Abstract
Negative pressure wound therapy (NPWT) contracts the wound and alters the pressure in the tissue of the wound edge, which accelerates wound healing. The aim of this study was to examine the effect of the type (foam or gauze) and size (small or large) of wound filler for NPWT on wound contraction and tissue pressure. Negative pressures between −20 and −160 mmHg were applied to a peripheral porcine wound (n = 8). The pressure in the wound edge tissue was measured at distances of 0·1, 0·5, 1·0 and 2·0 cm from the wound edge and the wound diameter was determined. At 0·1 cm from the wound edge, the tissue pressure decreased when NPWT was applied, whereas at 0·5 cm it increased. Tissue pressure was not affected at 1·0 or 2·0 cm from the wound edge. The tissue pressure, at 0·5 cm from the wound edge, was greater when using a small foam than when using than a large foam. Wound contraction was greater when using a small foam than when using a large foam during NPWT. Gauze resulted in an intermediate wound contraction that was not affected by the size of the gauze filler. The use of a small foam to fill the wound causes considerable wound contraction and may thus be used when maximal mechanical stress and granulation tissue formation are desirable. Gauze or large amounts of foam result in less wound contraction which may be beneficial, for example when NPWT causes pain to the patient.
Keywords: Experimental surgery, Negative pressure wound therapy, Tissue pressure, Wound contraction, Wound healing
INTRODUCTION
Negative pressure wound therapy (NPWT) has remarkable effects on the healing of chronic and difficult wounds 1, 2. The technique entails the application of negative pressure to a sealed, airtight wound. One of the fundamental effects of negative pressure on the wound bed is believed to be the induction of mechanical deformation of the tissue 3, 4 as the wound contracts when negative pressure is applied (3). During this contraction, the wound edge tissue is drawn towards the vacuum, resulting in tissue compression and increased pressure on the wound edge 5, 6. In a previous study, using processed meat, it was shown that the tissue pressure increased 1 cm from the vacuum source, while at deeper locations, it was not affected (5). These findings were later verified in human subjects (7).
Compression of superficial tissue and an increase in tissue pressure have been suggested to decrease blood perfusion in the wound edge 8, 9, 10. Decreased blood flow may be one of the beneficial effects of NPWT, as factors released in response to hypoperfusion are strong stimulators of angiogenesis and granulation tissue formation. Another beneficial effect of the pressure on the wound wall may be to tamponade superficial bleeding during surgical procedures (11). However, in poorly vascularised tissue, there may be risk of ischaemia (12). In these cases, the level of negative pressure may need to be reduced to alleviate the pressure in the tissue, thereby reducing the degree of hypoperfusion (13). No study has yet been performed to examine whether tissue pressure can be affected by changing the type or size of wound filler used for NPWT.
Previous studies on tissue pressure during NPWT have been performed in vitro, using processed meat (5), and in vivo on human subjects (7), but only at limited positions around the wound edge. The aims of this study were to examine wound contraction and tissue pressure upon NPWT using different levels of negative pressure (ranging from −20 to −160 mmHg). Different types (foam or gauze) and sizes (small or large) of wound fillers were applied in vivo to a porcine peripheral wound and the pressure in the wound edge tissue was measured at distances of 0·1, 0·5, 1·0 and 2·0 cm from the wound edge and the wound diameter was determined.
MATERIALS AND METHODS
Animals
Eight healthy domestic pigs of both sexes, with a mean body weight of 70 kg, were fasted overnight with free access to water. The experimental protocol for this study was approved by the Ethics Committee for Animal Research at Lund University, Sweden. All animals received humane care in compliance with the European Convention on Animal Care.
Anaesthesia and surgical procedure
An intramuscular injection of xylazine (Rompun® vet. 20 mg/ml; Bayer AG, Leverkusen, Germany; 2 mg/kg) mixed with ketamine (Ketaminol® vet. 100 mg/ml; Farmaceutici Gellini S.p.A, Aprilia, Italy; 20 mg/kg) was used for premedication. Anaesthesia was then induced with intravenous sodium thiopental (Pentothal®; Abbot Scandinavia, Stockholm, Sweden; 8 mg/kg) and maintained with a continuous infusion of fentanyl (3·5 µg/kg/hour) in Ringer's acetate in combination with intermittent bolus doses of sodium thiopental. The animals were orally intubated with cuffed endotracheal tubes. Mechanical ventilation was established with a Siemens‐Elema ventilator (Siemens‐Elema AB, Solna, Sweden) in the volume‐controlled mode (65% nitrous oxide and 35% oxygen). Ventilatory settings were identical for all animals (respiratory rate, 15 breaths/minute; minute ventilation, 12 l/minute). A positive end‐expiratory pressure of 5 cm H2O was applied. A Foley catheter was inserted into the urinary bladder through a suprapubic cystostomy. After the experiments were finished, a lethal dose of potassium chloride was administered intravenously for euthanasia.
Wounds and treatment
Circular wounds, 6 cm in diameter, extending into the subcutaneous tissue, were created on the pig's back, 10 cm from the spine, towards the back leg. One wound was created on each pig for the performance of the experiments. Either AMD gauze (Kendall Healthcare, Mansfield, MA) or open pore structure polyurethane foam (VAC® black GranuFoam®, KCI, San Antonio, TX) was used as wound filler. The gauze was soaked in saline. The wound was sealed with transparent adhesive drape. A drain was connected to the vacuum source. Negative pressures between −20 and −160 mmHg were applied at 20 mmHg increments.
The effects of two different sizes of wound fillers were investigated. The foam pieces were circular and cut to a diameter of 50 or 70 mm (referred to as the ‘small’ and ‘large’ foam fillers, respectively). Two gauze sponges (each 150 × 170 mm) together constituted the ‘small’ gauze filler, and three gauze sponges constituted the ‘large’ gauze filler.
Pressure measurements
The negative pressure on the wound bed, underneath the wound filler, was measured using a saline‐filled pressure catheter. The tip of the catheter was sutured to the centre of the bottom of the wound (Figure 1). The pressure catheter was connected to a custom‐built pressure gauge that relies on pressure transduction via a fluid‐filled catheter. Owing to fluid accumulation at the end of the probe, this technique is not suitable for pressure measurements in the tissue as fluid may accumulate there. However, pressure measurements in the wound bed poses no problem as the fluid is evacuated continuously by the NPWT.
Figure 1.
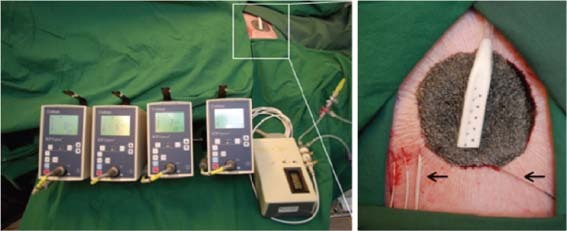
Left: the four Codman ICP express pressure monitors used to measure the tissue pressure around the wound edge and the custom‐built pressure gauge used to measure the pressure in the wound bed. Right: the porcine wound filled with foam showing the tissue pressure sensors (left arrow) 0·1, 0·5, 1·0 and 2·0 cm from the wound edge, and the pressure sensor sutured to the wound bed (right arrow). The drain is seen in the centre of the wound filler.
Intracranial tissue pressure microsensors (Codman/Johnson and Johnson Professional Inc., Raynham, Massachusetts, US) were used to measure the pressure in the tissue. These were inserted into the tissue, using an 18G Tuohy needle, at distances of 0·1, 0·5, 1·0 and 2·0 cm from the wound edge (Figure 1). The Codman ICP monitoring system was used to measure tissue pressures as it can record high positive pressures. This system was not used for measuring wound bed pressure as it only records negative pressures down to −99 mmHg. The resulting pressure was recorded after having stabilised at a constant level, which typically occurred within 2 minutes after application of NPWT.
Measurement of wound contraction
Four marks were made on the edge of the wound. The vertical and horizontal diameters of the wound were measured before and after the application of negative pressure. The horizontal diameter was here measured in parallel with the pig's spine and the vertical diameter was measured 90° from this. The mean of the two diameters measured (the horizontal and the vertical) was calculated.
Calculations and statistics
Eight pigs were used for this study. In cases where adverse events and experimental conditions inhibited the continuation of the experiments, the number had to be reduced. Calculations and statistics were performed using GraphPad 5.0 software. The sequence of applying the different NPWT modalities was varied between the experiments using a Latin square design. Statistical analysis was performed using the Mann–Whitney test. Significance was defined as P < 0·05. The P‐values in the interval 0·001–0·300 have been written out, while outside this interval the expressions ‘P < 0·001’ and ‘P > 0·30’ have been used. Results are presented as the mean ± the standard error of the mean and median (range).
RESULTS
Wound contraction
The mean wound diameter was initially 6·1 ± 0·2 cm (median 5·9 cm, range 5·4–6·7 cm). NPWT was applied and the wound diameter was reduced with increasing levels of negative pressure. Wound contraction was greater when using a small piece of foam [the mean wound diameter was 5·8 ± 0·1 cm, (median 5·7 cm, range 5·1–6·5 cm) at −120 mmHg] compared with when using a large piece of foam [the mean wound diameter was 6·2 ± 0·2 cm, (median 6·1 cm, range 5·6–7·6 cm) at −120 mmHg , P = 0 · 033]. Gauze resulted in an intermediate wound contraction that was not affected by the size of the gauze filler [the mean wound diameter was 6·1 ± 0·2 cm (median 5·9 cm, range 5·4–7·5 cm) for the small gauze filler and 6·1 ± 0·1 cm (median 5·9 cm, range 5·6–7·2 cm) for the large gauze filler at −120 mmHg, P > 0·30]. See Figure 2 for detailed results.
Figure 2.
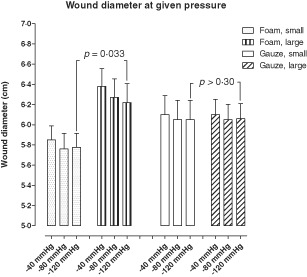
Wound diameter upon application of Negative pressure wound therapy using gauze and foam of different sizes (small or large). The diameter was measured in the horizontal and the vertical plane, as described in the Methods, and the mean values for each wound was calculated. Results are shown as mean ± standard error of the mean (SEM) of six experiments.
Pressure on the wound bed
Pressure transduction to the wound bed is equally good between the foam sizes tested. For example, at an applied pressure of −120 mmHg, the mean pressure recorded when using the small foam filler was −105 ± 2 mmHg (median −105 mmHg, range −110 to −99 mmHg) compared with −103 ± 2 mmHg (median −103, range −108 to −98 mmHg) when using the large foam filler (P > 0·30). The results were similar for gauze, for example when using the small gauze filler, the mean pressure was −104 ± 2 mmHg (median −104 mmHg, range −110 to −97 mmHg) compared with −108 ± 2 mmHg (median −108, range −112 to −101 mmHg) when using the large gauze filler (P > 0·30). See Figure 3 for detailed results.
Figure 3.
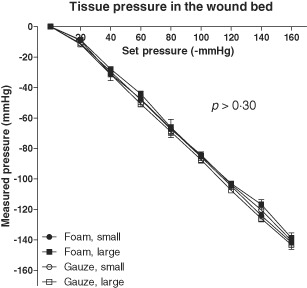
Wound bed pressures during Negative pressure wound therapy in wounds filled with foam or gauze of different sizes (small or large). Results are shown as mean ± standard error of the mean (SEM) of six experiments. Note that both sizes of foam and gauze offer similar pressure transduction to the wound bed.
Pressure in the wound edge tissue
The tissue pressure was measured at different distances from the wound edge. At 0·1 cm from the wound edge, the tissue pressure decreased during NPWT, regardless the type of wound filler [e.g. −26 ± 14 mmHg (median −17, range −91 to −3 mmHg), at −120 mmHg, using the small foam filler]. At 0·5 cm from the wound edge, the tissue pressure increased during NPWT, regardless the type of wound filler [e.g. 9 ± 1 mmHg (median 9, range 7–13 mmHg), at −120 mmHg, using the small foam filler]. At 1·0 cm and 2·0 cm from the wound edge, the tissue pressure was not affected by NPWT, regardless the type of wound filler (P > 0·30). See Figure 4 for detailed results.
Figure 4.
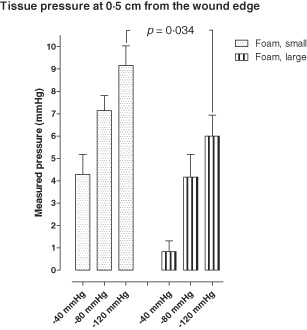
Tissue pressure measured 0·5 cm from the wound edge during Negative pressure wound therapy (NPWT) at −40, −80 and −120 mmHg, respectively in wounds filled with foam of different sizes (small or large). Results are shown as means of eight experiments. Note that the tissue pressure is positive at this distance from the wound edge. This is presumably the result of the compressive forces generated by NPWT.
The decrease in tissue pressure 0·1 cm from the wound edge was not affected by the size of wound filler [e.g. −26 ± 14 mmHg (median −17, range −91 to −3 mmHg), for small foam and −37 ± 16 mmHg (median −26, range −99 to −13 mmHg), for large foam, at −120 mmHg, P > 0·30]. However, the tissue pressure 0·5 cm from the wound edge was higher for a small foam than for a large foam [e.g. 9 ± 1 mmHg (median 9, range 7–13 mmHg), for the small and 6 ± 1 for large foam (median 6, range 3–9 mmHg), at −120 mmHg, P = 0·034, Figure 4].
DISCUSSION
This study showed that a small foam, under negative pressure, resulted in a greater wound contraction than a large foam. Gauze resulted in an intermediate wound contraction that was not affected by the size of the gauze filler. The reason for the difference in properties between foam and gauze may be that the porous structure of foam allows greater volume reduction under pressure.
At a distance of 0·1 cm from the wound edge, the tissue pressure decreased gradually with increasing suction pressure. These observations are in line with results from a previous study in mice (14). This finding suggests that NPWT induces a hypobaric environment not only in the wound bed but also in the superficial wound edge tissue. Subatmospheric tissue pressure and a pressure gradient over the wound edge may initiate a transport of interstitial fluid, which may facilitate the diffusion of oxygen and nutrients.
At 0·5 cm from the wound edge, the tissue pressure increased gradually with the applied negative pressure. Similar results have been reported in recent studies by Kairinos et al. 5, 7, where there was increased pressure in processed meat 1·0 cm from the wound edge, while the pressure was not affected at deeper locations (5). The reason for the increase in pressure 0·5 cm from the wound edge is not fully understood. It is believed that the negative pressure contracts the wound resulting in wound edge tissue compression, which causes increased tissue pressure around the edge of the wound (5).
The tissue pressure (at 0·5 cm from the wound edge) was dependent on the size of the foam filler, being higher for the small foam filler and lower for the large foam filler. One possible explanation for this is that a small foam filler results in a smaller wound diameter than a large foam filler. The negative pressure thus causes greater compression of the tissue when using a small foam filler, than when using a large one. Conversely, the large foam filler offers greater resistance against the wound edge wall, which prevents the tissue from being compressed.
The increase in tissue pressure may result in decreased blood flow (12). We have previously showed that the blood flow 0·5 cm from the wound edge decreased during NPWT (9). It is well known that reduced blood flow stimulates angiogenesis and granulation tissue formation, which facilitates the process of wound healing 15, 16. Furthermore, compression of the wound edge tissue may be beneficial during surgical procedures as it has been shown to tamponade superficial bleeding (11), and may reduce wound edge oedema. However, in poorly vascularised tissue, compression and decreased blood perfusion may cause ischaemia 12, 13.
The wound is more contracted during NPWT treatment at a high level of negative pressure or when using a small foam. Conversely, the wound is less contracted during NPWT treatment at a low level of negative pressure or when using gauze or a large foam filler. The biological effects in the wound edge may thus be adjusted by changing the conditions under which NPWT is applied. We know that mechanical wound contraction often causes pain to the patient (17). It is possible that this can be managed by reducing the level of negative pressure, or by using gauze or a large foam filler. In contrast, if the aim is to maximise granulation tissue formation, a high level of negative pressure 4, 9, 18 or a small foam filler may be used.
It is indeed interesting that wound contraction differs between different sizes and types of wound filler and it is most probable that this relates to healing during NPWT. In a previous study, it could be shown that wound contraction is greater when using foam than when using gauze in sternotomy wounds (19). Mechanical effects on the wound edge resulting from NPWT are believed to be one of the fundamental mechanisms by which NPWT promotes healing. Wound contraction creates deformational forces at the wound–foam interface (20), which is thought to initiate a series of inter‐related biological effects including the promotion of wound edge microvascular blood flow, the removal of bacteria and the stimulation of granulation tissue formation (21). Different amounts of wound contraction, offered by different types of wound fillers, may be desirable for different types of wounds. The advantage of greater wound contraction, such as that achieved with foam, may be massive stimulation of granulation tissue and, thus, faster healing. Large wound contraction may also be preferable in wounds dependent on reverse tissue expansion for secondary wound closure, for example for upper and lower limb compartment syndrome. However, when NPWT is applied to a wound that cannot be closed (e.g. because of an enlarged heart), a lower degree of wound contraction, such as that obtained with gauze, may be preferable. Also, a lower degree of wound contraction may result in a lower risk of the underlying organs becoming wedged between the sternal edges: This may reduce the risk of damage to the heart and lungs. Furthermore, patient's pain may be reduced by minimising the mechanical effects on the wound edge.
The choice of conditions for NPWT may be of even greater importance when treating poorly vascularised tissue, such as in diabetic patients with arteriosclerosis, or in patients with general arteriosclerosis. NPWT elicits hypoperfusion in the wound edge as a result of increased tissue pressure. We know that these effects can be governed by altering the level of negative pressure applied (13). The choice of filler material, or the size of the filler, may offer other means of governing wound edge perfusion, however, this needs to be studied in greater detail.
In conclusion, NPWT contracts the wound and alters the tissue pressure which may accelerate wound healing. NPWT creates a hypobaric environment in the superficial wound edge tissue (0·1 cm from the wound edge), which may cause the transport of interstitial fluid, facilitating the diffusion of oxygen and nutrients. Deeper in the wound edge (0·5 cm), tissue pressure increases that may be a result of wound contraction and wound edge tissue deformation. The use of a small foam to fill the wound causes considerable wound contraction and may thus be used when maximal mechanical stress and granulation tissue formation are desirable. Conversely, gauze or large amounts of foam result in less wound contraction which may be beneficial when NPWT causes pain to the patient.
REFERENCES
- 1. Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum‐assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117:121S–6S. [DOI] [PubMed] [Google Scholar]
- 2. Banwell PE, Teot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care 2003;12:22–8. [DOI] [PubMed] [Google Scholar]
- 3. Malmsjo M, Ingemansson R, Martin R, Huddleston E. Negative‐pressure wound therapy using gauze or open‐cell polyurethane foam: similar early effects on pressure transduction and tissue contraction in an experimental porcine wound model. Wound Repair Regen 2009;17:200–5. [DOI] [PubMed] [Google Scholar]
- 4. Borgquist O, Ingemansson R, Malmsjo M. The influence of low and high pressure levels during negative‐pressure wound therapy on wound contraction and fluid evacuation. Plast Reconstr Surg 2011;127:551–9. [DOI] [PubMed] [Google Scholar]
- 5. Kairinos N, Solomons M, Hudson DA. The paradox of negative pressure wound therapy – in vitro studies. J Plast Reconstr Aesthet Surg 2010;63: 174–9. [DOI] [PubMed] [Google Scholar]
- 6. Willy C, Gerngross H. Scientific background of the vacuum closure – an abstract. Zentralbl Chir 2004;129 Suppl 1:S6. [DOI] [PubMed] [Google Scholar]
- 7. Kairinos N, Solomons M, Hudson DA. Negative‐pressure wound therapy I: the paradox of negative‐pressure wound therapy. Plast Reconstr Surg 2009;123:589–98; discussion 599–600. [DOI] [PubMed] [Google Scholar]
- 8. Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum‐assisted closure therapy. Ann Thorac Surg 2005;79:1724–30; discussion 1730–1. [DOI] [PubMed] [Google Scholar]
- 9. Borgquist O, Ingemansson R, Malmsjo M. Wound edge microvascular blood flow during negative‐pressure wound therapy: examining the effects of pressures from −10 to −175 mmHg. Plast Reconstr Surg 125:502–9. [DOI] [PubMed] [Google Scholar]
- 10. Wackenfors A, Sjogren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum‐assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 2004;12(6):600–6. [DOI] [PubMed] [Google Scholar]
- 11. Sjögren J, Gustafsson R, Koul B, Ingemansson R. Selective mediastinal tamponade to control coagulopathic bleeding. Ann Thorac Surg 2003;75:1311–3. [DOI] [PubMed] [Google Scholar]
- 12. Kairinos N, Voogd AM, Botha PH, Kotze T, Kahn D, Hudson DA, Solomons M. Negative‐pressure wound therapy II: negative‐pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg 2009;123:601–12. [DOI] [PubMed] [Google Scholar]
- 13. Borgquist O, Ingemansson R, Malmsjo M. The effect of intermittent and variable negative pressure wound therapy on wound edge microvascular blood flow. Ostomy Wound Manage 56(3):60–7. [PubMed] [Google Scholar]
- 14. Murphey GC, Macias BR, Hargens AR. Depth of penetration of negative pressure wound therapy into underlying tissues. Wound Repair Regen 2009;17:113–7. [DOI] [PubMed] [Google Scholar]
- 15. Petzina R, Gustafsson L, Mokhtari A, Ingemansson R, Malmsjo M. Effect of vacuum‐assisted closure on blood flow in the peristernal thoracic wall after internal mammary artery harvesting. Eur J Cardiothorac Surg 2006;30:85–9. [DOI] [PubMed] [Google Scholar]
- 16. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76; discussion 577. [PubMed] [Google Scholar]
- 17. Krasner DL. Managing wound pain in patients with vacuum‐assisted closure devices. Ostomy Wound Manage 2002;48:38–43. [PubMed] [Google Scholar]
- 18. Borgquist O, Gustafsson L, Ingemansson R, Malmsjo M. Tissue ingrowth into foam but not into gauze during negative pressure wound therapy. Wounds 2009;21:302–9. [PubMed] [Google Scholar]
- 19. Malmsjö M, Lindstedt S, Ingemansson R. Effects of foam or gauze on sternum wound contraction, distension and heart and lung damage during negative‐pressure wound therapy of porcine sternotomy wounds. Interact Cardiovasc Thorac Surg 2011;12:349–54. [DOI] [PubMed] [Google Scholar]
- 20. Malmsjö M, Borgquist O. NPWT settings and dressing choices made easy. Wounds International 2010;1. [Google Scholar]
- 21. Malmsjo M, Ingemansson R, Sjogren J. Mechanisms governing the effects of vacuum‐assisted closure in cardiac surgery. Plast Reconstr Surg 2007;120:1266–75. [DOI] [PubMed] [Google Scholar]


