Abstract
Few studies have examined factors associated with diabetic foot ulcer (DFU) recurrence. Using data from patients enrolled in the prospective Eurodiale DFU study, we investigated the frequency of and risk factors for DFU recurrence after healing during a 3‐year follow‐up period. At our site, 93 Eurodiale‐enrolled patients had a healed DFU. Among these, 14 were not alive; of the remaining 79 patients we enrolled 73 in this study. On entry to the Eurodiale study, we assessed demographic factors (age, sex and distance from hospital); diabetes‐related factors [duration, and glycated haemoglobin (HbA1c) levels]; comorbidities (obesity, renal failure, smoking and alcohol abuse) and DFU‐related factors [peripheral arterial disease, ulcer infection, C‐reactive protein (CRP) and; foot deformities]. During the 3‐year follow‐up period, a DFU had recurred in 42 patients (57·5%). By stepwise logistic regression of findings at initial DFU presentation, the significant independent predictors for recurrence were plantar ulcer location [odds ratio (OR) 8·62, 95% confidence interval (CI) 2·2–33·2]; presence of osteomyelitis (OR 5·17, 95% CI 1·4–18·7); HbA1c > 7·5% ([DCCT], OR 4·07, 95% CI 1·1–15·6) and CRP > 5 mg/l (OR 4·27, 95% CI 1·2–15·7). In these patients with a healed DFU, the majority had a recurrence of DFU during a 3‐year follow‐up period, despite intensive foot care. The findings at diagnosis of the initial DFU were independent risk factors associated with ulcer recurrence (plantar location, bone infection, poor diabetes control and elevated CRP) and define those at high risk for recurrence, but may be amenable to targeted interventions.
Keywords: Diabetic foot ulcer; Risk factors for reulceration; Ulcer recurrence
Introduction
Ulcerations of the foot are among the most serious complications of diabetes mellitus as they are perhaps the most common reason for diabetes‐related hospitalisation and may lead to lower extremity amputations. Diabetic foot ulcers (DFUs) result from various factors that lead to breakdown of the skin, including peripheral neuropathy, peripheral arterial disease (PAD), foot deformity, limited joint mobility, hyperkeratosis, pedal oedema and ill‐fitting shoes 1, 2. Other factors may also lead to poor wound healing, such as soft tissue or bone infection, repetitive trauma, improper wound management, late presentation or poor patient adherence to the treatment regimens 3, 4, 5. Wound healing is the ultimate goal of treating patients with DFU and requires a complex approach (6). The annual risk of developing a DFU in most unselected diabetic populations is approximately 2% (7), but for a patient who has had a previous DFU the risk of another over the next 3 years increases to 17–60% (8).
Recurrent foot ulcerations are often long‐standing and associated with deterioration of the patient's health status; they may have negative effects on the patient's quality of life and adherence to recommended self‐care regimens, which are sometimes not recognised by their physicians (9). Furthermore, these recurrent ulcers increase the long‐term costs for DFU management, especially if they require home care services or result in an amputation (10). Because a history of a previous DFU is the strongest risk factor for developing another, the International Consensus for Diabetic Foot recommends that these patients have their feet inspected by a specialist every 1–3 months (1). Lower risk patients may, however, not need this intensive surveillance. There are currently only limited data concerning which patient‐related factors are most predictive for ulcer recurrence 1, 11, 12.
The recently conducted, large, prospective, multicentre, observational Eurodiale study assessed the risk factors for DFU (13). This study conducted in 14 European centres was designed to investigate the characteristics of diabetic patients with a foot ulcer and to assess factors that influence management strategies in diabetic foot disease. The study followed up over 1200 patients with a new DFU for 12 months (or until complete healing) to assess a wide variety of factors affecting healing (14). The mean minor amputation rate for all patients in the Eurodiale study was 18% and was mainly dependent on depth of the ulcer, presence of PAD or infection and male sex (15). The aim of our study was to assess the frequency of ulcer recurrence in patients with a healed DFU followed up in our centre for the 3 years after completing the Eurodiale study, and to identify the risk factors for recurrence.
Methods
All patients from our medical centre who were included in the original Eurodiale study and had a healed DFU (defined as fully epithelialised wound that remained closed for at least 6 weeks) were eligible for this study. The protocol for the study is shown in Figure 1. Of the original 120 patients we enrolled, 93 had a healed ulcer (after a minor amputation in 10); of these, 14 patients were not alive and 6 missed regular preventive visits at our foot clinic and declined further participation during the 3‐year follow‐up period. Among the remaining 73 patients who completed this study, 59 (80·8%) regularly visited our foot clinic to monitor the status of their foot. We contacted the remaining 14 patients who discontinued regular follow‐up at our foot clinic over telephone or by mail and conducted a structured interview to determine if they had sustained any foot lesions or ulcerations. These latter patients were also followed up by a local diabetologist every 3 months, who treated them for any new ulcer occurrence at a local surgical or foot clinic.
Figure 1.
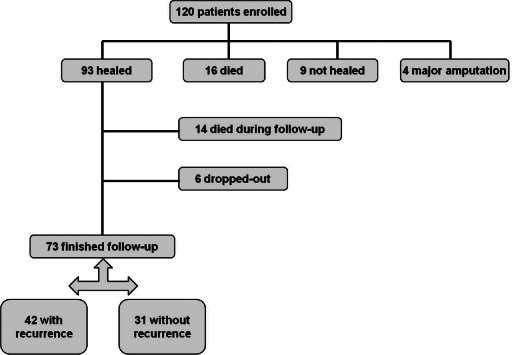
Design of our follow‐up study.
We investigated potential risk factors for ulcer recurrence, by selecting items similar to those in previously published studies of risks for developing a DFU 2, 5, 8. Using information obtained from the Eurodiale study (14), we divided these risk factors into four categories (Table 1).
Table 1.
Potential risk factors for ulcer recurrence
| Factors | With recurrence n = 42 | Without recurrence n = 31 | P | |
|---|---|---|---|---|
| Demographic factors | ||||
| Age (years) | 59·8 ± 8·9 | 62·5 ± 10·1 | NS | |
| Sex: male/female | 36/9 | 25/6 | NS | |
| Distance from hospital >15 km | 69·0% | 58·1% | NS | |
| Diabetes‐related factors | ||||
| Diabetes duration >10 years | 81% | 71% | NS | |
| Diabetes treatment with insulin | 81% | 74·2% | NS | |
| Poor glycaemic control (HbA1c >7·5%) | 83·3% | 54·8% | 0·079 | |
| Comorbidities | ||||
| Overweight (BMI >27 kg/m3) | 59·5% | 42·9% | NS | |
| Smoking (active) | 9·5% | 12·9% | NS | |
| Chronic alcohol usage | 42·9% | 48·4% | NS | |
| End‐stage renal disease | 11·9% | 6·5% | NS | |
| DFU‐related factors | ||||
| Peripheral arterial disease | 21·4% | 19·4% | NS | |
| Osteomyelitis | 54·8% | 22·6% | 0·0124 | |
| Charcot foot | 21·4% | 12·9% | NS | |
| Clinical signs of DFU infection | 21·4% | 29·0% | NS | |
| Elevated CRP (>5 mg/l) | 71·5% | 48·4% | 0·0454 | |
| Plantar location of DFU | 61·5% | 16·1% | 0·0001 | |
| Ulcer size >5 cm2 | 11·9% | 12·9% | NS | |
| Deep ulcer depth (subcutaneous) | 38·1% | 32·2% | NS | |
| Ulcer duration >3 months | 23·8% | 32·3% | NS | |
| Foot deformity | 71·4% | 67·7% | NS | |
| Previous ipsilateral amputation | 9·5% | 12·9% | NS | |
| Previous contralateral amputation | 7·1% | 9·7% | NS | |
| Multiresistant microorganisms | 4·8% | 6·5% | NS | |
| Days to complete healing | 154·4 ± 82·1 | 149·2 ± 83·9 | NS |
-
1
Demographic factors: age (in years), sex and distance of their residence from our medical centre.
-
2
Diabetes‐related factors: type of diabetes treatment (percentage receiving insulin), poor glycaemic control – HbA1c >7·5% (DCCT) and duration of diabetes.
-
3
Comorbidities: end‐stage renal disease (defined as needing dialysis), overweight or obesity (defined as a body mass index >27 kg/m2), chronic alcohol usage (defined as drinking more than 1 IU/day) and smoking (any amount of any type of tobacco).
-
4
DFU‐related factors: PAD [defined as an ankle‐brachial index (ABI) <0·9 or the absence of both foot pulses on the study foot] 13, 16; osteomyelitis (diagnosed by clinical features and plain X‐ray findings); Charcot foot (diagnosed by a presence of >2°C difference in skin foot temperature between the two feet and plain X‐ray and/or radionuclide bone scan compatible with Charcot foot); DFU infection [as defined by the International Working Group on the Diabetic Foot (17); elevated C‐reactive protein (CRP) level (defined as >5 mg/l]; ulcer location [‘plantar’ (covering plantar surface of the forefoot, mid‐foot or heel) or ‘non plantar’ on (dorsum of the foot, toes or heel)]; DFU size; DFU depth (a deep ulcer was defined as one extending below the subcutaneous tissue, e.g. down to muscle, fascia, tendon or bone); history of previous lower extremity amputation; culture with multiresistant organisms and days to complete DFU healing.
We assessed all DFU patients for these risk factors at their entry visit for the Eurodiale study – all blood samples (CRP) were collected at the time of enrolment. Once their ulcer healed, enrolled patients were treated by standardised methods on a regular basis, either in our foot clinic or at nearby foot centres. Those requiring pressure offloading received therapeutic shoes, orthotics or a total contact cast, as indicated. For infected ulcers, we prescribed appropriate antibiotic therapy. For those with severe PAD, a surgeon or radiologist performed an appropriate revascularisation procedure, if needed and technically possible. We also provided an individualised foot educational program for all patients, both initially and during the check‐up visits they had every 1–3 months (1). We defined ulcer recurrence after complete healing of the original DFU as the development of a new full‐thickness lesion on the studied foot during the follow‐up period.
We conducted statistical analyses using a χ 2 test and forward stepwise logistic regression and calculated univariate odds ratios (ORs) and their respective 95% confidence intervals (CIs). We also evaluated all potential predictors of DFU recurrence in a multivariable regression analysis. Finally, we compared the percentage of correct classifications based on a risk factor model we developed for patients with DFU recurrence (sensitivity) or without recurrence (specificity). The final model was calculated from the estimated logistic regression equation: logit (P) = a + b 1 x 1 + b 2 x 2 + b 3 x 3 + b 4 x 4, where x i is the explanatory variable (e.g. osteomyelitis) with value 1/0 corresponding with present/not present, logit (P) is the predicted value of logit (P), where P is the probability of reulceration [logit(P) = ln(P/(1 −P))], a is the constant term, b 1, b 2, b 3 and b 4 are the estimated logistic regression coefficients. The equation, based on the results of our study, was of the form: logit (P) = −3·0 + 2·15 × plantar location + 1·64 × osteom yelitis + 1·41 × HbA1c +1·50 × CRP. A case is predictive to be in group ‘recurrence’ if the probability is greater than the cut‐off point of P = 0·275.
We generated receiver operating characteristic (ROC) curves to determine the association of CRP levels with ulcer recurrence, and analysed the sensitivity, specificity, positive and negative predictive values for different CRP cut‐off levels. We calculated the Youden index, a summary measure of the ROC curve that both measures the effectiveness of a diagnostic marker and enables the selection of its optimal threshold value (cut‐off point), using the formula: sensitivity + specificity – 100.
Results
During the 3‐year follow‐up period, a foot ulcer recurred in 42 (57·5%) of 73 patients (Figure 1). The recurrence rate was significantly higher (39·7%) in the first year than in the second year (18·1%, P = 0·027) and third year (12·8%, P = 0·006; Figure 2). There was no significant difference in DFU recurrence rate in patients followed up regularly in our foot clinic [34/59 (57·6%)] when compared with those followed up at the other local foot or surgical clinics [8/14 (57·1%)]. The prevalence of potential predictors of ulcer recurrence in the patients with and without a new foot ulcer during follow‐up is shown in Table 1. Multivariate stepwise logistic regression of all potential risk factors for DFU recurrence showed that the only independent predictors were plantar location of the ulcer, presence of osteomyelitis, HbA1c > 7·5% and CRP > 5 mg/l (Table 2). The predicted probability of DFU recurrence based on the final estimated logistic regression equation model had a 90·5% sensitivity (correctly classifying the group with recurrent DFU) and 55% specificity (correctly classifying the group without recurrent DFU).
Figure 2.
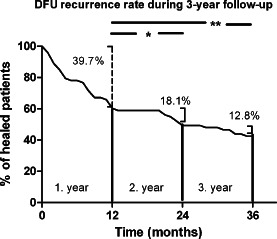
Timing of recurrence of diabetic foot ulcer during 3‐year follow‐up after initial ulcer healing.
Table 2.
Multivariate stepwise logistic regression – independent risk factors statistically significantly associated with ulcer recurrence
| Factors | Coefficient (ln OR) | OR | 95% CI |
|---|---|---|---|
| Plantar location of the ulcer | 2·15 | 8·62 | 2·2–33·2 |
| Osteomyelitis | 1·64 | 5·17 | 1·4–18·7 |
| HbA1c >7·5% | 1·4 | 4·07 | 1·1–15·6 |
| CRP >5 mg/l | 1·45 | 4·27 | 1·2–15·7 |
| Constant | −3·0 |
ln – decadic logarithm.
We performed a subanalysis of the association between osteomyelitis and reulceration. Among the 73 patients with a healed DFU, the 30 (42·9%) diagnosed as having osteomyelitis had a significantly higher risk of recurrence than those without osteomyelitis (54·6% versus 22·6%, respectively, P = 0·012) (Table 1). Of those with osteomyelitis, there was no significant difference in the rate of reulceration between those treated with antibiotic therapy alone [16/22 (73%)] or combined with surgical resection (7/8 [87%]). Similarly, there was no difference in the recurrence rate of osteomyelitis localised to the toes versus other foot locations [13/17 (76·5%) and 10/13 (77%), respectively]. In analysing different cut‐off values of CRP, a value above the upper limit of normal (>5 mg/l) was a better predictor of ulcer recurrence than using double or triple the upper normal range, with the highest sensitivity, acceptable specificity and Youden index (ROC curve in Figure 3).
Figure 3.
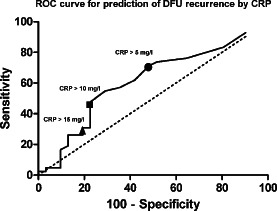
Predictive cut‐offs of CRP values.
Discussion
This 3‐year prospective follow‐up study of patients with a healed DFU showed a high incidence of ulcer recurrence (57·5%). These results are similar to a few previously reported studies investigating this issue. In a prospective study of a cohort of 81 diabetic patients who were at high risk for foot ulceration, Peters et al. reported a rate of reulceration of 60·5% during a mean follow‐up period of 31 months (18). Similarly, among 468 patients with a DFU that healed either primarily or after minor or major amputation, Apelqvist et al. showed 3‐year DFU recurrence rate of 61% (8). Ghanassia et al. also reported a similar recurrence rate (60·9%) among 89 patients during a mean follow‐up of 79 months (19).
We also identified that among wide spectrum of potential risk factors analysed in the Eurodiale study, those that were independently associated with recurrent foot ulcers were plantar location, presence of osteomyelitis, elevated level of HgA1c and elevated CRP. In light of the high risk of a recurrent ulceration, we believe that it would be appropriate to develop prevention programmes that focus on patients identified as being in the highest risk groups. This might also include efforts to educate the patients and their health care providers on methods that have been shown to be effective in preventing DFU (20).
In our study, as in the one reported by Peters et al.(18), the risk factor with the highest association with reulceration was plantar location of the initial ulcer. The likely explanation for this finding is that the ulcers located on plantar surface of an insensitive foot are exposed to repetitive injury and are under higher pressure than those in other locations (21). Most of the ulcers (75·3%) in our study were located on plantar surface of the foot, a finding that differs from the baseline results of the entire Eurodiale study (13), in which only 48% of ulcers were plantar. As the prevalence of PAD was similar among patients with plantar and non‐plantar location of the ulcer, plantar ulcers were not more often neuropathic than those in other locations. As reported by others 18, 22, we found that neither greater ulcer size nor depth at baseline was a significant predictor for reulceration.
Although clinical signs of infection, such as frank purulence, local warmth, erythema, lymphangitis, induration, pain, fever and foul smell 5, 17, did not significantly differ between the groups with and without reulceration, radiographically diagnosed osteomyelitis was a strong predictor of ulcer recurrence in our study. This finding may be related to the difficulty of effectively treating chronic osteomyelitis, which can persist despite antibiotic and surgical therapy. This ineffective treatment may result from poor penetration of antibiotics as well as the formation of biofilm into osteomyelitic bone. This persistent infection can lead to recurrent ulceration of the overlying skin, which was confirmed by the results of our study which showed that recurrent ulcers were near the site of the initial osteomyelitis and spreading of infection ‘per continuitatem’ is possible. Some previous studies have also reported that the presence of underlying osteomyelitis was a predictor of reulceration, as well as amputation, in patients with diabetic foot complications. In a study by Yesil et al. of 510 patients with a DFU, osteomyelitis was a significant risk factor for major amputation (23). Kowalski et al.(24) found that residual osteomyelitis at the surgical margin after bone resection in 111 patients was associated with a higher rate of treatment failure; more proximal amputation was required in 43% patients with positive margins versus 15% with negative margins (P = 0·001), despite the longer duration of antibiotic therapy for the former. Among 65 patients with surgically treated osteomyelitis, Aragon‐Sanchez et al. reported reulceration in 43% and a new episode of osteomyelitis in 17% (25). All patients at our centre diagnosed with osteomyelitis were treated with systemic antibiotics and surgical procedures, if needed, in accordance with the International Consensus recommendations (1). We found no significant difference in the recurrence rate of DFU in patients who did or did not have a surgical resection (87% versus 73%, respectively), which is in accordance with published data 26, 27.
We found that an elevated CRP level at the time of enrolment in the Eurodiale study was an independent risk factor for reulceration. The elevated CRP at presentation was likely attributable to infection in the foot ulcer, as the patients did not have evidence of other infectious or inflammatory diseases during our clinical examination. Several other studies have documented the usefulness of CRP for detection of DFU and for determining the prognosis for ulcer healing or amputation. Jeandrot et al.(27) have shown that an elevated CRP level was a good indicator in detecting infection among diabetic patients with a foot ulcer (17). In a prospective study of patients with a diabetic foot infection, Lipsky et al. reported that elevated levels of CRP were associated with failure of therapy of infected DFU (28). Some studies have demonstrated that an elevated CRP level is associated with an increased risk of lower extremity amputations in patients with a DFU 23, 29, but to our knowledge there are no previously published data on the predictive value of CRP for DFU recurrence. We found that the most sensitive CRP cut‐off for predicting recurrence was above the upper normal range (>5 mg/l).
Poor glycaemic control, defined as HbA1c > 7·5%, was also a significant risk factor for re‐ulceration in our study. In a 2‐year study by Mantey et al., HbA1c was significantly higher in those with a DFU recurrence compared to those without a recurrence (P = 0·03) (30).
Connor et al. also reported a relationship between HbA1c and a higher rate of ulceration per 10 years in patients with neuropathic foot ulcers (11). It is possible that poor long‐term glycaemic control may impair wound healing, but it may also reflect poorer patient compliance with various preventive measures, such as self‐monitoring of glycaemic control 11, 31 and adherence to treatment recommendations for DFU. A higher rate of ulcer recurrence may also be associated with insufficient patient education and lack of psychological support (32).
PAD has been found to increase the risk of DFU in several reports 1, 33. Unlike in some other studies 18, 23, we found that the presence of PAD was not a predictor for ulcer recurrence. This discrepancy may be explained by the use of different definitions of PAD in the studies and by their varied prevalence of previous revascularisation procedures [in the Eurodiale study revascularisation was performed before enrolment in 10 of 73 (13·7%) patients in our centre]. This may have led to these patients not being classified as having PAD (defined as an ABI of <0·9 and compatible clinical signs).
In general, for preventive programs, interventions with a higher sensitivity are preferable. In our study the final model for predicting ulcer recurrence, as determined by the estimated logistic regression equation, is in accordance with this prerequisite – the group with recurrent DFU was correctly classified in 90·5%, whereas the group without recurrent DFU was correctly classified in 55%. Our study however had several limitations. We enrolled a relatively small number of patients and not all our patients were regularly followed up after healing at our own foot clinic. Those who were not, however, were contacted by podiatric professionals to obtain and verify follow‐up information. This is one of the few studies that investigated the rate of, and risk factors for, recurrent ulceration in patients with a DFU. It was a single‐site study conducted by Eurodiale investigators with extensive experience in treating DFUs.
In summary, our results show a high recurrence rate of DFU during 3‐year follow‐up period in patients with a primarily healed ulcer, despite regular follow‐up and patient education. We also found that the independent risk factors for ulcer recurrence were plantar location of the ulcer, the presence of underlying osteomyelitis, poor glycaemic control and an elevated CRP at the time of diagnosis of the first foot ulcer. Knowing these risk factors may allow clinicians and health care systems to target heightened efforts at prevention of reulceration after healing to selected high‐risk patients.
Acknowledgements
We thank the members of Eurodiale group for realisation of first step of the study: L. Prompers, M. Huijberts, J. Apelqvist, E. Jude, A. Piaggesi, K. Bakker, M. Edmonds, P. Holstein, D. Mauricio, G. Ragnarson Tennvall, H. Reike, M. Spraul, L. Uccioli, V. Urbancic, K. Van Acker, J. van Baal and F. van Merode. This research was supported by grant MZO 00023001. The authors do not have any competing interests.
References
- 1. International Working Group on the Diabetic Foot. International Consensus on the Diabetic Foot & Practical Guidelines on the Management and Prevention of the Diabetic Foot. 2011.
- 2. Boulton AJ. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia 2004;47:1343–53. [DOI] [PubMed] [Google Scholar]
- 3. Edmonds M. Multidisciplinary care of the diabetic foot patient with infection. Int J Low Extrem Wounds 2010;9:6–8. [DOI] [PubMed] [Google Scholar]
- 4. Fosse S, Hartemann‐Heurtier A, Jacqueminet S, Ha Van G, Grimaldi A, Fagot‐Campagna A. Incidence and characteristics of lower limb amputations in people with diabetes. Diabet Med 2009;26:391–6. [DOI] [PubMed] [Google Scholar]
- 5. Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361: 1545–51. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong DG, Boulton AJ, Andros G, Attinger C, Eisenbud D, Lavery LA, Lipsky BA, Mills JL, Sibbald G, Smith AP, Wukich D, Margolis DJ. Defining success in clinical trials of diabetic foot wounds: the Los Angeles DFCon consensus. Int Wound J 2009;6:211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crawford F, McCowan C, Dimitrov BD, Woodburn J, Wylie GH, Booth E, Leese GP, Bekker HL, Kleijnen J, Fahey T. The risk of foot ulceration in people with diabetes screened in community settings: findings from a cohort study. QJM. 2011;104:403–10. [DOI] [PubMed] [Google Scholar]
- 8. Apelqvist J, Larsson J, Agardh CD. Long‐term prognosis for diabetic patients with foot ulcers. J Intern Med 1993;233:485–91. [DOI] [PubMed] [Google Scholar]
- 9. Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA 2009;301:603–18. [DOI] [PubMed] [Google Scholar]
- 10. Apelqvist J, Ragnarson‐Tennvall G, Larsson J, Persson U. Long‐term costs for foot ulcers in diabetic patients in a multidisciplinary setting. Foot Ankle Int 1995;16:388–94. [DOI] [PubMed] [Google Scholar]
- 11. Connor H, Mahdi OZ. Repetitive ulceration in neuropathic patients. Diabetes/Metab Res Rev. 2004;20 Suppl 1:S23–8. [DOI] [PubMed] [Google Scholar]
- 12. Ince P, Kendrick D, Game F, Jeffcoate W. The association between baseline characteristics and the outcome of foot lesions in a UK population with diabetes. Diabet Med 2007;24:977–81. [DOI] [PubMed] [Google Scholar]
- 13. Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, van Baal J, van Merode F, Schaper N. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50:18–25. [DOI] [PubMed] [Google Scholar]
- 14. Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, Uccioli L, Urbancic V, Bakker K, Holstein P, Jirkovska A, Piaggesi A, Ragnarson‐Tennvall G, Reike H, Spraul M, Van Acker K, Van Baal J, Van Merode F, Ferreira I, Huijberts M. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008;51:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Battum P, Schaper N, Prompers L, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Ragnarson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, van Acker K, van Baal J, Ferreira I, Huijberts M. Differences in minor amputation rate in diabetic foot disease throughout Europe are in part explained by differences in disease severity at presentation. Diabetic Med J Brit Diabetic Assoc 2011;28:199–205. [DOI] [PubMed] [Google Scholar]
- 16. Jirkovska A, Boucek P, Woskova V, Bartos V, Skibova J. Identification of patients at risk for diabetic foot: a comparison of standardized noninvasive testing with routine practice at community diabetes clinics. J Diabetes Complications 2001;15:63–8. [DOI] [PubMed] [Google Scholar]
- 17. Lipsky BA. A report from the international consensus on diagnosing and treating the infected diabetic foot. Diabetes/Metab Res Rev 2004;20 Suppl 1:S68–77. [DOI] [PubMed] [Google Scholar]
- 18. Peters EJ, Armstrong DG, Lavery LA. Risk factors for recurrent diabetic foot ulcers: site matters. Diabetes Care 2007;30:2077–9. [DOI] [PubMed] [Google Scholar]
- 19. Ghanassia E, Villon L, Thuan Dit Dieudonne JF, Boegner C, Avignon A, Sultan A. Long‐term outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: a 6.5‐year follow‐up study. Diabetes Care 2008;31:1288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 21. Boulton AJ, Hardisty CA, Betts RP, Franks CI, Worth RC, Ward JD, Duckworth T. Dynamic foot pressure and other studies as diagnostic and management aids in diabetic neuropathy. Diabetes Care 1983;6:26–33. [DOI] [PubMed] [Google Scholar]
- 22. Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K. Risk factors associated with adverse outcomes in a population‐based prospective cohort study of people with their first diabetic foot ulcer. J Diabetes Complications 2007;21:341–9. [DOI] [PubMed] [Google Scholar]
- 23. Yesil S, Akinci B, Yener S, Bayraktar F, Karabay O, Havitcioglu H, Yapar N, Atabey A, Kucukyavas Y, Comlekci A, Eraslan S. Predictors of amputation in diabetics with foot ulcer: single center experience in a large Turkish cohort. Hormones (Athens) 2009;8:286–95. [DOI] [PubMed] [Google Scholar]
- 24. Kowalski TJ, Matsuda M, Sorenson MD, Gundrum JD, Agger WA. The effect of residual osteomyelitis at the resection margin in patients with surgically treated diabetic foot infection. J Foot Ankle Surg 2011;50:171–5. [DOI] [PubMed] [Google Scholar]
- 25. Aragon‐Sanchez J, Lazaro‐Martinez JL, Hernandez‐Herrero C, Campillo‐Vilorio N, Quintana‐Marrero Y, Garcia‐Morales E, Hernandez‐Herrero MJ. Does osteomyelitis in the feet of patients with diabetes really recur after surgical treatment? Natural history of a surgical series. Diabetic Med J Brit Diabetic Assoc 2011; 29:813–8. [DOI] [PubMed] [Google Scholar]
- 26. Cuttica DJ, Philbin TM. Surgery for diabetic foot infections. Foot Ankle Clin 2010;15:465–76. [DOI] [PubMed] [Google Scholar]
- 27. Jeandrot A, Richard JL, Combescure C, Jourdan N, Finge S, Rodier M, Corbeau P, Sotto A, Lavigne JP. Serum procalcitonin and C‐reactive protein concentrations to distinguish mildly infected from non‐infected diabetic foot ulcers: a pilot study. Diabetologia 2008;51:347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lipsky BA, Sheehan P, Armstrong DG, Tice AD, Polis AB, Abramson MA. Clinical predictors of treatment failure for diabetic foot infections: data from a prospective trial. Int Wound J 2007;4:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Volaco A, Chantelau E, Richter B, Luther B. Outcome of critical foot ischaemia in longstanding diabetic patients: a retrospective cohort study in a specialised tertiary care centre. Vasa 2004;33:36–41. [DOI] [PubMed] [Google Scholar]
- 30. Mantey I, Foster AV, Spencer S, Edmonds ME. Why do foot ulcers recur in diabetic patients? Diabetic Med J Brit Diabetic Assoc 1999;16:245–9. [DOI] [PubMed] [Google Scholar]
- 31. Poolsup N, Suksomboon N, Rattanasookchit S. Meta‐analysis of the benefits of self‐monitoring of blood glucose on glycemic control in type 2 diabetes patients: an update. Diabetes Technol Ther 2009;11:775–84. [DOI] [PubMed] [Google Scholar]
- 32. Valk G, Kriegsman, DM , Assendfelft, WJ. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev 2005;25:CD001488. [DOI] [PubMed] [Google Scholar]
- 33. Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes – a review. Diabetic Med J Brit Diabetic Assoc 2010;27:4–14. [DOI] [PubMed] [Google Scholar]


