Abstract
Mediastinitis is treated with either vacuum‐assisted closure (VAC) or traditional closed drainage (TCD) with irrigation. The aim of the study was to determine the effect of the two treatments on mortality and re‐infection rate in a source population, using 21 314 consecutive patients undergoing isolated coronary artery bypass grafting (CABG) from January 1997 to October 2010. Median observation time was 2·9 years in the VAC group and 8·0 years in the TCD group. The epidemiological design was of an exposed (VAC, n = 64) versus non‐exposed (TCD, n = 66) cohort with two endpoints: (1) mortality and (2) failure of sternal wound healing or re‐infection. The crude effect of treatment technique versus endpoint was estimated by univariate analysis. Stratification analysis by the Mantel–Haenszel method was performed to quantify confounders and to pinpoint effect modifiers. Adjustment for confounders was performed using Cox regression analysis. Mediastinitis was diagnosed 6–105 (median 14) days after primary operation in the VAC group and 13 (5–29) days in the TCD group. There was no difference between groups in long‐term survival. Failure of sternal wound healing or re‐infection occurred less frequently in the VAC group (6%) than in the TCD group (21%; relative risk = 0·29, 95% CI = 0·06–0·88, P = 0·01). There are concerns for increase in right ventricle rupture in VAC compared with TCD. There was no difference in survival after VAC therapy and TCD therapy of post‐CABG mediastinitis. Failure of sternal wound healing or re‐infection was more common after TCD therapy.
Keywords: Coronary artery bypass grafting; Mediastinitis; Mortality; Re‐infection; Traditional closed drainage; Vacuum‐assisted closure
Introduction
Mediastinitis, or deep sternal infection, is a feared complication after cardiac surgery through a median sternotomy. The early mortality or in‐hospital mortality is reported to vary between 10% and 47% 1, 2, 3, 4, 5, and long‐term survival is also significantly reduced 6, 7, 8.
Traditional closed drainage (TCD) technique involved open debridement and Robicsek sternal rewiring, followed by prolonged closed irrigation with antibiotic solution and drainage 6, 9. Recently, we have shifted to vacuum‐assisted closure (VAC) method, based on topical negative pressure through a sponge across the sternal wound to promote bacterial removal and improve wound healing 10, 11. Failure of sternal wound healing, or re‐infection of the wound, requires repeated hospital admissions, prolonged antibiotic therapy and repeated wound debridement 12, 13. The aim of the study was to investigate the effect of treatment method – VAC therapy or TCD therapy – on survival time and on the frequency of failure of sternal wound healing or sternal re‐infection 14, 15.
Material and methods
Source population
The study was approved by the regional medical ethics committee and represented a multicentre collaboration between Rikshospitalet University Hospital, the Feiring Heart Clinic and Oslo Heart Center. The institutions maintain the same diagnostic criteria and treatment with active and prospective epidemiological surveillance of hospital infections. Between January 1997 and October 2010, a total of 21 314 adult patients undergoing coronary artery bypass grafting (CABG) were considered in our study. From 1997 to 2002, patients with poststernotomy mediastinitis were treated with TCD‐therapy; between 2002 and 2006, both TCD and VAC were used and after 2006, only VAC‐therapy was used. The diagnosis of poststernotomy mediastinitis was based on the criteria established by the Centres for Disease Control and Prevention 16, 17.
Study design
This is a cohort design of patients with CABG – surgery and postoperative mediastinitis. The epidemiological design was an exposed (VAC, n = 64) versus non‐exposed (TCD, n = 66) design and outcome, total mortality and re‐infection.
This is a dynamic cohort with date of operation as the entering time in the cohort. The closing date of the study was 1 October 2010. Total mortality was the main endpoint. Median observation time was 4·4 years, 2·9 years in the VAC group and 8·0 years in the TCD group.
The National Death Registry and information from the local hospital were used to assess death date and specific cause of death.
Statistical methods
Comparison between the two methods, VAC and TCD treatment, was carried out using the rate ratio (RR) and its 95% CL on the patients years model.
A stratification analysis using the Mantel–Haenszel using the patient years (time) model was carried out to quantify confounding effect of the potential variables and to pinpoint effect modification using the Breslow–Day test of heterogeneity (18). Control for multiconfounders was carried out using the Cox's multivariate model.
Kaplan–Meier method was used to differentiate long‐term survival between the two methods for surgical revision. Difference of survival was estimated by the log rank test and Wilcoxon's test (19). We used the log‐likelihood ratio test in the Cox's model for testing multi‐interactions (18). The results were reported in accordance with STROBE guidelines (20).
A priori power analysis for mortality endpoint
The a priori power analysis is based on the article by Sjøgren et al. (2005). The authors found at 5 years after operation an incidence of mortality of 18% for the VAC group and 41·3% for the TCD treatment. This means RR = 0·43 in favour of the VAC group, considering a type (I) error of 5% and a power of 80%. With RR = 0·43, we will need totally 2 × 67 patients and 67 patients in every group 12, 13.
Surgical technique in CABG
In all patients, the CABG approach was through a median sternotomy, using cardiopulmonary bypass, systemic moderate hypothermia and antegrade crystalloid cardioplegic arrest. Standard technique included routine use of left internal mammary artery and supplemental saphenous vein grafts to obtain complete revascularisation. Mediastinal shed blood was retransfused. The patients received standard antibiotic prophylactics with four doses of 2 g of intravenous cephalotin for at least 24 hours or until all drains or monitor lines had been removed.
Surgical technique in revision of mediastinitis
In the TCD group, patients were treated with open debridement, irrigation and Robicsek rewiring of the sternum (9). The irrigation, containing antibiotic solution, was continued until effluent drainage was free from bacterial growth. If this technique did not provide healing, surgical reconstruction with pectoralis muscle flaps was used.
In the VAC group, open debridement and removal of all sternal wires was performed. As measures to prevent right ventricle rupture, specific efforts were made to free the sternum from the right ventricle, and paraffin gauze was placed between the sternum and right ventricle. These two steps were performed for all the patients. Then, a sterile polyurethane sponge with an open‐pore structure of 400–600 µm (KCI, Copenhagen, Denmark) was trimmed to fit in the wound. The first layer was placed between the sternal edges onto the anterior of the heart, and the second layer was placed subcutaneously. The wound and the sponge were sealed with transparent adhesive drape, which overlapped the wound by 8–10 cm. An evacuation tube connected the sponge to a custom‐built vacuum source. A continuous negative pressure of 125 mm Hg was used in all cases, and a canister pump unit collected the exudates from the wound 10, 11. The sponge was changed twice a week until there was no bacterial growth in the wound and the concentration C‐reactive protein was below 50 mmol/l. The VAC dressing changes were performed in the operating room under conscious sedation by the surgeon. All patients stayed in hospital during the treatment. None of them was sent home with VAC dressing.
In the VAC group, sternum was rewired with steel wires without reinforcement in 48 patients, with Robicsek technique in 7 cases, and surgical reconstruction with pectoralis major muscle flaps in 9 patients. In TCD therapy, in 13 patients wounds were closed with simple steel wires without reinforcement, 43 with Robicsek technique and 10 received pectoralis muscle flap reconstruction. Indications for pectoralis muscle flap reconstruction were sternal dehiscence, chronic osteomyelitis and chondritis.
At revision for mediastinitis, specimens for bacterial cultures were collected from soft tissue and sternal edges. Bacterial growth was positive in 85 patients (79·4%). Staphylococcus aureus made up for 74 (57·0%) of all organisms. Seventeen (13· 0%) of the cases had positive growth of Staphylococcus epidermidis, four (3·7%) had Escherichia coli and Enterobacteriae, two (1·5%) had Pseudomonas airegonosa and 1 (0·8%) had Bacteroides fragilis. In 28 patients, there was no growth. Duration of antibiotic treatment was median 46 days for both the VAC and TCD groups.
The two treatment groups were comparable with respect to majority of the pre‐, intra‐ and postoperative variables (Table 1). Age was higher in the VAC group, probably reflecting acceptance of older patients for CABG in recent years (VAC era). Emergency surgery (within 24 hours) was also more common in the VAC group. This is probably due to a shift in recent years to earlier intervention for unstable angina or non‐ST‐elevation myocardial infarction. Mediastinitis occurred in 135 (0·6%) of 21 314 patients, where 64 patients were treated with VAC therapy and 66 patients underwent TCD therapy. Five patients were excluded from the study, three had both VAC and TCD therapy and two were treated with open sternum and wound packing. Time between CABG and surgical revision was median 14 (6–29) days for VAC patients and 13 (5–105) days for TCD patients (P = 0·26). Treatment duration was median 8 (2–84) days in the VAC group and 8 (2–99) days in the TCD group (P = 0·79). VAC patients had median 4 (3–4) operations, including initial opening of sternum, subsequent revisions with changes in sponges and the final wound closing. The TCD therapy patients required only one operation.
Table 1.
Characteristics of patients with post‐CABG mediastinitis treated with VAC or TCD *
| VAC (n = 64) | TCD (n = 66) | P value † | |
|---|---|---|---|
| Preoperative | |||
| Age (years) | 68·2 (±9·2) | 63·3 (±10·2) | <0.01 |
| Male gender (%) | 90·9 | 85·9 | 0.27 |
| Vessels involved (n) | |||
| I | 3 | 2 | 0.99 |
| II | 10 | 3 | |
| III | 51 | 61 | |
| Left main stenosis | 23/64 | 16/66 | 0.28 |
| Emergency surgery | 35/64 | 16/66 | <0.01 |
| Unstable angina | 34/64 | 20/66 | 0.03 |
| Body mass index (kg/m2) | 29·1 (±3·8) | 28·1 (±4·1) | 0.17 |
| S‐creatinine > 200 µmol/l | 2/64 | 3/66 | 0.39 |
| Previous cardiac surgery (n) | 0 | 2/66 | 0.14 |
| Previous myocardial infarction (n) | 41/64 | 39/66 | 0.56 |
| Preoperative atrial fibrillation (n) | 5/64 | 4/66 | 0.69 |
| Chronic obstructive pulmonary disease (n) | 14/64 | 18/66 | 0.27 |
| Diabetes (n) | 25/64 | 20/66 | 0.29 |
| Functional NYHA classification (I–IV) | |||
| I–II | 16/64 | 20/66 | 0.67 |
| III–IV | 48/64 | 46/66 | |
| General atheroschlerosis (n) | 16/64 | 12/66 | 0.15 |
| Left ventricular ejection fraction (%) | 59 (±15·8) | 61·4 (±16·4) | 0.42 |
| Operative | |||
| Left internal mammarial artery (n) | 62/64 | 65/66 | 0.30 |
| Right internal mammarial artery (n) | 0 | 2/66 | 0.13 |
| Aortic cross‐clamp time (min) | 37·1 (±20·9) | 42·3 (±37·5) | 0.35 |
| Cardio‐pulmonary bypass time (min) | 64·5 (±41·6) | 69·2 (±23·8) | 0.44 |
| Postoperative | |||
| Mediastinal drainage (ml) | 709 (±354) | 761 (±515) | 0.50 |
| Reoperation for bleeding | 4/64 | 2/64 | 0.21 |
| Ventilation time (hours) | 3·1 (±2·8) | 3·1 (2·1) | 0.94 |
| Blood transfusion (units) | 1·8 (±3·8) | 2·0 (±6·2) | 0.89 |
VAC, vacuum‐assisted closure; TCD, traditional closed drainage; NYHA, New York Heart Association.
*Numbers are mean ± standard deviation or percentage.
†two‐sided t‐test or Mann–Whitney test for continuous numerical data and χ 2 test for categorical data.
Results
Effect of VAC versus TCD on 30‐day mortality and morbidity
There was no difference between groups on 30‐day mortality (Table 2). In the VAC group, two patients died within 30 days: one from myocardial infarction and sepsis 17 days after the sternal reopening and the other due to right ventricle rupture during sternal reopening 22 days after CABG. Two other patients had right ventricle rupture 1 week after initiation of VAC treatment. The laceration of the right ventricle was closed, and they both survived. In the TCD group, there was no 30‐day mortality. Hospital stay was median 14 days in both treatment groups. There was no difference between groups on the appearance of postoperative arrhythmia, use of inotropic agents or intra‐aortic balloon pump, cerebral stroke or myocardial infarction. However, 30‐day mortality and morbidity were rare, and the study was not powered enough to identify eventual differences between groups on these events.
Table 2.
Long‐term mortality: crude and adjusted rate ratio using stratification person years and Cox's *
| Crude effect | RRc | Confounding effect (%) |
|---|---|---|
| Mediastinitis (n = 130) | 1·10 (0·56–2·180) | |
| Simultaneously control for multiconfounders † | RRa | |
| Using the Cox's model | 0·80 (0·39–1·62) | 27·3 |
RRa, adjusted rate ratio; RRc, crude rate ratio.
*0; was replaced by 0·5. The confounding effect was quantified by comparing (RRa − RRc/RRc) × 100.
†Age and emergency surgery.
Effect of VAC versus TCD on long‐term survival
Five‐year survival was 65·4 (±8·9)% in the VAC group and 71·2 (±5·6)% in TCD group. Using univariate analysis, there was no statistically significant difference between groups in long‐term survival (log rank test, P = 0·77 and Breslow test, P = 0·99; Figure 1). When adjusted for the two confounders' age and emergency surgery, long‐term survival did not differ between groups using Cox regression analysis (Table 2).
Figure 1.
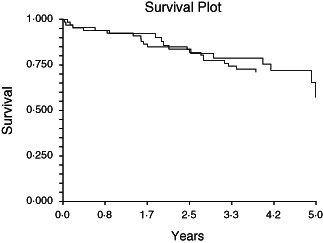
Survival curves using the Kaplan–Meier method for patients treated with VAC and patients treated with TCD.
Cause of death was identified using information from the death registry and local hospitals. Cause of death was ischemic heart disease in 21 (47·7%) patients, cerebral disease in 6 (13·6%) patients, pneumonia and respiratory insufficiency in 5 (11·4%), sepsis in 3 (6·8%), diabetes mellitus 3 (6·8%), malignant disease in 3 (6·8%), gastrointestinal disease in 2 (4·5%) and renal insufficiency in 1 (2·4%).
Failure of sternal wound healing or occurrence of sternal re‐infection
Failure of wound healing with the need of muscle flap reconstruction or later occurrence of sternal re‐infection was seen in 14 cases (21·2%) in the TCD group and in 4 cases (6·3%) in the VAC group (Table 3). The wound was closed by use of pectoralis major muscle flaps in all the VAC cases and in 9 of the 14 TCD patients (Table 4).
Table 3.
Re‐infection of sternum within one year of follow‐up, univariate analysis
| VAC | TCD | OR | 95% CI | P value | |
|---|---|---|---|---|---|
| Re‐infection | 4/64 (6·25%) | 14/66 (21·2%) | 0·25 | 0·076–0·79 | 0·013 |
VAC, vacuum‐assisted closure; TCD, traditional closed drainage; OR, odd ratio; CI, confidence interval.
Table 4.
Failure of wound healing or sternal re‐infection after revision for mediastinitis, univariate analysis
| Failure of wound healing < 2 weeks | Re‐infection between 2 and 12 weeks | Re‐infection after 12 weeks | Pectoralis major muscle flap | |
|---|---|---|---|---|
| VAC (n = 4) | 1 | 2 | 1 | 4 |
| TCD (n = 14) | 3 | 7 | 4 | 9 |
VAC, vacuum‐assisted closure; TCD, traditional closed drainage.
Discussion
The present report of post‐CABG mediastinitis compares two different surgical treatment modalities – VAC and TCD – on survival rate and occurrence of sternal re‐infection. We found no difference in survival, while sternal re‐infection was less frequent after VAC therapy. Previous studies have reported that VAC therapy is superior to TCD therapy on early and late survival (14) and on the occurrence of sternal re‐infection (15). Another study has suggested that CABG patients with VAC‐treated mediastinitis have similar long‐term survival as CABG patients without mediastinitis (21). In this study, 30‐day mortality after surgery for mediastinitis was only 1·5% (2 deaths out of 130 cases). The study was therefore grossly underpowered to detect any difference between groups on early mortality. During follow‐up, there were fewer total deaths in the VAC group compared with the TCD group, but when adjusted for multiconfounders and for the different observation time between the two groups, there was no statistically significant difference in total mortality rate. Our survival analysis may, however, be disturbed by several factors. First, the retrospective and non‐randomised design of the study may lead to selection bias, and there may exist unknown confounders for which it is impossible to adjust for. Second, our study covered a period of nearly 14 years, where TCD therapy dominated in the first half of the period and VAC therapy in the second. The quality of pre‐, per‐ and postoperative care may have improved during these 14 years, which would favour outcome in the VAC group, especially on early outcome. On the other hand, TCD treatment was a well‐established procedure during the study period, while VAC therapy was introduced as a new procedure. This could represent a possible bias in favour of TCD therapy due to learning‐curve effect in VAC therapy.
Failure of wound healing after sternal revision or sternal re‐infection with chronic osteomyelitis and osteochondritis is difficult to deal with (22). Because of which the patients undergo repeated hospital admissions with wound debridements and sustained antibiotic therapy 12, 13, 14, 15. Our study reveals that VAC therapy represents a step forward to reduce the problem of residual or recurrent sternal infection. The negative wound pressure in VAC therapy continually removes bacteria, debris and exudates, enhances microcirculation and accelerates tissue granulation and stabilises the sternal wound edges 10, 11. Although VAC patients undergo more wound revisions (median 4), the length of hospital stay was not longer than that for TCD patients. Repeated revisions in VAC therapy offer good inspection control of the infection and serial microbiological tests. VAC therapy results in a stable and painless sternal wound, making mobilisation easier than for TCD cases, in which patients are more or less bound to the bed and the wall suction unit. Several studies have reported high rates of re‐infection after TCD therapy 9, 10. The explanation to these failures may be increased bacterial resistance in a closed system and a relative devascularisation of the sternum after harvesting the mammary artery and using Robicsek rewiring as routine procedures. Therapeutic failure of mediastinitis treatment with recurrent infections aggravates an already difficult situation and may result in complications and higher mortality 6, 7, 8. In VAC therapy, special attention must be given to the underlying heart, especially the fragile right ventricle. If VAC treatment is planned, it is mandatory to mobilise at least one of the sternal edges from the right ventricle during sternal reopening and to protect the anterior of the right ventricle with several layers of paraffin gauze dressing. If both sternal edges stay adherent to the heart, this may shear or cut the right ventricle during accelerated sternal movement as in forced respiration or episodes of coughing 23, 24. In this study, three patients experienced this complication. One patient had right ventricular rupture at primary sternal reopening, and died 2 days later because of continuous bleeding. Two more patients had right ventricular rupture during VAC treatment on days 7 and 8 after primary revision. The laceration of the right ventricle was sutured and sternum was rewired, and they both survived.
There is concern for increases in right ventricular rupture in VAC as compared with TCD, but our study is not powered enough to answer this question. Our results are not conclusive, because of the low prevalence of mediastinitis; we will need a multicentre randomised clinical trial to answer our research hypothesis properly and conclusively.
In conclusion, there is no difference between VAC and TCD therapy, as far as mortality is concerned. There is a lower rate of re‐infection in favour of VAC treatment, but there are concerns for increase in right ventricle rupture in VAC as compared with TCD.
Acknowledgements
The authors would particularly like to thank Ulla Bella and Mona Bekken Vold at Feiring Heart Center for continuous support and for their contribution in organising the data. We are indebted to the chief of Oslo Heart Center, Eivind Øvrum, MD, PhD, to Nihal D. Perera, MSCI, and Rolf Øystese, CCPP, for computer and programming assistance and to Christine Schei for her work with figures and tables.
References
- 1. Milano CA , Kesler K , Archbald N , Sexton DJ , Jones RH. Mediastinitis after coronary bypass graft surgery. Risk factors and long‐term survival. Circulation 1995. ; 92 : 2245 – 51. [DOI] [PubMed] [Google Scholar]
- 2. Abbod CS , Wey SB , Baltar VT. Risk factors for mediastinitis after cardiac surgery. Ann Thorac Surg 2004. ; 77 : 676 – 83. [DOI] [PubMed] [Google Scholar]
- 3. Loop FD , Lytle BW , Cosgrove DM , Mahfood S , McHenry M24C , Goormastic M , Stewart RW , Golding LA , Taylor PC. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990. ; 49 : 179 – 87. [DOI] [PubMed] [Google Scholar]
- 4. Braxton JH , Marrin CA , McGrath PD , Morton JR , Norotsky M , Charlesworth DC , Lahey SJ , Clough R , Ross CS , Olmstead EM , O'Connor GT. 10‐Year follow‐up of patients with and without mediastinitis. Semin Thorac Cardiovasc Surg 2004. ; 16 : 70 – 76. [DOI] [PubMed] [Google Scholar]
- 5. Baillot R , Cloutier D , Montalin L , Côté L , Lellouche F , Houde C , Gaudreau G , Voisine P. Impact of deep sternal infection management with vacuum‐assisted closure therapy followed by sternal osteosynthesis: a 15‐year review of 234499 sternotomies. Eur J Cardiothorac Surg 2010. ; 37 : 880 – 87. [DOI] [PubMed] [Google Scholar]
- 6. Risnes I , Abdelnoor M , Almdahl SM , Svennevig JL. Mediastinitis after coronary artery bypass grafting risk factors and long term survival. Ann Thorac Surg 2010. ; 89 : 1502 – 10. [DOI] [PubMed] [Google Scholar]
- 7. Toumpoulis IK , Anagnostopoulos CE , DeRose JJ , Swistel DG. The impact of deep sternal wound infection on long‐term survival after coronary artery bypass grafting. Chest 2005. ; 127 : 464 – 71. [DOI] [PubMed] [Google Scholar]
- 8. Braxton JH , Marrin CA , McGrath PD , Ross CS , Morton JR , Norotsky M , Charlesworth DC , Lahey SJ , Clough RA , O'Connor GT. Mediastinitis and long‐term survival after coronary artery bypass graft surgery. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg 2000. ; 70 : 2004 – 7. [DOI] [PubMed] [Google Scholar]
- 9. Robiscek F , Daugherty HK , Cook JW. The prevention and treatment of sternum separation following open heart surgery. J Thorac Cardiovasc Surg 1977. ; 73 : 267. [PubMed] [Google Scholar]
- 10. Morykwas MJ , Argenta LC , Shelton‐Brown EL , McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997. ; 38 : 553 – 62. [DOI] [PubMed] [Google Scholar]
- 11. Wackenfors A , Sjøgren J , Gustafsson R , Ingemansson R , Malmsjø M. Effects of vacuum‐assisted closure therapy on inguinal wound edge microvascular blood flow. Reg 2004. ; 12 : 600 – 6. [DOI] [PubMed] [Google Scholar]
- 12. Sjøgren J , Malmsjø M , Gustafsson R , Ingemansson R. Poststernotomy mediastinitis: a review of conventional surgical treatments, vacuum‐assisted closure therapy and presentation of the Lund University Hospital mediastinitis algorithm. Eur J Cardiothorac Surg 2006. ; 30 : 898 – 905. [DOI] [PubMed] [Google Scholar]
- 13. Raja SG , Berg GA. Should vacuum‐assisted closure‐therapy be routinely used for management of deep sternal wound infection after cardiac surgery? Interact Cardiovasc Thorac Surg 2007. ; 6 : 523 – 8. [DOI] [PubMed] [Google Scholar]
- 14. Sjøgren J , Gustafsson R , Nilsson J , Malmsjø M , Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum‐assisted closure versus conventional treatment. Ann Thorac Surg ; 2005. ; 79 : 2049 – 55. [DOI] [PubMed] [Google Scholar]
- 15. Petzina R , Hofmann J , Navasardyan A , Malmsjö M , Stamm C , Unbehaun A , Hetzer R. Negative pressure wound therapy for post‐sternotomy mediastinitis reduces mortality rate and sternal re‐infection rate compared to conventional treatment. Eur J Cardiothorac Surg 2010. ; 38 : 110 – 3. [DOI] [PubMed] [Google Scholar]
- 16. Garner J , Jarvis WR , Emori GT , Horan TC , Huges J. CDC definitions for nosocomial infections 1988. Am J Infect Control 1988. ; 16 : 128 – 40. [DOI] [PubMed] [Google Scholar]
- 17. El Oakley RM , Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996. ; 61 : 1030 – 6. [DOI] [PubMed] [Google Scholar]
- 18. Kleinbaum DG , Kupper LL , Morgenstern H. Epidemiologic research, principles and quantitative methods. John Wiley and Sons; , New York , 1982. . [Google Scholar]
- 19. Lee ET. Statistical methods for survival data analysis. Belmont (CA) : Lifetime Learning Publications; , 1984. . [Google Scholar]
- 20. Von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007. ; 370 : 1453 – 7. [DOI] [PubMed] [Google Scholar]
- 21. Sjøgren J , Nilsson J , Gustafsson R , Malmsjø M , Ingemansson R. The impact of vacuum‐ assisted closure on long‐term survival after poststernotomy mediastinitis. Ann Thorac Surg . 2005. ; 80 : 1270 – 5. [DOI] [PubMed] [Google Scholar]
- 22. Steingrimsson S , Gustafsson R , Gudbjartsson T , Mokhtari A , Ingemansson R , Sjøgren J. Sternocutanous fistulas after cardiac surgery: incidence and late outcome during a ten‐year follow up. Ann Thorac Surg 2009. ; 88 : 1910 – 5. [DOI] [PubMed] [Google Scholar]
- 23. Sartipy U , Lockowandt U , Gabel J , Jideus L , Dellgren G. Cardiac rupture during vacuum‐assisted closure therapy. Ann Thorac Surg 2006. ; 82 : 1110 – 1. [DOI] [PubMed] [Google Scholar]
- 24. Malmsjø M , Petzina R , Ugander M , Engblom H , Torbrand C , Mokhtari A , Hetzer R , Arheden H , Ingemansson R. Preventing heart injury during negative pressure wound therapy in cardiac surgery: assessment using real‐time magnetic resonance imaging. J Thorac Cardiovasc Surg 2009. ; 138 : 712 – 7. [DOI] [PubMed] [Google Scholar]


