesthetic Plastic Surgery at the University Hospital San Giovanni Battista of Turin during the period from May 2008 to September 2010.
The treatment with V1STA™ uses a PHMB pre‐impregnated gauze with saline, placed on the bottom of the wound. This method needs a negative pressure of 80 mm Hg transferred by a drain according to the Chariker–Jeter method (2). We used this level of pressure constantly. In this study, for the treatment with V.A.C Therapy, we always used a PU foam with a pore size of 400–600 mm (V.A.C.® Pack Dressing) to transfer a constant negative pressure of 125 mm Hg (1). In both methods a transparent adhesive was used to fix the dressing around the drain to complete the seal in accordance with the manufacturer's guidelines 1, 2. Dressing was changed once every 3 days and a wound measuring system with laser and a digital camera was used weekly to evaluate the macroscopic changes on the wound bed.
For this study, we compared a pool of 7 gauze‐treated patients with a pool of 11 foam‐treated patients after following the inclusion/exclusion criteria. Inclusion criteria were acute post‐traumatic (car/motorcycle accident or surgical complication) wounds up to the muscular band, age range 18–80 and wound size from 30 cm2. Exclusion criteria were chronic wounds, diabetes and pregnancy.
After approval by the ethics committee and obtaining informed consensus, 12–15 days after the traumatic event (or else after NPWT application) 18 biopsies were taken on nine adult patients. Out of the 18 biopsies of the granulation tissue, nine were taken on four patients treated with gauze (three female and one male, average age: 84·36, average time of treatment: 56·48 days); nine were taken on five patients treated with foam (five male, average age: 41·76, average time of treatment: 33·89 days) (Table 1). In the four patients treated with gauze there were two cases of lower extremity post‐traumatic wounds, one case of nectrotising fasciitis of the lower leg after heart transplantation and one scalp wound case after complication of selective embolisation of the internal maxillary artery for epistaxis. In all the five patients treated with foam, the wounds were located at the lower extremity (Table 1).
Table 1.
Patients' baseline characteristics, and examinations performed on each patient
| Patient | Age | Sex | Wound location | Filler used | Duration (days) of NPWT | Healed | Type of healing | Biopsies (n) taken | Ultrasonographic examination performed (Yes/No) | Echocontrastography performed (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | M | Leg | Gauze | 49 | Yes | Skin graft | 3 | No | No |
| 2 | 79 | F | Leg | Gauze | 6 | Yes | Skin graft | 2 | Yes | No |
| 3 | 83 | F | Scalp | Gauze | 27 | Yes | Skin graft | 2 | No | No |
| 4 | 80 | F | Leg | Gauze | 30 | Yes | Skin graft | 2 | Yes | No |
| 5 | 29 | M | Foot | Gauze | 21 | Yes | Skin graft | 0 | Yes | Yes |
| 6 | 79 | F | Leg | Gauze | 30 | Yes | Skin graft | 0 | No | Yes |
| 7 | 30 | F | Leg | Gauze | 10 | Yes | Skin graft | 0 | Yes | No |
| 8 | 43 | M | Leg | Foam | 27 | Yes | Skin graft | 3 | Yes | No |
| 9 | 34 | M | Leg | Foam | 16 | Yes | Skin graft | 1 | No | No |
| 10 | 60 | M | Foot | Foam | 5 | Yes | 2nd intention | 2 | No | No |
| 11 | 81 | M | Foot | Foam | 29 | Yes | Skin graft | 1 | No | No |
| 12 | 33 | M | Leg | Foam | 36 | Yes | Skin graft | 2 | No | No |
| 13 | 69 | M | Leg | Foam | 39 | Yes | Skin graft | 0 | Yes | Yes |
| 14 | 44 | M | Leg | Foam | 18 | Yes | Skin graft | 0 | Yes | Yes |
| 15 | 63 | M | Foot | Foam | 50 | Yes | Skin graft | 0 | Yes | Yes |
| 16 | 40 | M | Foot | Foam | 5 | Yes | Skin graft | 0 | Yes | No |
| 17 | 20 | M | Leg | Foam | 45 | Yes | Skin graft | 0 | Yes | No |
| 18 | 67 | M | Foot | Foam | 15 | Yes | Skin graft | 0 | Yes | No |
Bioptic samples were fixed in 4% formalin and were put in paraffin. Three microsections were coloured with haematoxylin–eosin and by Masson's trichrome staining. The following parameters were considered when using haematoxylin–eosin: hemorrhage, necrosis, hyaline–granulocytic–lymphocytic vasculitis, vessel size, wall thickness of the vessels, acute inflammation, chronic inflammation and the presence of apoptotic nuclei in the vascular endothelium. With Masson's trichrome staining, we considered the amount of collagen fibres and their orientation and organisation. Immunohistochemical evaluation was performed by avidin–biotin technique using the anti‐actina flat muscle monoclonal antibody (AG clone) to mark myofibroblasts and pericytes. Another monoclonal antibody anti CD‐34 (QBEnd 10 clone) was used to mark the endothelial cells, the size and shape of the vessels and the number of myofibroblasts. They were analysed by a pathologist blinded to the treatment.
From 6 to 15 months after healing, on selected and homogeneous patients, an US examination was made to evaluate the thickness and the ultrasonographical pattern of the newly reconstructed skin, comparing it with the contralateral physiological one. We analysed a total of 12 areas in 11 patients. Four areas in four patients treated with gauze (one male and three female, average age: 53, average time of treatment: 16·75 days) and eight areas in seven patients treated with foam (all male, average age: 48·86, average time of treatment: 16·4 days). In all patients the wounds were located at the lower extremities: they were all post‐traumatic wounds with loss of tissue up to the muscular band. The US examination was performed using ESAOTE TECHNOS device (ESAOTE S.p.A, Geneva, Italy) supported by a high‐frequency probe (10–13 MHz) to study the superficial structures and colour‐Doppler module to evaluate tissue vascularisation. We evaluated thickness, echoicity and skin vascularisation after reconstructive treatment. The evaluation was performed by scanning different planes and comparing neighbouring and contralateral symmetric cutaneous normal areas with the new regenerated tissue. They were analysed by a sonographer blinded to the treatment.
To view and analyse the newly formed vessels, from 12 to 22 months after healing, we continued our study using the echocontrastography to assess the neovascularisation of the newly regenerated tissue. The echocontrastography examination was performed in single blind using the Mylab 70 xVG ESAOTE (Esaote SpA) equipment supported by two probes with 6–18 MHz and 4–13 MHz frequencies. Before the examination, we injected the contrast agent SonoVue (Bracco International, Amsterdam, The Netherlands), consisting of microbubbles of sulphur hexafluoride, into the veins of selected patients. SonoVue is a contrast agent for ultrasound used in the exploration of the great vessels or organs. It is a loss of millions of microbubbles, each one smaller than a red blood cell. The bubbles reflect the ultrasound signal and increase the echogenicity of blood with respect to other body tissues. The agent consists of powder and solvent for injection. We analysed a total of five areas in five patients; two areas in two patients treated with gauze (one male and one female, average age: 50 years, average time of treatment: 25 days) and three areas in three patients treated with foam (all male, average age: 59 years, average time of treatment: 12 days) (Table 1). In all patients, the wounds were located in the lower extremities. They were all post‐traumatic wounds with loss of tissue up to the muscular band, onto which a medium thickness graft taken from the upper third of the thigh was applied.
To support these data, 12 to 15 months after healing, we also took five biopsies from the newly formed tissue after NPWT and skin graft application. Then we led histological and immunohistochemical analyses on these biopsies. The difficulty in achieving biopsy is to perform a surgical procedure on a healthy person; hence, the number of the biopsies from scar tissue is low. These biopsies were taken from patients who were already having other surgical procedures done close to the lesion, so it was possible to achieve the biopsies without any further anaesthesia. From the five biopsies taken on scar tissue of three patients (in all three cases skin grafts were taken from the anterolateral side of the thigh), three biopsies were taken from two patients treated with gauze (one male and one female, mean age: 54, mean time of treatment: 13·5 days) and two from a patient treated with foam (male, age: 63, duration of treatment: 50 days). In the two patients treated with gauze they were leg and foot healed post‐traumatic wounds. The wound of the patient treated with foam was located at the leg and was post‐traumatic too.
RESULTS
All foam patients were treated at −125 mm Hg for an average of 25·9 days before skin grafts were applied. All gauze patients were treated at −80 mm Hg for an average of 24·7 days before skin grafts were applied.
The clinical results obtained after application of NPWT using gauze are similar to the clinical results of our long‐term case history of NPWT using foam. We observed an increased stimulation of granulation tissue formation and reduction of oedema with good exudate management, resulting in local condition improvement of wounds after the use of the two fillers.
Biopsies of granulation tissue prior to skin grafting from five foam and four gauze‐based NPWT patients did not reveal any obvious histological differences between the two treatments. The histological and immunohistochemical analyses (1, 2, 3, 4, 5, 6) of the biopsies of the granulation tissue evidence a similar pattern in patients treated with gauze compared with those treated with foam in regard to inflammatory cells, myofibroblast and new blood vessels.
Figure 1.
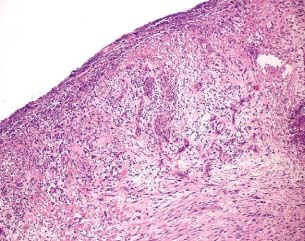
Haematoxyline–eosin evaluation of the granulation tissue after treatment with gauze (patient 2 from Table 1).
Figure 2.
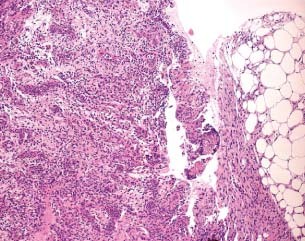
Haematoxyline–eosin evaluation of the granulation tissue after treatment with foam (patient 8 from Table 1).
Figure 3.
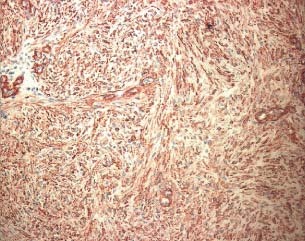
Immunohistochemical evaluation using anti‐actina antibody after treatment with gauze (patient 4 from Table 1).
Figure 4.
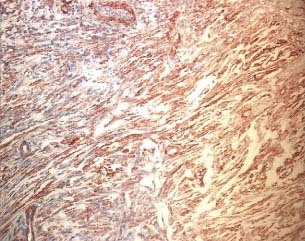
Immunohistochemical evaluation using anti‐actina antibody after treatment with foam (patient 9 from Table 1).
Figure 5.
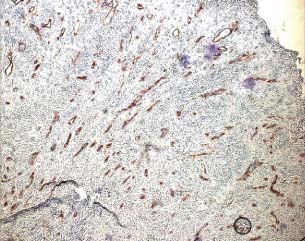
Immunohistochemical evaluation using the antibody anti CD‐34 after treatment with gauze (patient 4 from Table 1).
Figure 6.
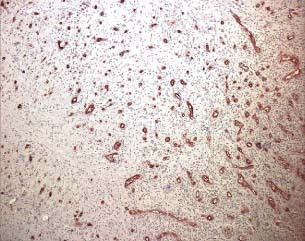
Immunohistochemical evaluation using the antibody anti CD‐34 after treatment with foam (patient 11 from Table 1).
In spite of the histological data, the US examination of selected and homogeneous patients reveals that the newly reconstructed tissue of patients treated with gauze is more similar to the physiological one. Ultrasound analysis of the skin‐grafted wounds showed an average depth of scar tissue (mean) of 18 mm, median 20 and SD 5 in the beds of the foam‐treated wounds and an average depth of 7 mm, median 7 and SD 0·8 in the gauze‐treated ones. Ultrasonography showed that the scar tissue thickness after treatment with foam is approximately twice the scar tissue obtained after treatment with gauze (7, 8). Hypoechoicity is much more evident in foam‐treated patients (9, 10): it means that the fibrotic tissue is more represented (Table 2; 9, 10).
Figure 7.
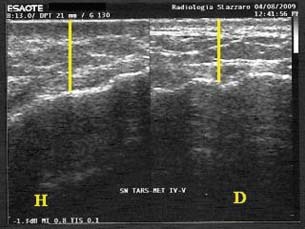
Thickness of the scar tissue after treatment with gauze (US exam: on the left healthy tissue, on the right scar tissue; patient 5 from Table 1).
Figure 8.
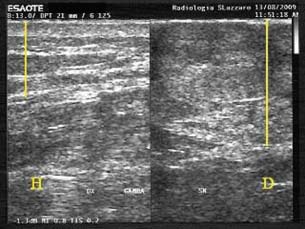
Thickness of the scar tissue after treatment with foam (US exam: on the left healthy tissue, on the right scar tissue; patient 13 from Table 1).
Figure 9.
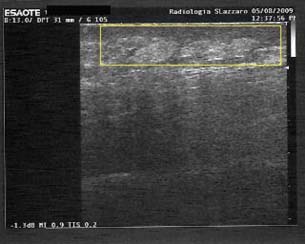
Low hypoechoicity of the scar tissue after treatment with gauze (US exam; patient 4 from Table 1).
Figure 10.
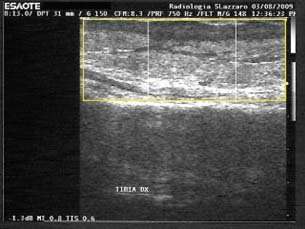
High hypoechoicity of the scar tissue after treatment with foam (US exam; patient 8 from Table 1).
Table 2.
Ultrasonography: semi‐quantitative evaluation of the thickness and detection or not detection of hypoechoicity of the new reconstructed tissue after NPWT (6 to 15 months after healing)
| Patient * | Months after healing | Thickness | Hypoechoicity |
|---|---|---|---|
| 2 | 9 | + | No |
| 4 | 12 | + | No |
| 5 | 15 | + | No |
| 7 | 6 | + | No |
| 8 | 6 | +++ | Yes |
| 13 | 9 | +++ | Yes |
| 14 | 6 | +++ | Yes |
| 15 | 6 | ++ | Yes |
| 16 | 15 | ++ | Yes |
| 17 | 15 | +++ | Yes |
| 18 | 15 | + | Yes |
*The numeration of the patients is taken from Table 1.
+ 5–10 mm, ++ 15–20 mm, +++ 20–25 mm.
From the little data gathered from echocontrastography, it appears that revascularisation after treatment with gauze is higher. The presence of less scar tissue after NPWT with gauze is accompanied by an increased formation of new mini‐vessels. The presence of this blood supply leads to the restoration of the physiological condition.
In the three biopsies taken on the scar tissue after treatment with gauze we observed a minor tissue thickness and disorganisation and less sclerotic components compared with the two biopsies taken on the new reconstructed tissue after treatment with foam.
DISCUSSION
The aim of this study is to compare the two different fillers presently available, used to apply negative pressure on the wound bed and to identify if there are different indications for their use. Nowadays, NPWT is safe and practical to use with a real effectiveness in the treatment of difficult wounds, both acute and chronic (3).
The first studies on NPWT were published in Russian medical literature 4, 5, 6, 7, 8 and collected with the so called ‘Kremlin Papers'. Meanwhile, in 1989 the Americans Chariker and Jeter et al. published their experience on the use of NPWT in the treatment of incisional and cutaneous fistulae (9). In 1997, Argenta and Morykwas published their clinical experience on vacuum‐assisted closure using foam (10). Later, in 2007, Smith and Nephew introduced gauze as a new filler to transfer negative pressure on the wound.
The effectiveness of NPWT is well known, but its working process still remains to be fully understood. NPWT results in mechanical deformation of the wound, which is widely believed to play a role in the mechanism through which NPWT stimulates the formation of new tissue.
Experimental studies, clinical experience and the latest randomised clinical trials have shown that NPWT increases formation of granulated tissue, reduction of the wound area, cell division, perfusion of peri‐wound area and decreases local and interstitial tissue oedema 11, 12, 13, 14, 15, 16. Moreover, a recent study performed on diabetic mice evidences that NPWT stimulates cell‐proliferation (17).
Generally, in our department two types of fillers are used to apply the negative pressure: PU foam and PHMB pre‐impregnated gauze.
Our present study confirms that gauze is effective in the cure of complicated wounds. These patients showed a progressive healing resulting in mechanical deformation of the wound as referred in the literature 18, 19.
Concerning the level of negative pressure, the optimal pressure is not known, but the literature suggests that different pressures exist for different fillers. The recommended negative pressure used for V.A.C. therapy using foam is from −80 to −125 mm Hg (1). This pressure can be increased or decreased on demand between 50 and 200 mm Hg with the V.A.C. device. A decrease in blood flow below baseline was shown when negative pressure reached 400 mm Hg (20). For the gauze, the optimal pressure is from −40 to −80 mmHg (2). Wackenfors et al. in 2004 used a laser Doppler device to measure microvascular blood flow to an inguinal wound in a pig during V.A.C therapy and they concluded that a low negative pressure during the treatment may be beneficial, especially in soft tissue, to minimise possible ischaemic effects (20). Kairinos et al. in 2009 21, 22 concluded that NPWT should be used with caution on tissue with compromised vascularity, particularly when it is used circumferentially. In NPWT perfusion decreases for increasing suction pressure (22) according to the different levels of pressure used for the fillers; we prefer using foam in hyperexudating wounds and in lesions with slough, and gauze in ischaemic and granulated wounds.
Furthermore, from the patient data we have observed that gauze is more manageable and more useful in the treatment of irregular wounds and round surface wounds with undermined edges and subcutaneous fistulae. As for flat wounds and great loss of tissue, both fillers are equally useful.
Moreover, gauze is capable of staying on for 72 hours (2) (the reason why it is previously impregnated with polyhexamethylene biguanide, in case the device switches off) while foam has to be changed after 2 hours (1).
We used either, gauze or foam, to speed up wound healing after skin grafting.
In our case studies, we noticed a different granulation tissue on the wound bed; using foam we observed an irregular patchy wound bed which needed a second surgical procedure to flatten the receiving area before skin grafting; using gauze we observed a uniform wound bed which was then more adaptable for skin graft. Considering this clinical evidence, we studied if these data coincided with histological specimens. It was the clinical statement that urged us to perform this study. This statement was also supported by the experimental studies obtained from literature which state that there are anatomical differences between the two fillers. The foam because of micropores present on it permits the ingrowth of the granulation tissue in the micropores (this probably is the reason for the irregular patchy wound bed observed when using the foam) while the gauze because of its dense lines does not permit this ingrowth of the granulation tissue (23).
To this purpose, after approval by the ethics committee and obtaining informed consent from patients, we performed the histological and immunohistochemical study on 18 biopsies of adult patients, taken from the granulation tissue located on the wound bed after NPWT, during reconstructive surgical procedures (before skin grafting). In the 18 biopsies taken from the granulation tissue we compared the histological pattern concerning the tissue organisation and the vascular morphology in patients treated with gauze and the pattern in patients treated with foam, and they were found to be similar. The immunohistochemical pattern regarding the inflammatory cells, myofibroblast and new blood vessels was also similar.
After skin graft reconstruction, the tissue obtained after NPWT with gauze seemed to be more pliable than that obtained with foam. Less pliability of the wounds means less scar tissue: certain anatomical areas like flexor, extensor regions, the main function of which is movement, are more important than the permanent mechanical strength of the wound. In the areas where the mechanical strength of the wound is more important we used foam as it gives a better clinical result.
To study the newly reconstructed tissue we led an US examination of the scar tissue of these patients, from 6 to 15 months after healing. Physiologically, the US pattern of normal skin is represented as follows:
-
–
a hyperechoicity layer determined by entrance point of US beam (probe‐gel‐skin interface);
-
–
a middle layer characterised by medium echoicity, represented by the dermis, delimited in depth by another hypoechoicity layer which constitutes the surface anchored layer dermis
-
–
hypodermis; and
-
–
a lobular hypoechoicity layer represented by hypodermis with thin hyperechoicity fibres demarked in depth by the muscular band. Ultrasonography showed that the scar tissue thickness after treatment with foam was approximately twice the scar tissue obtained on treatment with gauze (Table 2; 7, 8). Hypoechoicity is much more evident in those cases treated with foam: which means that the fibrotic tissue is more represented (Table 2; 9, 10). In both cases, through colour‐Doppler evaluation, an increase in flow was found with respect to the surrounding healthy skin areas.
These data were confirmed after analysis with echocontrastography, which showed a higher degree of physiological restoration of the blood flow in patients treated with gauze. However the study was carried out in a short time and there are only few data available.
On the basis of the histological and immunohistochemical analyses achieved from the biopsies of the newly formed tissue after NPWT and skin graft application. This analyses can be considered the ideal technique to support our data, but the difficulty of having to perform a surgical procedure in a healthy person has to be considered. From the limited data achieved, in the biopsies taken on the scar tissue after treatment with gauze, we observed minor tissue thickness and disorganisation and fewer sclerotic components compared with the biopsies taken on newly reconstructed tissues after treatment with foam. Moreover, the less representation of the sclerotic component in the new reconstructed tissue after treatment with gauze seems to permit an easier blood vessel growth. In fact, the vessel number is higher, the diameter of the vessels is larger and the morphology of the vessels is more similar to the physiological one. All these data are confirmed by the recent experimental studies of Malmsjo et al. (19) and Borgquist et al. (24).
From the analysis of these preliminary data, we can imagine that the scar tissue after treatment with gauze seems to be more similar to the physiological one than the scar tissue after treatment with foam. The findings of this preliminary analysis suggest that foam‐based NPWT may induce a thicker layer of scar tissue beneath skin grafts than gauze‐based NPWT, which possibly explains the reduced pliability of the reconstructed bed. At present it is unclear which factor might be responsible for the difference in pressure (−125 versus −80 mm Hg), the length of time taken to reconstruct the wound bed or the intrinsic nature of the foam or gauze on the tissue surface. Prospective studies are needed to investigate whether these preliminary observations are confirmed and to investigate what the mechanism might be.
Therefore, it can be concluded that different indications exist to use negative pressure either with foam or gauze depending on the different types of wounds, different types of patients and perhaps different anatomical regions (19).
ACKNOWLEDGEMENTS
All authors disclose that they do not have any financial and personal relationships with other people or organisations that could influence this work. This study was also not sponsored by any company.
REFERENCES
- 1. KCI. V.A.C.: recommended guidelines for use. Physician & Caregiver Reference Manual . San Antonio, TX: KCI, 2002, 2‐B‐128.
- 2.Smith&Nephew. V1STA™. Recommended using protocol., authors
- 3. Argenta LC, Morykwas MJ, Marks MW, DeFranzo AJ, Molnar JA, David LR. Vacum‐assisted closure: state of clinical art. Plast Reconstr Surg 2006;117:127S–42S. [DOI] [PubMed] [Google Scholar]
- 4. Kostiuchenok II, Kolker VA, Karlov VA. The vacuum effect in the surgical treatment of purulent wounds. Vestnik Khirurgii 1986;9:18–21. [PubMed] [Google Scholar]
- 5. Davydov YA, Malafeeva AP, Smirnov AP. Vacuum therapy in the treatment of purulent lactation mastitis. Vestnik Khirurgii 1986;9:66–70. [PubMed] [Google Scholar]
- 6. Usupov YN, Yepifanov MV. Active wound drainage. Vestnik Khirugii 1987;4:42–5. [Google Scholar]
- 7. Davydov YA, Larichev AB, Menkov KG. The bacteriological and cytological assessment of vacuum therapy of purulent wound. Vestnik Khirugii 1988; 10:48–52. [Google Scholar]
- 8. Davydov YA, Larichev KG, Abramov AY. Concepts for clinical biological management of the wound process in the treatment of purulent wounds using vacuum therapy. Vestnik Khirugii 1991; 2:132–5. [Google Scholar]
- 9. Chariker ME, Jeter KF, Tintle TE. Effective management of incisional and cutaneous fistulae with closed suction wound drainage. Contemp Surg 1989;34:59–63. [Google Scholar]
- 10. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–77. [PubMed] [Google Scholar]
- 11. Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum‐assisted closure device. Plast Reconstr Surg 2008;122:786–97. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 13. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–37; discussion 1038. [DOI] [PubMed] [Google Scholar]
- 14. Moues CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004;12:11–7. [DOI] [PubMed] [Google Scholar]
- 15. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96; discussion 1097–8. [DOI] [PubMed] [Google Scholar]
- 16. Timmers MS, Le Cessie S, Banwell P, Jukema GN. The effects of varying degrees of pressure delivered by negative‐pressure wound therapy on skin perfusion. Ann Plast Surg 2005;55:665–1. [DOI] [PubMed] [Google Scholar]
- 17. Moues CM, van Toorenenbergen AW, Heule F, Hop WC, Hovius SER. The role of topical negative pressure in wound repair: expression of biochemical markers in wound fluid during wound healing. Wound Rep Reg 2008;16:488–94. [DOI] [PubMed] [Google Scholar]
- 18. Campbell PE, Smith GS, Smith JM. Retrospective clinical evaluation of gauze‐based negative pressure wound therapy. Int Wound J 2008;5: 280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malmsjo M, Ingemansson R, Martin R, Huddleston E. Negative‐pressure wound therapy using gauze or open‐cell polyurethane foam: similar early effects on pressure transduction and tissue contraction in an experimental porcine wound model. Wound Rep Reg 2009;17:200–5. [DOI] [PubMed] [Google Scholar]
- 20. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. [DOI] [PubMed] [Google Scholar]
- 21. Kairinos N, Voogd AM, Botha PH, Kotze T, Kahn D, Hudson DA, Solomons M. Negative‐pressure wound therapy II: negative‐pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg 2009;123:601–12. [DOI] [PubMed] [Google Scholar]
- 22. Kairinos N, Solomons M, Hudson DA. Negative‐pressure wound therapy I: the paradox of negative‐pressure wound therapy. Plast Reconstr Surg 2009;123:589–98; discussion 599–600. [DOI] [PubMed] [Google Scholar]
- 23. Borgquist O, Gustafsson L, Ingemansson R, Malmsjö M. Tissue ingrowth into foam but not into gauze during negative pressure wound therapy. Wounds 2009;21:302–9. [PubMed] [Google Scholar]
- 24. Borgquist O, Gustafsson L, Ingemansson R, Malmsjö M. Micro‐ and macromechanical effects on the wound bed of negative pressure wound therapy using gauze and foam. Ann Plast Surg 2010;64:789–93. [DOI] [PubMed] [Google Scholar]


