Abstract
The focus on quality of life issues in wound care has justly taken a far greater importance. With the acceptance that pain can be a major factor to the patient, and in particular, pain at dressing change comes the opportunity for avoidance and/or reduction strategies. Whilst pain has been associated with wound infection for millennia, it is only much more recently that this has received due attention from research and clinical practice. In this study, the nature of pain, changes in pain and pain associated with infection are the focal points. A Delphi approach, now a frequently used tool in wound care research, has been used to obtain expert opinion on these aspects of management.
Keywords: Delphi, Wound infection, Wound pain
INTRODUCTION
Over the past decade, there has been an increasing awareness of the occurrence and significance of wound‐related pain. Numerous research studies have been reported showing the association of pain with wound infection, and, with the dressing change procedure in particular 1, 2, 3, 4. The link between wound pain, stress, delayed healing and patient quality of life has also been established 5, 6, 7. This interest has resulted in two best practice documents from the World Union of Wound Healing Societies (WUWHS 2004, 2008). These define the occurrence, assessment and management/avoidance of wound‐related pain.
The estimated worldwide burden of wounds may be viewed in Table 1, (8).
Table 1.
Estimation of the worldwide burden of wounds (8)
| Surgical wounds | 40–50 million |
| Leg ulcers | 8–10 million |
| Burns | 7–10 million |
| Pressure ulcers | 7–8 million |
Viewed in isolation, this estimate clearly avoids qualifying the personal wound burden of patients but rather hints at the encumbrance sustained by society as a whole. One of the more troublesome facets of a wound that is frequently encountered is that of pain. Wound‐related pain provides the patient with regular, sometimes constant and intense reminders that healing has yet to be accomplished. We have learnt that the pain experience is best described by the patient from their own subjective stand point and is not something that can be easily or accurately described to, or by, a third party who by definition is unable to place themselves in that self‐same pain experience (9). Although the epidemiological evidence on wound‐related pain is limited it is recognised that wound pain is a common experience (10) and continues to be a major concern of patients and clinicians 2, 11, 12, 13.
The morbidity/mortality associated with wound infection is a global issue that can affect patients with either acute or chronic wounds. Epidemiological data across all wound types are not readily available because of the disseminated and disparate populations. However, robust data have been collected for surgical site infection 14, 15 possibly prompted by the value of such data used as a measure of the quality of patient care.
The wound inflammatory response provides a causal association between pain and infection as the soft tissue responds to the invading microorganisms 1, 10, 16 and leads to the expression of enzymes and free radicals (17) causing tissue damage.
Clarity in respect of the character and nature of pain that is associated with wound infection has yet to be realised. However, we are aware that
-
•
an increase in pain or a change in the nature of pain (pressure ulcers);
-
•
unexpected pain/tenderness (acute/surgical wounds) and
-
•
onset of pain in a previously pain‐free wound (partial/full thickness burn wounds)
have all been identified (18) and may be accepted as clinical signs of wound infection.
WOUND INFECTION, DRESSINGS AND PAIN
It is now widely recognised that wound infection causes pain (16), that there is an association between pain and stress (19) and that stress interferes with healing 20, 21. What is not so clear is whether patients with a wound infection experience more pain in general than those patients with non infected wounds. This requires clarification, especially in relation to dressing change procedures and related intervention such as debridement as this will facilitate/result in improved care. When infection intervenes, irrespective of how that infection is managed a dressing is required that will obviate tissue trauma and avoid exacerbating the patients' pain experience. Wound‐related pain may be considered from two broad aetiologies, that is, somatic and event‐related pain. Somatic pain is nociceptive pain where the nerves detect alterations in temperature, vibration and swelling/pressure in the skin, joints, muscles and deep tissue. Sensory neurones respond to stimuli and often the result is pain that is dull, intense and ongoing in nature. Event‐related pain is a result of intervention that is operational, procedural or incident (O.P. or I) in origin being derived from exogenous sources, for example, dressing change procedures and/or debridement, unlike somatic pain which is endogenous. The authors of this study have sought to clarify some of the similarities and differences between these two sources of pain and to therefore indicate that intervention by the clinician will need to be adjusted accordingly. Irrespective of pain aetiology the routine procedures of wound care including debridement, cleansing and dressing changes still have to be conducted. Clinicians should consider, what does pain aetiology mean in practical terms? Does the patients pain impact on clinician thinking, and influence the treatment plan? Importantly, what is the impact of topical applications – including choice of dressing on the patient? Answers to these questions support and provide direction in clinical guidance and best practice. To investigate if a relationship exists between wound infection, dressings and pain in the chronic wound, a Delphi study was devised and completed during 2009–2010 with the intention of:
-
•
highlighting any correlation found between infected wounds and increased pain/sensitivity,
-
•
identifying expert clinician response to event‐related pain episodes,
-
•
reporting on the impact of dressings/ antiseptics on the somatic and operative influences of wound infection associated pain and
-
•
reporting the Delphi panel rankings of treatments related to wound infection and pain.
METHODOLOGY
A Delphi approach, or Delphi procedure, is designed to obtain a dependable consensus from a selected expert panel who respond to several ‘rounds’ of set questions or statements that are interspersed with controlled feedback (22). Between rounds, panel responses are collated and statistically summarised and used as a basis for the preparation of the next round. These data are then fed back to the individual panel members and provision is made for revision of answers (in light of the group response) returned in the previous round (23). In this study, three rounds were used.
Four characteristics typify the Delphi process:
-
1
anonymity (panel members are unaware of their comembers identity),
-
2
iteration (the process involves a series of rounds),
-
3
controlled feedback (the responses are analysed and fed back) and
-
4
statistical group response (articulation of the degree of group consensus) (23).
These four features provide rigour for this qualitative process where decisions are made by individual panel members and refined by the group as a whole thus ensuring that each participant has a ‘equal voice’. Thus, contact between the researcher(s) and the panel members takes place only on an individual basis, usually via email, letter or the internet and where anonymity of panel members is strictly maintained. In this study, the data derived from the Delphi approach are reported generally using summary statistics. Where appropriate, Cronbach's α has been used to test the level of consensus achieved by the panel. Thus Cronbach's α provides an unbiased check on the consistency (consensus) of the panel responses (24).
Delphi panel members
Thirty international experts were selected on the basis of their clinical/publication profiles and were invited to participate. Twenty‐one internationally recognised multiprofessional respondents accepted the invitation. The distribution of panel members together with their country of residence may be seen in Table 2.
Table 2.
Distribution of panel members with their country of residence
| Country | Number of participants (n = 21) |
|---|---|
| Australia | 1 |
| Belgium | 2 |
| Canada | 1 |
| Eire | 1 |
| Italy | 2 |
| The Netherlands | 1 |
| UK | 9 |
| UAE | 1 |
| USA | 3 |
The panel members were from three disciplines (Table 3).
Table 3.
Panel members by discipline
| Discipline | Number of participants |
|---|---|
| Nurses | 14 |
| Physicians | 6 |
| Physical therapists | 1 |
The experience of the panel members in relation to tissue viability ranged from 5 to 29 years with a mean of 18·5. Four worked in a primary care facility/clinic that was based in the community. Thirteen were in a secondary care hospital either inpatient or outpatient facility. One worked in hospice/palliative care sector. One university based, one in a home health care setting and one participant followed patients through from hospital to community care. The number of wound care patients seen in a typical week by each member ranged from 3 to 150 with a mean of 38.
All members had extensive experience in caring for patients with painful and infected wounds.
To explore their views on wound care practice in relation to chronic wounds/infection/ pain/dressings a questionnaire was prepared with two distinct sections. The first section related to somatic or background pain and the second focussed on event‐related pain that is O.P.I. pain.
RESULTS
Somatic and event‐related pain
Somatic pain associated with chronic wounds is likely to be multifarious in origin, associated with chronic inflammation, the generation of direct pain mediators together with sensitisation of the wound through the lowering of nociceptor thresholds via the production of indirect pain mediators (25). Event‐related pain is the direct result of clinical intervention and therefore careful planning prior to intervention is required.
The precise relationship between wound infection and the occurrence of pain has, to the best of our knowledge, not been thoroughly investigated. This gap in our knowledge led to the construction and inclusion of the following questions which we anticipate will encourage further investigation.
It is clear from these responses (Table 4) that the majority identified a causal relationship between wound infection and the occurrence of pain. This is consistent with the wound infection criteria developed by Cutting and Harding (26) and other studies such as Cutting et al. (18) which focussed on the development of clinical criteria of wound infection by indication. However, the relationship expressed overall is not quite so demonstrative in the event‐related pain responses. An element of uncertainty appears to be expressed within the four ‘not sure’ responses (Table 5).
Table 4.
Do you consider that a causal relationship exists between wound infection and the onset of, or a change in, the nature of pain?
| Response | Event‐related pain | Somatic‐related pain |
|---|---|---|
| Yes | 15 | 19 |
| No | 2 | 1 |
| Not sure | 4 | 1 |
Table 5.
Do you consider that patients with a wound infection generally experience more pain than those with non infected wounds?
| Response | Event‐related pain | Somatic‐related pain |
|---|---|---|
| Yes | 17 | 17 |
| No | 1 | 1 |
| Not sure | 3 | 3 |
The responses are broadly similar to those of the previous question. Of note, however, is the fact that this is the first published objective evidence of clinicians' views on the degree of pain experienced by patients with and without wound infection viewed from event‐ and somatic‐related perspectives (6, 7).
Table 6.
In what proportion of patients do you regard the occurrence of wound pain as diagnostic of infection?
| Response (%) | Somatic‐related pain |
|---|---|
| 0–25 | 4 |
| 25–50 | 8 |
| 50–75 | 7 |
| 75–100 | 2 |
Table 7.
In what proportion of patients do you regard the occurrence of pain in a previously painless wound diagnostic of infection?
| Response (%) | Somatic‐related pain |
|---|---|
| 0–25 | 0 |
| 25–50 | 2 |
| 50–75 | 6 |
| 75–100 | 12 |
The following responses were obtained in the somatic‐related pain category.
The responses reported in Table 6 (somatic pain) are consistent with the work published by Cutting and Harding (26) and Cutting et al. (18)
The responses in Table 6 suggest that the majority (15) of panel members (n = 71%) consider that pain is diagnostic of infection in 25–75% of patients.
A slightly modified question was posed in respect to event‐related pain to take account of the circumstances that apply – O.P.I. pain.
As presented in Table 8, 17 experts (81%) considered that pain in a previously painless wound is diagnostic of infection in 50–100% of patients. This is also consistent with the wound infection criteria developed by Cutting and coworkers 18, 26 and validated by Gardner et al. (27) who found that increasing pain was diagnostic of wound infection. It is interesting to note that four (19%) of panel members regard the occurrence of pain in a previously painless wound diagnostic of infection in fewer than 50% of cases.
Table 8.
In what proportion of patients do you regard the occurrence of pain in a previously painless wound diagnostic of infection?
| Response (%) | Event‐related pain |
|---|---|
| 0–25 | 1 |
| 25–50 | 3 |
| 50–75 | 12 |
| 75–100 | 5 |
When considering somatic‐related pain it can be seen that the majority of respondents, 16 (76%) considered an alteration in pain to be diagnostic of infection in 50–100% of patients. These findings have important implications for patient management and indicate that this feature of ‘an increase or change in the nature of pain’ should be an obligatory component of wound assessment documentation (9, 10). This is an important tenet of care which is echoed in recent WUWHS consensus documents 28, 29 and may also be found in relevant US Federal regulations (30).
Table 9.
In what proportion of patients do you regard an increase or change in the nature of pain diagnostic of infection?
| Response (%) | Event‐related pain |
|---|---|
| 0–25 | 1 |
| 25–50 | 4 |
| 50–75 | 10 |
| 75–100 | 6 |
Table 10.
In what proportion of patients do you regard an increase or change in the nature of pain diagnostic of infection?
| Responses (%) | Event‐related pain | Somatic‐related pain |
|---|---|---|
| 0–25 | 4 | 1 |
| 25–50 | 6 | 4 |
| 50–75 | 6 | 10 |
| 75–100 | 5 | 6 |
Although the majority deemed that a change in therapeutic intervention was justified there appears to be a degree of vacillation within the panel, especially in the event‐related responses (Table 11). In this study, we have not sought to achieve unanimity of opinion as that would frustrate the Delphi process. A key value of this research methodology is that trends of opinion including majority/minority splits may well come to the fore and this allows individuality of opinion to persevere and not be hidden as may happen in other consensus processes.
Table 11.
Is a change in the nature of wound pain sufficient indication to justify change in therapeutic intervention (excluding dressings)?
| Responses | Event‐related pain | Somatic‐related pain |
|---|---|---|
| Yes | 13 | 17 |
| No | 4 | 3 |
| Not sure | 4 | 1 |
Having established the panel's views on the links between pain in both somatic and event‐related circumstances the emphasis in the questions changed towards treatment (Table 12).
Table 12.
If you answered yes to the previous question would you treat specifically for pain, infection or pain and infection?
| Responses | Event‐related pain | Somatic‐related pain |
|---|---|---|
| Pain | 2 | 1 |
| Infection | 1 | 0 |
| Pain and infection | 10 | 16 |
Previous publications have brought to our attention the fact that dressing change procedures can be most painful for the patient 31, 32 and that the effective management of wound‐related pain is not an established or widely held skill 25, 33. The association of wound infection with pain is, however, established (25) and this has been explored further through the medium of the expert panel. Likewise, the relationship of dressings to wound pain is also recognised (34) and the expert panel explored in relation to wound infection.
Event‐related pain and dressings
The responses indicate that the panel members have independently formed a very strong association between pain in a previously painless wound and the need for a change in topical management.
Somatic only – the panel members were asked the following:
The unanimous yes response in 13, 14 underlines the need for every patient and their wound to have a personalised management plan (35) and also indicates that the clinician should regularly reconsider treatment options. The panel responses also provide a strong association between new pain and the need for a review of wound dressing.
Table 13.
Would the occurrence of pain in a previously painless wound require a review of dressing?
| Responses | Event‐related pain |
|---|---|
| Yes | 20 |
| No | 0 |
| Not sure | 1 |
Table 14.
Would the occurrence of pain in a previously painless wound or an increase in existing pain require a review of dressing?
| Responses | Somatic‐related pain |
|---|---|
| Yes | 21 |
| No | 0 |
| Not sure | 0 |
Event‐related pain only– the panel members were asked the following (Table 15):
Table 15.
Would an increase in existing pain require a review of dressing?
| Responses | Event‐related pain |
|---|---|
| Yes | 21 |
| No | 0 |
| Not sure | 0 |
The responses clearly indicate that the panel members have independently formed a very strong association between pain in a previously painless wound and the need for a potential change in topical management. The importance of appropriate selection of dressing in relation to reducing/avoiding pain has been noted before (36).
These findings reflect the experience of the experts, based on their personal clinical practice. It is of interest to note that for event‐related pain, only 3 of 21 (14%) were sufficiently convinced to state that there was no positive effect on wound pain. Whereas, 12 (57%) were sufficiently confident to state that topical antimicrobials did have a positive impact on wound pain. Systematic reviews 37, 38, 39, 40, 41 of clinical trial data for topical antimicrobials in wound care query the relevance of topical agents in wound care (Table 16). The evidence that we have gathered in this study provides objective verification using a technique with a recognised validity (23) and, in addition, external validity.
Table 16.
In your experience, do you think that topical antimicrobials have a positive impact on treating wound pain?
| Responses | Event‐related pain | Somatic‐related pain |
|---|---|---|
| Yes | 12 | 18 |
| No | 3 | 0 |
| Not sure | 6 | 2 |
| No response | 0 | 1 |
Prior to these systematic reviews disagreement arose concerning the safety of topical antiseptics 42, 43, 44, subsequently their use in wound care became highly controversial (45). More recently additional research, clinical findings and commentary 46, 47, 48 have helped to clarify the situation allowing a more informed approach to their use. This article has already established a firm link between wound infection and the occurrence of pain so it was considered relevant to ask the panel members to consider their own experience regarding use of topical antiseptics.
The panel's unanimous response supports the notion that some dressings have the potential to cause pain reflects the stark association that is already found in the literature (Table 17) 28, 31, 49.
Table 17.
Do you think some dressing types have the potential to cause pain at dressing change (dressing application or removal)?
| Responses | Event‐related pain |
|---|---|
| Yes | 20 |
| No | 0 |
| Not sure | 1 |
Clinical trial data support the use of dressings impregnated with an anti‐inflammatory analgesic (Ibruprofen) (Table 18) 50, 51, 52. The pragmatic approach taken by this current Delphi panel is not wholly consistent with this and reflects that product innovation do not always readily translate into widespread use.
Table 18.
In your experience, do you think that dressings containing an analgesic are effective in treating wound pain associated with dressing change?
| Responses | Event‐related pain |
|---|---|
| Yes | 4 |
| No | 7 |
| Not sure | 3 |
| No response | 7 |
These results reflect the panel's consideration for holistic care within wound management (Table 19). The narrow approach often advocated towards healing as being the only important outcome 53, 54 obviously fails to consider the patient centred quality of life issues.
Table 19.
In your experience, do you think that wound dressings have a role to play in managing wound pain that is associated with the onset of wound infection?
| Responses | Somatic‐related pain |
|---|---|
| Yes | 17 |
| No | 0 |
| Sometimes | 4 |
Event‐related pain
Panel members were asked to rate dressings which in their opinion had the potential to cause pain at dressing change. Ratings were on a scale of 1–9, where 1 represented no potential and 9 represented high potential (Table 20).
Table 20.
Dressing – rate mean and SD
| Dressing | Mean | SD |
|---|---|---|
| Foams | 4·2 | 2·4 |
| Super absorbent dressings | 4·4 | 1·8 |
| Cadexomer iodine | 4·6 | 2·3 |
| Alginates | 4·7 | 2·2 |
| Silver dressings | 4·8 | 1·6 |
| Fibrous dressings | 5·0 | 2·1 |
| Capillary dressings | 5·1 | 2·0 |
| Honey | 5·7 | 2·1 |
| Semi‐permeable films | 5·8 | 2·0 |
| Basic contact dressings | 6·0 | 2·7 |
| NPWT drapes/foams | 6·4 | 2·2 |
| Tulle dressings | 6·4 | 2·2 |
| Gauze | 8·2 | 1·0 |
| Adhesives (dressings or tapes) | 8·4 | 0·7 |
NPWT, negative pressure wound therapy; SD, standard deviation.
Not all authorities support the view that traditional dressings are more likely to be associated with causing pain (55). Others have associated this pain with trauma at dressing change (36) as a result of adhesion to the wound bed (dressing drying out) or adverse effects of dressing adhesive border to the periwound skin. Our findings, however, indicate that pain can be caused not only by these factors but also by the dressing materials used, for example, tulle and gauze. A Cronbach's α was calculated at 0·80. Nunnally and Bernstein (56) postulate that a value of 0·70 or greater can be considered as an acceptable reliability coefficient, therefore this value indicates a high level of agreement between the panel members.
Panel members were asked to rank the significance of dressings in managing wound pain for the 11 dressings that had been previously identified. Ratings were on a scale of 1–9, where 1 represented no potential and 9 represented high potential. The summary statistics [mean and standard deviation (SD)] for these rankings shown in Table 21, a Cronbach's α of 0·77 again indicates a high level of agreement between the panel members.
Table 21.
Dressing – rank mean and SD
| Dressing | Mean | SD |
|---|---|---|
| Hydrophobic dressing | 3·53 | 1·93 |
| Hydrocolloids | 3·90 | 1·97 |
| Honey | 4·10 | 1·92 |
| Povidone iodine | 4·71 | 1·90 |
| SSD topical cream | 5·52 | 1·99 |
| Ibruprofen impregnated dressings | 5·57 | 2·13 |
| Cadexomer iodine | 5·62 | 1·66 |
| PHMB | 5·65 | 1·73 |
| Sheet hydrogel | 5·70 | 2·15 |
| Silver dressings | 5·76 | 1·67 |
| Silicone | 5·86 | 1·96 |
PHMB, polyhexamethylene biguanide; SD, standard deviation; SSD, silver sulfadiazine.
The panellists were asked to rank those dressings previously identified which they considered to be relevant to managing wound pain associated with the onset of wound infection.
Although the overlapping SDs may suggest that there is not a substantial difference in rankings of the dressings as shown in Figure 1, Cronbach's α was calculated as 0·77 indicating a strong level of agreement between the panel members for each dressing type.
Figure 1.
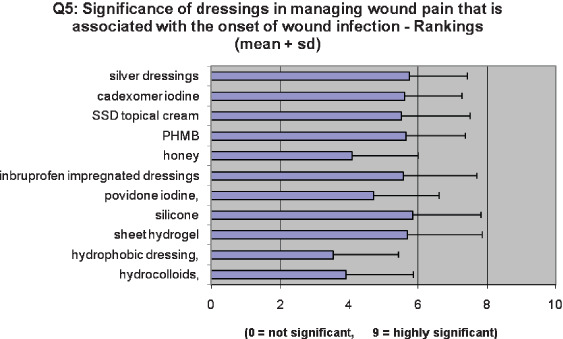
Significance of dressings in managing wound pain that is associated with the onset of wound infection – rankings (mean + standard deviation).
The panel members were asked to identify and then rank any important dressing‐related issues that would influence dressing selection (Figure 2). It is interesting to note that the ‘value’ to patients features heavily and echoes the sentiments as portrayed in Table 19.
Figure 2.
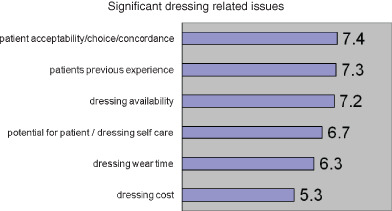
Significant dressing related issues.
DISCUSSION
In this study, we have gathered objective evidence that a causal relationship exists between wound infection and the onset of, or a change in, the nature of pain from 21 international wound experts. Likewise, expert opinion that patients with a wound infection generally experience more pain than those patients with non infected wounds. To the best of our knowledge this is the first objective evidence to that effect. This merits incorporation into care plans.
In light of the evidence concerning an increase in existing pain and review of dressing it is clear that it is no longer acceptable to neglect regular patient/wound evaluation, and, is a requirement to reconsider dressing selection. The panel is emphatic that topical antimicrobials have a positive impact on treating wound pain. This is widely regarded as being attributable to reduction of bioburden. A systematic review of the evidence for antimicrobial agents has been judged to be lacking at an evidential level (57). However, their objective was to establish the effects of silver containing wound dressings in ‘preventing wound infection and healing of wounds' neither of which is recommended purpose of these products.
CONCLUSIONS
Wound dressings are generally regarded as either ‘traditional’ or ‘advanced’ in design and function with traditional dressings been relatively unsophisticated, often gauze‐based and inexpensive. Advanced dressings are designed on the principle of moist wound healing, are manufactured from a range of materials both natural and synthetic and relatively speaking more expensive. Advocates of the traditional dressings approach claim that use of advanced wound dressings in terms of cost and time to healing outcomes cannot be justified. This stance does not take into account the patient experience in relation to quality of life.
This study was designed to compare event‐related pain with somatic pain and, without prejudgement, we were intrigued to see what the experts would conclude. For optimal clinical management of wound‐related pain it is important that the practitioner be aware of both event‐related and of somatic pain as this will facilitate best practice in the management of the patient.
As a result of this research, clinicians are therefore duty bound to differentiate the cause of wound‐related pain, both event‐related and somatic, and then to adjust intervention accordingly.
REFERENCES
- 1. White RJ. Wound infection‐associated pain. J Wound Care 2009;18:245–9. [DOI] [PubMed] [Google Scholar]
- 2. Price PE, Fagervik‐Morton H, Mudge EJ, Beele H, Ruiz JC, Nystrom TH, Lindholm C, Maume S, Melby‐Østergaard B, Peter Y, Romanelli M, Seppänen S, Serena TE, Sibbald G, Soriano JV, White W, Wollina U, Woo KY, Wyndham‐White C, Harding KG. Dressing‐related pain in patients with chronic wounds: an international patient perspective. Int Wound J 2008;5:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woo KY, Harding K, Price P, Sibbald G. Minimising wound‐related pain at dressing change: evidence‐informed practice. Int Wound J 2008;5:144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morris C, Emsley P, Marland E, Meuleneire F, White R. Use of wound dressings with soft silicone adhesive technology. Paediatr Nurs 2009;21:38–43. [DOI] [PubMed] [Google Scholar]
- 5. Solowiej K, Upton D. The assessment and management of pain and stress in wound care. Br J Community Nurs 2010;15:26–33. [Google Scholar]
- 6. Solowiej K, Upton D. Managing stress and pain to prevent patient discomfort, distress and delayed wound healing. Nurs Times 2010;106:21–3. [PubMed] [Google Scholar]
- 7. Solowiej K, Mason V, Upton D. Review of the relationship between stress and wound healing: part 1. J Wound Care 2009;18:357–66. [DOI] [PubMed] [Google Scholar]
- 8. George G. Wound management. Richmond: PJB Publications, 1996. [Google Scholar]
- 9. Arber A. Is pain what the patient says it is? Interpreting an account of pain. Int J Palliat Nurs 2004;10:491–6. [DOI] [PubMed] [Google Scholar]
- 10. European Wound Management Association E. Position document: pain at wound dressing changes. London: MEP Ltd, 2002. [Google Scholar]
- 11. Goncalves ML, de Gouveia Santos VL, de Mattos Pimenta CA, Suzuki E, Komegae KM. Pain in chronic leg ulcers. J Wound Ostomy Continence Nurs 2004;31:275–83. [DOI] [PubMed] [Google Scholar]
- 12. Rastinehad D. Pressure ulcer pain. J Wound Ostomy Continence Nurs 2006;33:252–7. [DOI] [PubMed] [Google Scholar]
- 13. Palfreyman S. Assessing the impact of venous ulceration on quality of life. Nurs Times 2008;104:34–7. [PubMed] [Google Scholar]
- 14. Weiss CA, Statz CL, Dahms RA, Remucal MJ, Dunn DL, Beilman GJ. Six years of surgical wound infection surveillance at a tertiary care centre. Arch Surg 1999;134:1041–8. [DOI] [PubMed] [Google Scholar]
- 15. Reilly J, Allardice G, Bruce J, Hill R, McCoubrey J. Procedure‐specific surgical site infection rates and postdischarge surveillance in Scotland. Infect Control Hosp Epidemiol 2006;27:1318–23. [DOI] [PubMed] [Google Scholar]
- 16. Bjarnsholt T, Kirketerp‐Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N, Givskov M. Why chronic wounds won't heal: a novel hypothesis. Wound Repair Regen 2008;16:1–10. [DOI] [PubMed] [Google Scholar]
- 17. Cooper R. Understanding wound infection. In: Cutting K, Gilchrist B, Gottrup F, editors. Identifying criteria for wound infection EWMA position document. London: MEP Ltd, 2005:2–5. [Google Scholar]
- 18. Cutting KF, White RJ, Mahoney P. Clinical identification of wound infection: a Delphi approach. In: Cutting K, Gilchrist B, Gottrup F, Leaper D, Vowden P, editors. Identifying criteria for wound infection EWMA position document. London: MEP, 2005:6–9. [Google Scholar]
- 19. Soon K, Acton C. Pain‐induced stress: a barrier to wound healing. Wounds UK 2006;2:92–101. [Google Scholar]
- 20. Glaser R, Kiecolt‐Glaser JK, Marucha PT. Stress‐related changes in pro inflammatory cytokine production in wounds. Arch Gen Psychiatry 1999;56:450–6. [DOI] [PubMed] [Google Scholar]
- 21. Kiecolt‐Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry 2005;62:1377–84. [DOI] [PubMed] [Google Scholar]
- 22. Graham G, Regehr G, Wright JG. Delphi as a method to establish consensus for diagnostic criteria. J Clin epidemiol 2003;56:1150–6. [DOI] [PubMed] [Google Scholar]
- 23. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gasquet I, Villeminot S, Estaquio C, Durieux P, Ravaud P, Falissard B. Construction of a questionnaire measuring outpatients' opinion of quality of hospital consultation departments. Health Qual Life Outcomes 2004;4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clay CS, Chen WYJ. Wound pain: the need for a more understanding approach. J Wound Care 2005;14:181–4. [DOI] [PubMed] [Google Scholar]
- 26. Cutting KF, Harding KG. Criteria for identifying wound infection. J Wound Care 1994;3:198–201. [DOI] [PubMed] [Google Scholar]
- 27. Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen 2001;9:178–86. [DOI] [PubMed] [Google Scholar]
- 28. World Union of Wound Healing Societies. Principles of best practice: minimising pain at wound dressing‐related procedures. A consensus document. London: MEP, 2004. [Google Scholar]
- 29. WUWHS. Principles of best practice: minimising pain at wound dressing‐related procedures. A consensus document. London: MEP, 2008. [Google Scholar]
- 30. State Advisory Council on Pain Management ptTOSS‐Aa‐B. 42 Code of Federal Regulations Parts 483, 2005.
- 31. King B. A review of research investigating pain and wound care. J Wound Care 2003;12:219–23. [DOI] [PubMed] [Google Scholar]
- 32. Reddy M, Keast D, Fowler E, Sibbald RG. Pain in pressure ulcers. Ostomy Wound Manage 2003;49:30–5. [PubMed] [Google Scholar]
- 33. Bell C, McCarthy G. The assessment and treatment of wound pain at dressing change. Br J Nurs 2010;19:S4–10. [DOI] [PubMed] [Google Scholar]
- 34. Hollingworth H. Pain at wound dressing‐related procedures: a template for assessment. URL http://www.worldwidewounds.com/2005/august/Hollinworth/Framework‐Assessing‐Pain‐Wound‐Dressing‐Related.html [accessed on 31 January 2010].
- 35. Societies WUoWH. Principles of best practice: minimising pain at wound dressing‐related procedures. A consensus document. London: MEP, 2004. [Google Scholar]
- 36. Hollingworth H, Collier M. Nurses' views about pain and trauma at dressing changes: results of a national survey. J Wound Care 2000;9:369–73. [DOI] [PubMed] [Google Scholar]
- 37. O’Meara SM, Al‐Kurdi D, Ovington LG. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2008:CD003557. [DOI] [PubMed] [Google Scholar]
- 38. Hinchliffe RJ, Valk GD, Apelqvist J. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 2008;24 Suppl 1:S119–44. [DOI] [PubMed] [Google Scholar]
- 39. Bergin SM, Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database Syst Rev 2006;CD005082. [DOI] [PubMed] [Google Scholar]
- 40. Vermeulen H, van Hatton JM, Storm‐Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev 2007; CD005486. [DOI] [PubMed] [Google Scholar]
- 41. Jull AB, Rodgers A, Walker N. Honey as a tpical treatment for wounds. Cochrane Database Syst Rev 2008;CD005083. [DOI] [PubMed] [Google Scholar]
- 42. Lineaweaver W, Howard R, Soucy D, McMorris S, Freeman J, Crain C, Robertson J, Rumley T. Topical antimicrobial toxicity. Arch Surg 1985;120:267–70. [DOI] [PubMed] [Google Scholar]
- 43. Brennan SS, Leaper DJ. The effect of antiseptics on the healing wound: a study using the rabbit ear chamber. Br J Surg 1985;72:780–2. [DOI] [PubMed] [Google Scholar]
- 44. Faddis D, Daniel D, Boyer J. Tissue toxicity of antiseptic solutions. A study of rabbit articular and periarticular tissues. J Trauma 1977;17:895–7. [PubMed] [Google Scholar]
- 45. Cutting KF. A dedicated follower of fashion? Topical medications and wounds. Br J Nurs 2001;10:9–16. 12170491 [Google Scholar]
- 46. Kaspar D, Schwarz W, Claes L, Ignatius A. Study of the toxicity of povidone‐lodine for fibroblast‐like cells (BALB‐3T3) and primary human chondrocytes. Arzneimittelforschung 2006;56:605–11. [DOI] [PubMed] [Google Scholar]
- 47. Cooper RA. Iodine revisited. Int Wound J 2007;4: 124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. White RJ, Cutting K, Kingsley A. Topical antimicrobials in the control of wound bioburden. Ostomy Wound Management 2006;52:26–58. [PubMed] [Google Scholar]
- 49. Pediani R. What has pain relief to do with acute surgical wound healing? World Wide Wounds. URL www.worldwidewounds.com/2001/march/Pediani/backup.html [accessed on 3 January 2001]. [Google Scholar]
- 50. Sibbald RG, Coutts P, Fierheller M, Woo K. A pilot (real‐life) randomised clinical evaluation of a pain‐relieving foam dressing: (ibuprofen‐foam versus local best practice). Int Wound J 2007;4 Suppl 1:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cigna E, Tarallo M, Bistoni G, Anniboletti T, Trignano E, Tortorelli G, Scuderi N. Evaluation of polyurethane dressing with ibuprofen in the management of split‐thickness skin graft donor sites. In Vivo 2009;23:983–6. [PubMed] [Google Scholar]
- 52. Jorgensen B, Friis GJ, Gottrup F. Pain and quality of life for patients with venous leg ulcers: proof of concept of the efficacy of Biatain‐Ibu, a new pain reducing wound dressing. Wound Repair Regen 2006;14:233–9. [DOI] [PubMed] [Google Scholar]
- 53. Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, Amery CM, Edmonds ME, Gibby OM, Johnson AB, Jones GR, Masson E, Patmore JE, Price D, Rayman G, Harding KG. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technol Assess 2009;13:1–10. [DOI] [PubMed] [Google Scholar]
- 54. Jones AM, San Miguel L. Are modern wound dressings a clinical and cost‐effective alternative to the use of gauze? J Wound Care 2006;15:65–9. [DOI] [PubMed] [Google Scholar]
- 55. Ubbink DT, Vermeulen H, Goossens A, Kelner RB, Schreuder SM, Lubbers MJ. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg 2008;143:950–5. [DOI] [PubMed] [Google Scholar]
- 56. Nunnally JC, Bernstein IH. Psychometric theory, 3rd edn. New York: McGraw‐Hill, 1994. [Google Scholar]
- 57. Storm‐Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database Syst Rev 2010:CD006478. [DOI] [PubMed] [Google Scholar]


