Abstract
Ionic silver has a long history as an antimicrobial in human health care. This article is a review of the published literature on how ionic silver may enter the body from exposure to silver‐containing wound care products and its eventual metabolic fates, in an assessment of the safety during normal use of these products in wound care. Following the application to breached skin, there appears to be little evidence of localised or systemic toxicity, and this is borne out by the continuous use of silver sulfadiazine formulations for more than 50 years. Consequently, following normal use, the risk of silver ion toxicity locally and systemically is considered to be low or negligible.
Keywords: Absorption, Detoxification, Elimination, Silver, Wound dressing
Introduction
Silver has a long history as an antimicrobial in human health care. It has been developed for use in water purification, wound care, bone prostheses, reconstructive orthopaedic surgery, cardiac devices, catheters and surgical appliances, and has been the subject of extensive reviews [e.g. reviews by Klasen 1, 2], so will not be discussed further here, except that these are mostly in the metallic form. Dilute silver nitrate solution has been used in wound and skin disinfection since the early part of the 19th century, and one of the often used silver formulations, silver sulfadiazine (SSD) used particularly for the treatment of burns, has been available for some 50 years, as reviewed by White 3. During the past 25 years, use of silver in medical health care devices has seen a varied increase and this has been largely driven by innovations in wound dressings, and particularly in the variety of forms that ionic silver is presented in the formulations.
The case of safe and effective use in this application relies heavily on historical data of industrial exposure – mainly by inhalation, ingestion and absorption through intact skin. For exposure through open skin (i.e. skin wounds), a substantial body of scientific research and clinical experience with SSD formulations for the treatment of burns provides a good overview of its general safety but also points out its limitations on use. SSD formulations provide low levels of silver ions (Ag+) that can be absorbed through skin and open wounds where they are rapidly bound by proteins. The distribution and metabolic routes of absorbed silver ions are quite well understood and are described in more detail in the latter part of this article. Many modern silver‐containing dressings or wound treatment formulations are also designed to provide low levels of silver ions, by either presenting the silver as silver salts, silver coatings or as metallic silver, which give rise to silver ions in an aqueous environment. This article is a review of the published literature on how ionic silver may enter the body from exposure to silver‐containing wound care products and its eventual metabolic fates. The safety implications of the use of modern silver‐containing wound care products are also discussed.
Type of silver‐containing medical materials
The first use of silver directly as an antiseptic is perhaps silver nitrate, typically as a 1% (w/v) solution. This use dates back to the first part of the 19th century and continues to be used, albeit minimally, until now as it can cause transient irritation and staining 3, 4.
SSD was introduced over 40 years ago 5, and is typically presented as a 1% (w/w) cream formulation. It is the silver salt of sulfadiazine, a short‐acting sulphonamide bacteriostatic agent that slowly dissociates and dissolves to produce silver ions.
Since the 1990s, there has been a vast increase in innovations in wound care products containing silver, partly owing to concerns over increasing bacterial resistance to antibiotics, but also owing to the safety and efficacy profile of silver. Modern silver‐containing wound care compositions can be broadly classified into two distinct types by the chemical state in which the silver is presented – metallic silver or silver salts.
Metallic silver is generally accepted to be biologically inert 6 and immobile, and therefore not absorbed into the body through ingestion, inhalation or skin absorption. However, a number of modern wound care products contain nanoparticulate (i.e. metallic) silver. Because of their small size, it is possible at least theoretically, that some nanoparticulate silver may be directly absorbed into the body. Little is known about how or even if nanoparticulate matter can translocate through intact or breached skin 7. This is an area in which further research should be considered and therefore will not be conducted as part of this review as the focus will be specifically on the antiseptic function of silver ions and their subsequent distribution in a relatively aqueous environment 6.
While silver salts in aqueous solutions (e.g. silver nitrate, silver acetate or silver sulphate) are used as antiseptics, it is usually more convenient to present these silver salts as part of dressing formulations. Dressings in which the silver is present as a silver salt are varied but can be subdivided as dressings that have incorporated a silver salt and dressings which are themselves silver salts. The method of incorporation and the type and form of the counter ion can, to a large extent, govern the availability of silver ions for antiseptic purposes.
Examples of silver‐containing wound dressings and formulations, and their compositions are shown in Table 1.
Table 1.
Examples of the main types of silver‐containing wound care formulations
| Silver form | Formulation type | Example |
|---|---|---|
| Finely divided in carbonised cloth | Actisorb® | |
| Coated onto nylon fibre | Silverlon®and SILVERCEL®products | |
| Metallic silver | Coated onto polyester fibre | Atrauman®Ag |
| Nanoparticulate coated foam, film or fibre | Acticoat™and PolyMem®Silver products | |
| Silver salt solution | Silver nitrate (0·5–1% solutions) | No commercial preparations |
| 1% SSD in a cream | Flamazine™ and Silvadene® Tegaderm™Ag (on gauze mesh) | |
| SSD in Vaseline gauze | Urgotul®products | |
| SSD in foam | Allevyn™Ag products | |
| Silver salts incorporated into dressings or creams | Silver sulphate particulate in foam | Mepilex® Ag products |
| Calcium silver phosphate in adhesive on film | Arglaes® | |
| Silver chloride in hydrogel | Silvasorb™products | |
| Silver complex with sodium hydrogen zirconium phosphate in alginate fabric | Maxsorb® Extra Ag, Seasorb® Ag, Algisite™ and Sorbsan® Silver products | |
| Silver salt dressings | Silver sodium carboxymethylcellulose fibre | AQUACEL®Ag |
| Silver sodium collagen/CMC | Promogran Prisma® |
CMC, carboxymethylcellulose; SSD, silver sulfadiazine.
Dissolution of silver salts to free silver ions
For the silver to be bioabsorbable and to have biocidal properties, it has to be in the form of free silver ions in solution. Iconic silver is free to interact with various body constituents (e.g. proteins, cell membranes) and microbial cell walls, and can be transported in body fluids bound to proteins or peptides.
A number of different silver salts have been used in wound treatment formulations. They have different solubility characteristics, which mean that they differ in the capability of supplying free silver ions to the wound environment.
Silver nitrate has a high degree of aqueous solubility. In dilute aqueous solution, all the silver and nitrate are in the form of free silver cation (Ag+ the positively charged ion) and nitrate anion (NO3 − the negatively charged ion). Application of silver nitrate solution can deliver huge amounts of silver ions to the patient, with multiple undesirable effects including skin irritancy and argyria, as reviewed by Lansdown 4, so even though the undesirable effects are generally transient, its use for wound antiseptic purposes is rapidly being replaced by other antiseptic formulations.
Silver chloride and silver sulphate have been used in dressing formulations as both are sparingly soluble in aqueous solution, so can provide small amounts of silver ions at any one point in time unlike the uncontrolled availability that silver nitrate provides. In water, while most remains as a solid, small amounts dissolve and exist as free silver cations and chloride/sulphate anions, respectively, in equilibrium with the solid form. A complex of silver calcium phosphate, which is also sparingly soluble in water, is similarly used in some dressing formulations.
SSD 5 is a salt of silver and sulfadiazine. Like silver chloride, it is a sparingly soluble salt, so in an aqueous environment it will give rise to a small amount of free silver ions in equilibrium with the solid form.
The amount of silver ions generated by these sparingly soluble silver salts is dependent on how soluble these salts are and the composition of the liquid they are dissolving into. In pure water at ambient temperature, the solubility of several common silver salts in increasing degrees of aqueous solubility are shown in Table 2.
Table 2.
Solubility constants of common silver salts in increasing degrees of solubility
| Salt | Aqueous solubility product (Ksp)* | References |
|---|---|---|
| Silver sulphide | 10−50 | 54 |
| Silver phosphate | 8·88 × 10−17 | 54 |
| Silver sulfadiazine | 8·12 × 10−12 | 10 |
| Silver chloride | 1·56 × 10−10 | 10 |
| Silver sulphate | 1·2 × 10−5 | 54 |
| Silver nitrate | 51·6 (very soluble) | 55 |
Ksp quoted are approximate values.
Some silver‐containing dressings are formed from silver ions bound to macromolecular polyanions, that is, polymers with multiple negatively charged groups, for example, carboxymethylcellulose. Silver ions can similarly be complexed with collagen or gelatine (see Table 1). Chemically these compounds are also described as salts, because the silver ions are bound to the polyanions by ionic binding. In an aqueous environment, there is equilibrium between free silver ions in solution and silver ions bound to the polymer. The attraction between silver ions and the polymer is very strong, so the amount of free silver ions is kept low at any one point in time. If the free silver ions are removed from the aqueous environment, for example, by binding to proteins or precipitated as silver chloride in the presence of free chloride ions, the equilibrium is reestablished by further dissolution of free silver ions from the dressings to replenish the free silver ion pool (see Figure 1). Thus, these dressings can maintain a low, continual level of free Ag+ in the solution phase.
Figure 1.
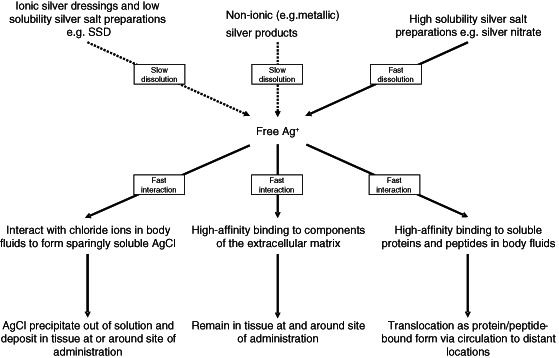
The possible multiple interactions of free silver ions (Ag+) at the site of application. The supply of free Ag+ is from the various silver‐containing products. All common formulations except silver nitrate release Ag+ at low rates of dissolution. Free Ag+ has strong binding reactions with chloride ions in biological fluids [forming silver chloride (AgCl)], and with soluble proteins and peptides in biological fluids and extracellular matrix proteins. These result in the rapid sequestration of silver ions released from dressing formulations into bound forms. Slow release and multiple strong binding reactions all contribute to limit the free Ag+ concentration to very low levels, except when silver nitrate is used – it is highly soluble and can load the site of administration with high levels of Ag+ within a short period of time.
Factors controlling free silver ion concentrations in the wound environment
While modern silver‐containing dressings are already designed to provide slow availability of free silver ions, there are multiple constituents in the wound environment that can readily interact and bind the free silver ions, which equally help maintain a low concentration of free silver ions in the environment. The multiple interactions of free silver ions from silver‐containing wound care products at the site of application are shown in Figure 1.
One of the main constituents of the wound environment is the chloride anion, which is present in substantial concentration (approximately 103 mM or 0.37%w/v) as one of the main ionic constituents of body fluids. As silver chloride is sparingly soluble in water, the excess amount of chloride ions alone will effectively control the maximum amount of free silver ions to the aqueous solubility limit of silver chloride – in the µg/ml region. Experimentally, the concentration of free silver ions in normal saline is determined to be in the region of 0·5 to 1 µg/ml 7.
Proteins also play an important role in controlling the level of free silver ions at the interface of the dressing and the wound surface. Silver ions bind strongly to proteins, peptides and amino acids, and cell surface structures of both host and microbial cells. These are abundant in the wound environment. Silver ions bind to proteins via several functional groups – carboxylic acid groups, imidazole, sulfhydryls and amines, with varying degrees of affinity, the strongest of which is with sulfhydryls groups 8, 9. The structures and amino acid composition of proteins are extremely variable; therefore, the affinity of silver ions to different classes of proteins is expected to be extremely variable. However, the binding – particularly to proteins with abundance of sulfhydryls groups – is likely to be very strong. In a physiological environment, the presence of proteins (e.g. albumin) could increase silver release from silver‐containing dressings. This has been demonstrated experimentally by Tsipouras et al. 10 who showed that human serum substantially increases the amount of silver dissolution from SSD cream, by approximately 300‐fold and this additionally released silver is protein‐bound (i.e. not in the form of free silver ions). Canada et al. 11 also showed that the presence of 5% bovine serum albumin substantially increases the availability of silver from silver dressings. Substantial silver binding to membranes composed of collagen can also be demonstrated in an experimental system that mimics silver binding to proteins of the extracellular matrix (ECM) 12.
The constituents of a wound environment are likely to be the key players in controlling the free silver ion concentration, and likely to be sufficient in controlling the free silver ion levels from all current types of silver‐containing formulations for wound care, except silver nitrate that delivers a large amount of silver ions within a short period of time. The latter is, however, rarely used now, so is unlikely to present as a medical problem.
Routes of silver absorption
There is substantial evidence for systemic silver absorption through the use of topical silver products, particularly SSD. Experimental and clinical studies have shown that while most of the topically applied silver remains at or around the application site (e.g. the skin or wound surface), silver can also be detected in the circulatory system and deposited in internal organs 13.
Skin barrier function and silver absorption to the local skin environment
As previously described, silver ions have very high affinity for many body substances, particularly proteins. This affinity essentially constitutes the main barrier to systemic silver absorption, and explains why most of the silver ions released from silver‐containing wound care products are bound to the ECM at or around the site of administration 13. Figure 2 summarises the possible multiple interactions of silver ions released from silver‐containing wound care products at or around the site of application.
Figure 2.
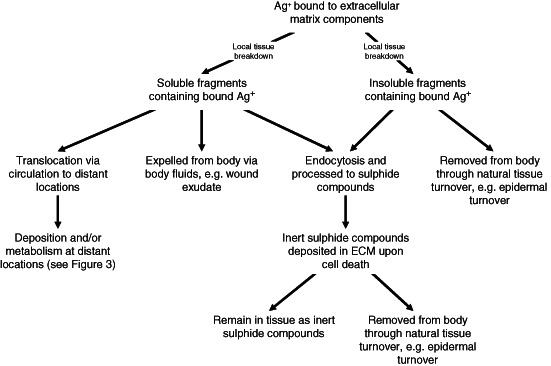
Possible fates of silver ions (Ag+) bound to extracellular matrix (ECM) at or around the site of administration.
On contact with the skin, silver ions will first encounter the metabolically ‘dead’stratum corneum and be exposed to its constituents that include substantial amounts of ECM proteins particularly rich in sulfhydryl (–SH) groups, therefore will readily bind silver ions 14. In the lower, living layers of the epidermis, silver ions will also interact with a variety of substances with high affinity for metallic cations. In addition to ECM proteins as described above, these include also binding to reduced glutathione. The binding of silver to the latter initiates the glutathione efflux system leading ultimately to its subsequent metabolism and detoxification. These will be described in more detail below. Evidence for this comes from in vitro studies on organotypic skin 15. Silver ions penetrating as far as the dermis will encounter the connective tissues rich in fibrous proteins collagen and elastin, which also contain many silver‐binding sulfhydryl groups 14.
While skin wounds do not have the outer (epidermal) layer, wound tissue shares many structural and biochemical similarities with the dermal tissue, so will have strong affinity for any silver ions that are present. The abundance of ECM and soluble proteins will similarly sequester any free silver ions rapidly contributing to keeping the concentration of free silver ions low.
The high affinity of silver ions with proteins of the skin layers would explain why most of the topically applied silver is observed to be bound to the skin layers or around the vicinity of the wound 13. This is consistent with in vivo experimental studies using radiolabelled silver (Ag110) in SSD, which showed that accumulation occurred in superficial layers of the skin and in a short time period after exposure (2–8 hours); clearance was complete within 28 days 16. The conclusion from this is that silver binds superficially and has low absorption from a single application.
Substantial skin accumulation of silver is likely to result in skin discolouration and has been demonstrated in both in vitro testing 7, and in the clinical use of silver‐containing wound care products 17, 18.
Systemic absorption of silver and potential increases in systemic silver levels
Silver ions, if applied topically over a prolonged period or at high dosage such as in treatment of burns, can enter the systemic circulation. Although the exact route of absorption is not clear, considering that there is strong binding of silver ions to proteins it is likely to be absorbed as a complex with mobile proteins, for example, proteins of body fluids, which are then carried through the skin drainage systems that include the lymphatic system. On reaching the blood circulation, it can be further distributed to other organs, or become metabolised in the liver and kidney. Figure 3 summarises the possible routes for silver transportation, metabolism, detoxification and elimination following systemic absorption.
Figure 3.
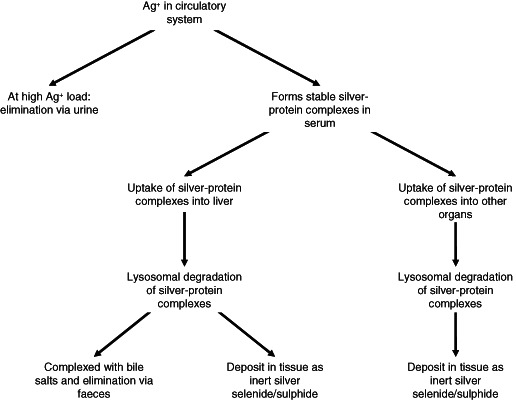
Possible routes of silver transportation, metabolism, detoxification and elimination following systemic absorption.
When applied over large body surface areas such as an extensive burn systemic absorption can be quite rapid. Wan et al. 13 reported that in patients receiving SSD cream for treatment of burns, elevated silver levels were detectable in blood after 6 hours and in urine within 24 hours.
In general, it is clear that prolonged use of silver‐containing dressings may result in local and systemic silver uptake, which in turn can lead to transiently raised levels in blood and urine, and deposition of silver both at the site of application and in body organs. However, any risks under the normal application of silver‐containing wound dressings are still generally regarded as low 4, 19, 20. Consequently, the low incidences of elevated systemic levels of silver ions can be explained by the multiple pathways that exist in the body for eliminating and/or detoxification of the absorbed silver ions.
Silver clearance from the body
Normal silver concentration in the tissues is considered to be very low, with measurements reported by Wan et al. 13, demonstrating concentrations of silver in blood, urine, liver and kidney of subjects without industrial or medicinal exposure to be <2·3 µg/l, 2 µg/day and 0·05 µg/g per organ of wet tissue, respectively.
Clinical and scientific studies have shown that some of the absorbed silver made available from silver‐containing wound care products enters the blood circulation quite rapidly and can be detected in the urine within 24 hours 13.
While silver can be detected in urine following use of silver‐containing wound care products, the predominant elimination route for systemically absorbed silver is considered to be via faeces. This was shown by radioisotope studies that followed the fate of silver radioisotope (Ag110) experimentally administered or through accidental inhalation exposure 21, 22, 23. Studies on workers exposed to silver in industrial processes also came to similar conclusions of the faecal route being the predominant elimination route 24. Following accidental inhalation of Ag110, most of the silver disappeared very rapidly with a half‐life of about one day, except about 15% which had a biological half‐life of 52 days similar to the biological half‐life of silver in the liver (50 days) 21.
Although following exposure to silver‐containing wound care products, silver can be detected in urine 13, 25, 26, and it would appear that this only happens after high levels of exposure following prolonged use. Coombs et al. 27 reported that the level of silver in urine was shown to be low if the serum level is less than 100 µg/l but increases when above this level. In a study on patients with second‐degree or third‐degree burns (∼60% total body surface area), Boosalis et al. 28 found the mean urinary silver excretion was found to be ∼1100 µg/l, compared with control levels of <1 µg/l. Silver secretion in urine does not appear to correlate with proteinurea 27. However, the high affinity of silver ions for proteins and peptides suggests that the silver secreted via urine is likely to be bound to small proteins and/or peptides. The exact nature of the silver secreted via urine is at present unclear, so it is not possible to decipher the metabolic routes that lead to urinary elimination of silver.
Chemical nature of absorbed silver
At the point of initial absorption, the silver is most likely to be in the form of silver ions. Consequently, owing to its high affinity for a variety of anions present in the wound environment, including chloride ions and proteins, the free silver ions are likely to become rapidly bound. Those bound to mobile proteins (e.g. body fluid proteins) have potential for being transported to other parts of the body via the circulation system. Silver binding to immobile proteins (e.g. to connective tissue proteins in the skin and organs) is likely to remain at the site of binding. Over long periods of time, silver deposited in skin or other organs could result in the formation of highly insoluble salts such as silver sulphide or silver selenide, which have the dark grey colour that is characteristic of argyria. Silver is transformed into these highly insoluble silver salts through the body's detoxification systems, which are discussed below. It is interesting to note that silver deposits in skin appear to be largely in the form of silver sulphide 29, 30, whereas those in kidney and liver are largely in the form of silver selenide 31, 32. These findings may represent different routes of metabolism in different organs.
Silver deposition in skin and routes of elimination
As reported by Wan et al. 13, a large proportion of topically applied silver is sequestered in the various layers of skin. The epidermal layer of skin is constantly turning over so those bound to this layer, as protein‐bound or as particulate matter, for example silver sulphide, will eventually be eliminated as a consequence of this turnover, and indeed superficial skin staining is known to gradually disappear over periods of weeks to months. There is, however, substantially less turnover in the dermal and subdermal skin layers and so deep argyria is generally considered to be long‐lasting.
The work reported by Reymond et al. 29 suggested that in subjects with recent silver exposure, the skin‐associated silver is mainly intracellular and in lysosomes. This would suggest that at this point of time the silver is protein‐bound and being metabolised intracellularly. However, in subjects where silver exposure has taken place over many years, the main location of silver was found to be extracellular, on fibrillar components of connective tissue and in the basal material of sweat glands, and bound with sulphur 30. This would suggest that the protein–silver complexes cannot be completely digested by the lysosomal enzymes – the non‐digestible part is the highly insoluble silver sulphide. Initially stored intracellularly, the silver sulphide is eventually deposited in the extracellular environment after the death of the cell. Similar findings were reported by Kristiansen et al. 15 who used organotypic cultures of human breast skin exposed to silver‐containing wound care products and demonstrated that silver sulphide, because of its extreme insolubility was simply not bioavailable.
Silver in blood circulation
One of the routes by which silver ions are sequestered and ultimately transformed to the non‐reactive silver complexes such as silver sulphide and selenide is mediated through a quite well‐recognised metabolic pathway. Gluathione, a small – SH containing peptide substance, has multiple cellular functions particularly that of maintaining intracellular oxidative potential 33. Silver ions bind directly to reduced glutathione via the sulfhydryl groups 9, 10, 34. An extracellular pool of reduced glutathione is present in blood plasma 34, 35 as well as other tissues such as in lung lining fluid and small intestinal lumen 35 and this extracellular pool of glutathione is considered very important in protection against chemically induced injury not limited to silver itself. Reduced glutathione can be experimentally demonstrated to protect against silver ion induced cytotoxicity 36. Binding to reduced glutathione is considered to be one of the first stages of detoxifying absorbed silver ions. Once bound to glutathione, glutathione‐S‐transferases 37, 38 facilitate the first steps of silver translocation from glutathione to other sulphur‐containing proteins, forming silver–protein complexes, which are ultimately internalised and metabolised in lysosomes.
Selenoprotein P (SPP), a secreted glycoprotein produced by many organs and contains most of the selenium in blood 39, 40, may also have a key role in sequestering silver ions in the blood stream. The work of Sasakura and Suzuki 41 showed that transition metal ions in serum, including silver ions, form complexes with selenide or sulphide, which are then bound to SPP. Selenide is itself formed in blood plasma through metabolic reduction of selenium to hydrogen selenide 42. SPP has a high affinity for plasma membranes through its ability to bind heparan sulphate, which facilitates endocytosis 39, 40, and this may be one mechanism by which circulating silver ions are taken up for metabolism by the liver and kidney and may help to explain why silver deposits in these organs are predominantly silver selenide 31, 32. This protein also has receptor‐mediated binding to various body cells including brain and testicular tissue 39, 40. These cellular interactions may explain why absorbed silver can be distributed to other body organs, although this mechanism is not proven. A short form of SPP is small enough to be filtered through the kidney glomerulus 40, which may, although not proven, explain the presence of silver in urine after high‐level exposure.
The rate of silver elimination from blood circulation is slow. In a small clinical study, the half‐life for silver elimination from serum is estimated to be 46·4 days, with a median elimination rate of 1·5% per day 43. This is similar to the estimated rate of silver elimination from the liver through bile excretion 21, which would suggest that the silver in blood circulation, unless in vast excess, does not get eliminated by filtration in the kidney, but has to be taken up by the liver, processed and eliminated through bile excretion in faeces. The hepatic processing of silver‐containing compounds is described the subsequent section.
Elimination of silver deposited in other organs
The dissociation of SSD following application to burn patients has led to silver depositions being reported in gingiva, cornea, liver and kidneys in addition to skin 13. The normal tissue turnover in these organs is very slow, so any silver that may be deposited there is likely to persist for long periods. Although there is currently no clinical or experimental evidence to verify this, a useful indicator of the possible persistence duration can be gained from the study by Newton and Holmes 21 that showed the half‐life of Ag110 in liver following accidental exposure to be in the region of 50 days. As tissue turnover in different organs and even different parts of organs are extremely variable, it is expected that the half‐life of silver accumulated in different organs is likely to be extremely variable. For example, in skin cells, the silver is likely to be initially retained in the lysosomes as sulphide complexes, but eventually deposited into the ECM when the cell eventually dies 29, 44.
Some of the absorbed silver could ultimately be taken up intracellularly, as already discussed through silver binding to glutathione or as protein complexes (e.g. SPP), such that the subsequent disposal of these ‘damaged’ proteins would be expected to be through intracellular degradation via the glutathione efflux system, described above. Dutczak and Ballatori 45 showed the elimination of glutathione‐bound mercury via this mechanism as well as mercury being eliminated from liver cells via bile excretion. As the glutathione efflux system is present in all mammalian cells, it is suspected that this is a common system for removal of metals, including silver, from all cells 45.
In the liver, the silver is thought to be removed via bile, and consequently via faeces; however, the liver may also possess another mechanism for metal detoxification. This mechanism is described through observations made in a study of marine animals exposed to environmental heavy metal pollutants including mercury and silver. Ikemoto et al. 32 reported that silver, along with mercury and selenium, was preferentially accumulated in nuclear, lysosomal and mitochondrial fractions of hepatocytes after uptake of the protein‐bound silver, possibly as selenoprotein complexes, from the circulation system. Studies of the subcellular localisations and metabolic intermediates, suggest that silver may become associated with metallothionein and complexes with high molecular weight proteins 46, 47, 48, 49, 50, 51. Whereupon it is then passed through several subcellular locations and eventually transferred to the lysosome where the protein component is degraded, leaving the mineralised silver selenide compounds as deposits. Studies of the distribution of metabolic intermediates in subcellular fractions in several species of marine animals suggest substantial metabolic similarities between these species, so it is reasonable to expect that a similar metabolic route would be present also in humans. Silver selenide complexes, such as silver sulphide, are also extremely stable and insoluble. While these are likely to remain as effectively permanent deposits, the processing of the silver into such stable complexes, where the silver is effectively compartmentalised and not bioavailable, can also be regarded as a form of detoxification, even though it is not physically eliminated from the body.
Elimination of silver via wound exudate
Wound exudate is rich in proteins, so it is highly likely that some of the silver bound to proteins will be eliminated from the body via wound exudate.
Conclusions
The published literature contains a wealth of information on silver and its salts as antimicrobials, for topical use. In consideration of silver absorption through the medium of silver ions, most usage of topical silver formulations is to damaged skin (i.e. breached dermis), and the safety profile shows very few toxicity problems both locally and/or systemically. Cutaneous toxicity following topical application, manifested as local argyria, is rare and where it has occurred, it has been through the prolonged topical application of silver nitrate or SSD, usually to large body surface areas in acute burns. Consequently systemic toxicity is considered to be very rare indeed.
Valid commentary on the safety of topically applied silver must refer to the widespread use of silver‐containing dressings on open wounds. Such dressings are routinely used on deep wounds, and for many weeks at a time. This is a rigorous examination of safety from which evidence presented in the literature over the past decade has shown to be generally good.
While it is accepted and can be shown that prolonged use of silver dressing in large or deep wounds will result in systemic absorption, with silver distribution to circulation and to organs distant from the sites of application, studies have also shown the existence of several mechanisms by which the body removes excess silver. These mechanisms include natural tissue turnover that occurs in most body tissues and particularly in the epidermis, and the host metal detoxification mechanisms involving metallothioneins and glutathione occurring in the liver and kidney, where the silver is excreted ultimately in faeces and urine.
Overall, the safety record of the modern silver‐containing wound dressings has been excellent 20, 52, 53. While some permanent retention of silver from exposure to silver‐containing dressings cannot be ruled out, there is good biological basis to suggest that the retained silver will ultimately be in the forms of extremely stable silver selenide and silver sulphide complexes that are effectively non‐bioavailable. The conversion of silver into these stable forms can be considered as forms of detoxification, even though the silver is not physically eliminated from the body.
Acknowledgements
The authors wish to thank WY John Chen PhD, for his excellent comments and review of the manuscript. This work is funded from internal sources of ConvaTec Ltd. All authors are employees of ConvaTec Ltd, who manufactures and markets silver‐containing wound care products. AQUACEL® is a registered trademark of ConvaTec Inc. All other trademarks are the property of their respective owners.
References
- 1. Klasen HJ. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000;26:117–30. [DOI] [PubMed] [Google Scholar]
- 2. Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000;26:131–38. [DOI] [PubMed] [Google Scholar]
- 3. White RJ. A historical overview of the use of silver in wound management. Br J Nurs 2002;(Silver Suppl):S3–8. [Google Scholar]
- 4. Lansdown ABG. Uptake and metabolism of silver in the human body. In Silver in healthcare: its antimicrobial efficacy and safety in use. Issues in Toxicology No 6. Cambridge: Royal Society of Chemistry, 2010:43–71. [Google Scholar]
- 5. Fox CL. Silver sulfadiazine – a new topical therapy for Pseudomonas in burns. Arch Surg 1968;96:184–8. [DOI] [PubMed] [Google Scholar]
- 6. Leaper DJ. Silver dressings: their role in wound management. Int Wound J 2006;3:282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker M, Cochrane CA, Bowler PG, Parsons D, Bradshaw P. Silver deposition and tissue staining associated with wound dressings containing silver. Ostomy Wound Manage 2006;52:42–50. [PubMed] [Google Scholar]
- 8. Gruen LC. Interaction of amino acids with silver (I) ions. Biochim Biophys Acta 1975;386:270–4. [DOI] [PubMed] [Google Scholar]
- 9. Zegzhda GD, Zegzhda TV, Shul’man VM. Compounds of silver with glutathione. Zh Neorg Khim 1969;14:134–9. [Google Scholar]
- 10. Tsipouras N, Rix CJ, Brady PH. Solubility of silver sulfadiazine in physiological media and relevance to treatment of thermal burns with silver sulfadiazine cream. Clin Chem 1995;41:87–91. [PubMed] [Google Scholar]
- 11. Canada TA, Wiencek KM, Cowan ME, Lindsay BJ. Challenging silver – influence of extraction medium on the release of silver from commercial silver dressings. Spartanburg, SC: Milliken & Company, 2007. [Google Scholar]
- 12. Tsipouras N, Rix CJ, Brady PH. Passage of silver ions through membrane‐mimetic materials, and its relevance to treatment of burn wounds with silver sulfadiazine cream. Clin Chem 1997;43:290–301. [PubMed] [Google Scholar]
- 13. Wan AT, Conyers RAJ, Coombs CJ, Masterton JP. Determination of silver in blood, urine, and tissues of volunteers and burn patients. Clin Chem 1991;37:1683–7. [PubMed] [Google Scholar]
- 14. Murayama M. On the nature of the interaction between binding sites for heavy metals (mercapto–mercapto interactions) in normal human hemoglobin. J Biol Chem 1959;234:3158–62. [PubMed] [Google Scholar]
- 15. Kristiansen S, Ifversen P, Danscher G. Ultrastructural localization and chemical binding of silver ions in human organotypic skin cultures. Histochem Cell Biol 2008;130:177–84. [DOI] [PubMed] [Google Scholar]
- 16. Harrison HN. Pharmacology of sulfadiazine silver. Its attachment to burned human and rat skin and studies of gastrointestinal absorption and extension. Arch Surg 1979;114:281–5. [DOI] [PubMed] [Google Scholar]
- 17. Wang X‐Q, Chang H‐E, Francis R, Olszowy H, Liu P‐Y, Kempf M, Cuttle L, Kravchuk O, Phillips GE, Kimble RM. Silver deposits in cutaneous burn scar tissue is a common phenomenon following application of a silver dressing. J Cutan Pathol 2009;36:788–92. [DOI] [PubMed] [Google Scholar]
- 18. Wang X‐Q, Kempf M, Mott J, Chang HE, Francis R, Liu PY, Cuttle L, Olszowy H, Kravchuk O, Mill J, Kimble RM. Silver absorption on burns after the application of Acticoat™: data from pediatric patient and a porcine burn model. J Burn Care Res 2009;30:341–8. [DOI] [PubMed] [Google Scholar]
- 19. Landsdown ABG, Williams A. How safe is silver in wound care? J Wound Care 2004;13:131–6. [DOI] [PubMed] [Google Scholar]
- 20. Lansdown AB, Williams A, Chandler S, Benfield S. Silver absorption and antibacterial efficacy of silver dressings. J Wound Care 2005;14:155–60. [DOI] [PubMed] [Google Scholar]
- 21. Newton D, Holmes A. A case of accidental inhalation of zinc‐65 and silver‐110m. Radiat Res 1966;29:403–12. [PubMed] [Google Scholar]
- 22. Furchner JE, Richmond CR, Drake GA. Comparative metabolism of radionuclides in mammals‐iv. Retention of silver‐110 in the mouse, rat, monkey, and dog. Health Phys 1968;15:505–14. [DOI] [PubMed] [Google Scholar]
- 23. Phalen RF, Morrow PE. Experimental inhalation of metallic silver. Health Phys 1973;24:509–18. [DOI] [PubMed] [Google Scholar]
- 24. DiVincenzo GD, Giordano CJ, Schriever LS. Biologic monitoring of workers exposed to silver. Int Arch Occup Environ Health 1985;56:207–15. [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Han CM, Yu CH. Change in silver metabolism after the application of nanometer silver on burn wound. Zhonghua Shao Shang Za Zhi 2004;20:161–3. [PubMed] [Google Scholar]
- 26. Trop M, Novak M, Rodl S, Hellbom B, Kroell W, Goessler W. Silver‐coated dressing Acticoat caused raised liver enzymes and argyria‐like symptoms in burn patient. J Trauma 2006;60:648–52. [DOI] [PubMed] [Google Scholar]
- 27. Coombs CJ, Wan AT, Masterton JP, Conyers RA, Pedersen J, Chia YT. Do burn patients have a silver lining? Burns 1992;18:179–84. [DOI] [PubMed] [Google Scholar]
- 28. Boosalis MG, McCall JT, Ahrenholz DH, Solem LD, McClain CJ. Serum and urinary silver levels in thermal injury patients. Surgery 1987;101:40–3. [PubMed] [Google Scholar]
- 29. Reymond JL, Stoebner P, Amblard P. Cutaneous argyria: an electron microscopic study of four eases with microanalysis X study of one case (author's transl). Ann Dermatol Venereol 1980;107:251–5. [PubMed] [Google Scholar]
- 30. Jonas L, Bloch C, Zimmermann R, Stadie V, Gross GE, Schad SG. Detection of silver sulfide deposite in the skin of patients with argyria after long‐term use of silver‐containing drugs. Ultrastruct Biol 2007;31:379–84. [DOI] [PubMed] [Google Scholar]
- 31. Aaseth J, Olsen J, Hovig T. Argyria‐tissue deposition of silver as selenide. Scand J Lab Invest 1981;41:247–51. [DOI] [PubMed] [Google Scholar]
- 32. Ikemoto T, Kunito T, Tanaka H, Baba N, Miyazaki N, Tanabe S. Detoxification mechanism of heavy metals in marine mammals and seabirds: interaction of selenium with mercury, silver, copper, zinc, and cadmium in liver. Arch Environ Contam Toxicol 2004;47:402–13. [DOI] [PubMed] [Google Scholar]
- 33. Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med 1999;27:916–21. [DOI] [PubMed] [Google Scholar]
- 34. Khan H, Khan MF, Asim ur R, Jan SU, Ullah N. The protective role of glutathione in silver induced toxicity in blood components. Pak J Pharm Sci 2011;24:123–8. [PubMed] [Google Scholar]
- 35. Jones DP, Brown LA, Sternberg P. Variability in glutathione‐dependent detoxication in vivo and its relevance to detoxication of chemical mixtures. Toxicology 1995;105:267–74. [DOI] [PubMed] [Google Scholar]
- 36. Baldi C, Minoia C, Di Nucci A, Capodaglio E, Manzo L. Effects of silver in isolated rat hepatocytes. Toxicol Lett 1988;41:261–68. [DOI] [PubMed] [Google Scholar]
- 37. Habig WH, Pabst MJ, Jakoby WB. Glutathione‐S‐transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–9. [PubMed] [Google Scholar]
- 38. Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: Implications for classification of non‐mammalian members of an ancient enzyme superfamily. Biochem J 2001;360:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burk RF, Hill KE, Motley AK. Selenoprotein metabolism and function: evidence for more than one function for selenoprotein P. J Nutr 2003;133:1517S–20S. [DOI] [PubMed] [Google Scholar]
- 40. Burk RF, Hill KE. Selenoprotein P – expression, functions, and roles in mammals. Biochim Biophys Acta 2009;1790:1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sasakura C, Suzuki KT. Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J Inorg Biochem 1998;71:159–62. [DOI] [PubMed] [Google Scholar]
- 42. Nuttall KL, Allen FS. Selenium detoxification of heavy metals: a possible mechanism for the blood plasma. Inorganica Chimica Acta 1984;92:187–9. [Google Scholar]
- 43. Moiemen NS, Shale E, Drysdale KJ, Smith G, Wilson YT, Papini R. Acticoat dressings and major burns: systemic silver absorption. Burns 2011;37:27–35. [DOI] [PubMed] [Google Scholar]
- 44. Matsumura T, Kumakiri M, Ohkawara A, Himeno H, Numata T, Adachi R. Detection of selenium in generalized and localized argyria: report of four cases with x‐ray microanalysis. J Dermatol 1992;19:87–93. [DOI] [PubMed] [Google Scholar]
- 45. Dutczak WJ, Ballatori N. Transport of glutathione‐methylmercury complex across liver canalicular membranes on reduced glutathione carriers. J Biol Chem 1994;269:9746–51. [PubMed] [Google Scholar]
- 46. Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Ann Rev Pharmacol Toxicol 1999;39:267–94. [DOI] [PubMed] [Google Scholar]
- 47. Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. J Nutr 2000;130:1085–8. [DOI] [PubMed] [Google Scholar]
- 48. Hahn SH, Yoo OJ, Gahl WA. Effect of metal ions on the stability of metallothionein in the degradation by cellular fractions in vitro. Exp Mol Med 2001;33:32–6. [DOI] [PubMed] [Google Scholar]
- 49. Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci 2002;59:627–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ikemoto T, Kunito T, Anan Y, Tanaka H, Baba N, Miyazaki N, Tanabe S. Association of heavy metals with metallothionein and other proteins in hepatic cytosol of marine mammals and seabirds. Environmental Toxicol Chem/SETAC 2004;23:2008–16. [DOI] [PubMed] [Google Scholar]
- 51. Inoue K, Takano H, Shimada A, Satoh M. Metallothionein as an anti‐inflammatory mediator. Mediat Inflamm 2009;2009:101659. DOI: 10.1155/2009/101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schaller M, Laude J, Bodewaldt H, Hamm G, Korting HC. Toxicity and antimicrobial activity of a hydrocolloid dressing containing silver particles in an ex vivo model of cutaneous infection. Skin Pharmacol Physiol 2004;17:31–6. [DOI] [PubMed] [Google Scholar]
- 53. Brett DW. A discussion of silver as an antimicrobial agent: alleviating the confusion. Ostomy Wound Manage 2006;52:34–41. [PubMed] [Google Scholar]
- 54. CRC Handbook of Chemistry and Physics. In: Lide DR, editor. 72nd edn, Boca Raton, Ann Arbor, Boston: CRC Press, 1991. [Google Scholar]
- 55. www.saltlakemeatls.com [accessed on 29 October 2012].


