Abstract
Nearly all wounds are at risk for compromised healing due to excessive exudation, oedema, contaminants and presence of inflammatory mediators. Compromised wounds have the potential to develop complications, such as infection, which may lead to delayed wound healing, prolonged hospitalisation and more frequent readmissions. It is generally believed that the wound advances from contamination to colonisation when the bacteria on the wound's surface begin to replicate and increase their metabolic activity. Heavy bacterial bioburden increases the metabolic requirements, stimulates a proinflammatory environment and encourages the in‐migration of monocytes, macrophages and leukocytes – all of which can negatively impact wound healing. Bacteria also secrete harmful cytokines which can lead to vasoconstriction and decreased blood flow. Thus, controlling or preventing infections is essential for normal wound healing process to occur. While the mainstay of treating wound infection has historically included intravenous, oral and/or topical antimicrobials in addition to frequent gauze dressing changes, a shift towards wound management with advanced modalities, such as negative pressure wound therapy (NPWT), has occurred during the past decade. This review will provide expert opinion and scientific support for the use of NPWT with instillation (NPWTi; V.A.C. Instill® Wound Therapy and V.A.C. VeraFlo™ Therapy, KCI USA, Inc., San Antonio, TX) for the treatment of at‐risk and complicated wounds.
Keywords: At‐risk wounds, Infected wounds, Negative pressure wound therapy with instillation
INTRODUCTION
Negative pressure wound therapy with instillation (NPWTi) differs from standard NPWT systems in that topical solutions are cyclically fed into the foam dressing via an additional set of ingress tubing and held for a user‐selected period before removal under negative pressure. Solutions intended for topical use include topical cleansers, antibiotics, antifungals and antiseptics. Alternating topical wound solution instillation with NPWT may assist with wound cleansing, irrigation and removal of infectious material 1, 2, 3. Generally, instillation therapy is indicated for wounds, such as acute, traumatic, dehisced, chronic and ulcers (pressure, diabetic and venous), that would benefit from vacuum‐assisted drainage and controlled delivery of topical wound treatment solutions and suspensions over the wound bed.
More specifically, NPWTi has been reported to have been used in wounds with high levels of exudate and slough content, as well as acute traumatic wounds or wounds acutely debrided due to infected soft tissue (2). The combination therapy has also been reported to be successful in cases of large areas of post‐debrided exposed bone and cases of critical bacterial colonisation levels as an alternative to antibiotic‐impregnated beads, when appropriate (4).
Until recently, commercially available NPWT‐instillation systems (i.e. continuous or gravity‐fed) have been cumbersome to set up and limited in their ability to regulate solution volume delivery. The recent introduction of a system that combines NPWT and a new volumetrically controlled NPWTi (NPWT/NPWTi; V.A.C.ULTA™ Therapy, KCI USA, Inc., San Antonio, TX) has simplified and increased the precision of this technique. This instillation technology (V.A.C. VeraFlo™ Therapy; KCI USA, Inc.) uses reticulated open‐cell foam dressings that are less hydrophobic and specifically designed for instillation therapy [V.A.C. VeraFlo™ (ROCF‐V) and V.A.C. VeraFlo™ Cleanse (ROCF‐VC); KCI USA, Inc.]. Although NPWT alone provides adjunctive therapy that helps remove wound fluid and infectious material, NPWTi also includes added benefits, such as controlled, automated wound cleansing through instillation of topical antiseptic or antimicrobial wound solutions over the wound bed. Recent pilot studies using this new NPWT/NPWTi system have shown positive results for the treatment of complex wounds 5, 6.
REVIEW OF NPWTi FOR WOUND TREATMENT
A growing number of studies have reported on the effectiveness of NPWTi in various at‐risk wound types 2, 3, 4, 7. A pilot study of five post‐surgical diabetic foot wounds treated with NPWTi using a bacitracin‐polymyxin B solution showed successful outcomes with complete healing and no amputations, which resulted in early discharges to an outpatient setting. Authors reported that the addition of an instilled solution lowered wound fluid viscosity, facilitating more efficient removal into the canister (4).
Several more recent NPWTi studies have included larger patient groups with infected soft tissue or orthopedic wounds 2, 8, 9, 10. Gabriel et al. (2) published results from 15 patients with complex, infected wounds treated with NPWTi using silver nitrate compared to a retrospective historical Control group treated with moist gauze. NPWTi patients compared to Control patients required significantly fewer days of treatment (9·9 ± 4·3 versus 36·5 ± 13·1 days, P < 0·001), cleared clinical infection in a shorter time (6·0 ± 1·5 versus 25·9 ± 6·6 days, P < 0·001), achieved wound closure sooner (13·2 ± 6·8 versus 29·6 ± 6·5 days, P < 0·001) and had shorter in‐patient length of stay (14·7 ± 9·2 versus 39·2 ± 12·1 days, P < 0·001). The authors concluded that NPWTi ‘may reduce cost and decrease inpatient care requirements for these complex, infected wounds' (2).
Timmers et al. (8) also reported on a retrospective, case‐control cohort study of patients with osteomyelitis of the pelvis or lower extremity treated with systemic antibiotics and NPWTi with a polyhexanide antiseptic solution or treated with gentamicin polymethylmethacrylate beads and long‐term intravenous antibiotics (Control). The rate of infection recurrence was reduced with NPWTi compared to Control [3/30 (10%) versus 55/93 (58·5%), respectively P < 0·0001]. Mean duration of total hospital stay (36 days with NPWTi versus 73 days with Control; P < 0·0001) and number of required surgical procedures (two with NPWTi versus five with Control P < 0·0001) were also significantly less in the NPWTi group. In addition, Schintler et al. (9) reported on the successful NPWTi and polyhexanide treatment of 15 patients with complicated skin and soft tissue infection. Therapy duration ranged from 4 to 18 days. Infection was controlled and complete healing was achieved in all patients. The authors concluded that NPWTi may be a viable option for treating challenging, complex wounds at risk for compromised healing (9).
Positive outcomes have also been described in a pilot study of five patients with large infected venous stasis ulcers [multi‐drug‐resistant Pseudomonas (n = 2) and methicillin‐resistant Staphylococcus aureus (n = 3)] treated with NPWTi and 12·5% Dakin's solution. After 10 days of NPWTi, quantitative biopsies were negative for bacteria growth. Patients then received a split‐thickness skin graft followed by 4 days of standard NPWT. At 1‐month follow up, there was 100% graft take, and all wounds remained healed after 1 year (11). Additional case reports have described successful use of adjunctive NPWTi in infected open abdomen (12) and pyoderma gangrenosum post breast reconstruction (13).
WOUND PREPARATION AND INITIATION CRITERIA
A comprehensive patient and wound assessment should be performed (7). NPWTi should be used in conjunction with appropriate local wound care including irrigation, debridement and systemic antibiotics. Initially, all wounds should be visually assessed for devitalised tissue, and level of contamination and infection can be confirmed by punch‐wound biopsy cultures before and after debridement. After confirmation of infection, systemic antibiotic treatment should be administered if signs and symptoms of systemic infection exist and followed by initiation of NPWTi. Appropriate operative debridement should also be performed prior to NPWTi initiation in all wounds and reassessed at each dressing change for initiation of either NPWT or NPWTi. In this way, necrotic tissue, exudate and infectious material are removed from the wound bed, which will assist the wound in progressing through the normal phases of wound healing. Adequate haemostasis should also be achieved before NPWTi is used.
Clinical literature reports that wounds that have benefited from vacuum‐assisted drainage and controlled delivery of topical wound treatment solutions include contaminated or stalled wounds, colonised or critically colonised wounds, infected wounds, chronic wounds, high‐level exudating wounds and high‐risk wounds for amputation 2, 7, 14.
TREATMENT GOALS/OUTCOMES
The goal of managing any wound is to achieve closure or coverage efficiently while minimising donor site morbidity. Appropriate management should lead to downstaging of reconstruction technique and coverage, whether delayed primary closure, skin graft, skin substitute or vascularised flap.
Goals of NPWTi are helping to clean wounds, clear infection, enhance granulation tissue formation for primary closure or flap/graft coverage, thereby facilitating limb and implant salvage, hospital discharge and reducing complexity of required reconstructive procedures. Soaking the wound with topical solutions can also decrease the viscosity of thick exudates, easing their removal through the foam.
CONTRAINDICATIONS/WARNINGS
Contraindications and warnings for NPWTi include:
-
•
NPWT with or without instillation should not be used in the presence of untreated osteomyelitis, necrotic tissue with eschar present, non‐enteric or unexplored fistulas, or malignancy in the wound. NPWT/NPWTi dressings should never be placed in direct contact with exposed blood vessels, anastomotic sites, organs or nerves
-
•
NPWTi dressings should not be used with Octenisept® (Schülke & Mayr GmbH, Norderstedt, Germany), hydrogen peroxide or solutions that are alcohol‐based or contain alcohol
-
•
Fluids should not be delivered to the thoracic and abdominal cavity, due to the potential risk to alter core body temperature and the potential for fluid retention within the cavity
-
•
Solutions should not be infused into wounds with unexplored tunnels or unexplored undermining due to the possibility of inadvertently instilling topical wound solutions into adjacent body cavities
-
•
Due to periodic application of negative pressure, NPWTi should not be used on wounds requiring continuous negative pressure, such as over unstable structures, on patients at increased risk of bleeding, or over flaps or grafts
-
•
Some topical wound solutions or suspensions may adversely affect cellular or acellular bioengineered materials
-
•
NPWTi should not be initiated when haemostatic agents have been used in the wound bed
Additional warnings and precautions are provided in the Instructions for Use, which should always be consulted for complete safety information.
DISCONTINUATION CRITERIA
The wound should be assessed at every dressing change and a decision to continue NPWTi, convert to traditional NPWT, or close the wound should be made during this time. Plans for coverage can be made by consulting the appropriate clinician to evaluate the wound. If hardware and/or bone are still exposed, NPWTi should be continued until there is appropriate granulation coverage. If thick exudate persists and the wound has not improved, additional debridement followed by NPWTi should be considered. Once the wound has improved and the clinician judges that the wound has progressed, conversion to traditional NPWT may be considered. Therapy should be discontinued when the wound is ready for primary closure or coverage with a flap or graft. NPWTi should also be discontinued if any condition of gross infection, sepsis, recurrent infection or untreated osteomyelitis is revealed.
TECHNICAL PEARLS
NPWTi may allow surgeons to perform fewer complex reconstructive procedures for major soft tissue defects and reduce donor site morbidity compared to NPWT alone 2, 7. Most wounds appear to benefit from at least 1 or 2 days of instillation/irrigation, and use of NPWTi may lead to fewer trips to the operating room for washouts. Wounds can be washed out as frequently as needed at bedside with NPWTi, and improvement is typically seen as long as appropriate aggressive debridement is performed.
An NPWTi cycle includes solution volume, soak time and NPWT time – all of which should be adjusted according to patient wound care needs. The NPWTi device can be set to cleanse the wound as often as necessary to remove debris, and appropriate clinical judgment should be used to assess the frequency needed to achieve this goal. One way to assess optimal frequency and amount of solution delivery is to evaluate the colour and consistency of the exudate and fluid drawn into the canister.
CLINICAL CASES
Case study 1
A 43‐year‐old female presented with an infected chest wound after radiation. Prior to debridement, the wound was visually assessed for infection. Punch‐wound biopsy cultures were positive for bacterial bioburden. Patient received systemic antibiotics and wound was debrided. NPWTi/ROCF‐V was initiated, and Prontosan® (B.Braun Medical Inc., Bethlehem, PA) was instilled until the foam was filled, followed by a soak time of 3 minutes. Instillation was repeated every hour followed by continuous negative pressure at −125 mm Hg for 3 days. No complications occurred during therapy, and granulation tissue was present with negative cultures at the time of coverage with a latissimus flap (Figure 1).
Figure 1.
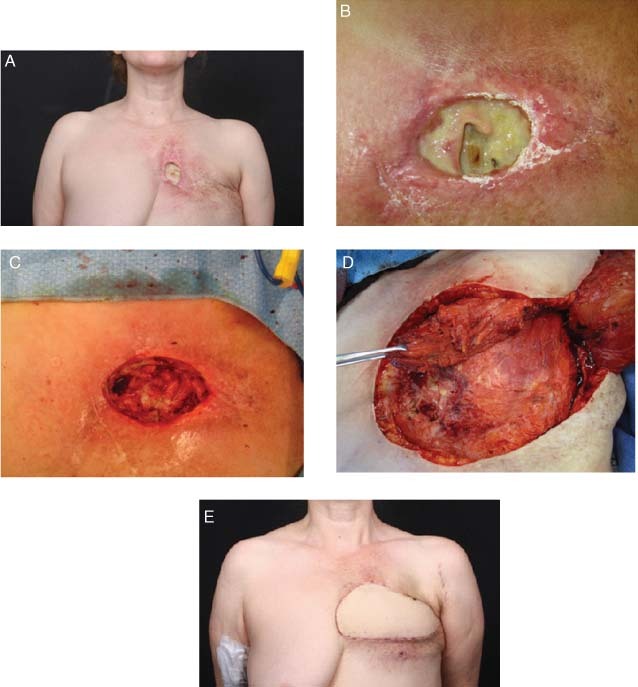
Case study 1: (A) Radiated chest wound. (B) Initial presentation of chest wound. (C) Wound after debridement of rib and cartilage and 4 days of negative pressure wound therapy with instillation (NPWTi/ROCF‐V). (D) Excision of radiated skin. (E) Six weeks following chest wall reconstruction with latissimus flap.
Case study 2
An 86‐year‐old female diabetic with peripheral vascular disease presented with a left foot abscess. Prior to debridement, the wound was visually assessed for infection. Punch‐wound biopsy cultures were positive for bacterial burden. Patient received systemic antibiotics and the wound was debrided. NPWTi/ROCF‐V was initiated, and saline was instilled until the foam was filled followed by a soak time of 3 minutes. Instillation was repeated every 2 hours followed by continuous negative pressure at −125 mmHg for 3 days. No complications occurred during therapy, and granulation tissue was present with negative cultures at the time of primary closure (Figure 2).
Figure 2.
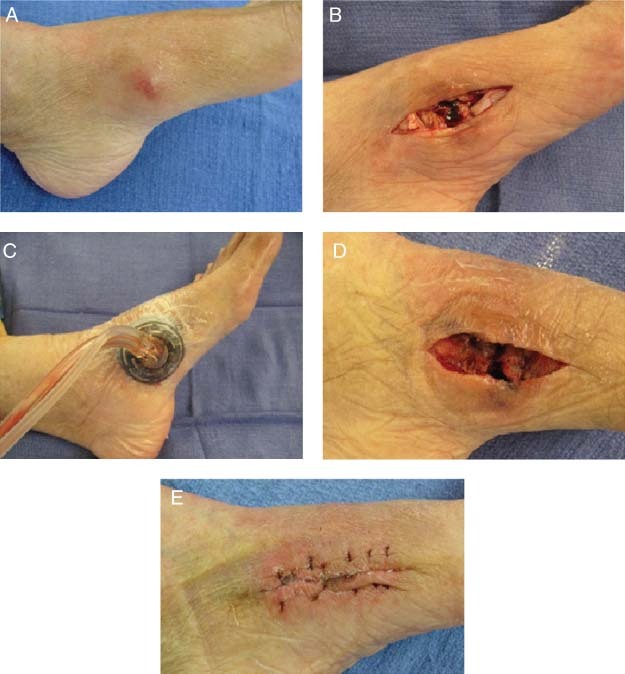
Case study 2: (A) Left foot abscess at presentation. (B) Abscess was drained and the wound debrided. (C) Application of negative pressure wound therapy with instillation (NPWTi/ROCF‐V). (D) After 3 days of NPWTi/ROCF‐V, wound was ready for primary closure. (E) Two weeks following primary closure.
ECONOMIC VALUE AND FUTURE DIRECTIONS
Costs of wound infection are enormous, both in terms of economics and morbidity. According to the US Centers for Disease Control and Prevention, each year, US hospitals alone experience 1·7 million healthcare‐associated infections (HAIs), leading to 99 000 deaths and costing between $37 and $45 billion (15). Although hospitals are making considerable strides in reducing HAIs, the incidence of complicated, at‐risk wounds remains on the rise and successful outcomes depend on management that is both aggressive and efficient. Rapid healing and early hospital discharge allow the patient to return more quickly to daily living, reducing costs for hospitals and society. Hence, controlling colonisation of the wound by various means, including NPWTi, is critical for cost control.
Numerous anecdotal studies have shown bioburden reduction and elimination of infection with adjunctive use of NPWTi, and its increased usage will likely continue for the foreseeable future. However, controlled studies that include wide‐ranging economic factors associated with hospital costs are needed to prove NPWTi's overall cost effectiveness.
ACKNOWLEDGEMENTS
We would like to thank Julissa Ramos, PhD (KCI, Inc.) for assisting with preparation of the manuscript.
CONFLICTS OF INTEREST
Dr AG has a Consulting agreement with Kinetic Concepts, Inc. This article is part of an educational supplement funded by Kinetic Concepts, Inc. to provide an overview of the V.A.C.® Therapy family of products for new users in developing markets. Targeted for distribution at the 2012 World Union of Wound Healing Societies (WUWHS) conference, this supplement article presents a brief literature review and clinical experience treating compromised wounds using V.A.C.® Therapy with instillation (i.e. VA.C. Instill® Wound Therapy and V.A.C. VeraFlo™ Therapy).
REFERENCES
- 1. Warriner R, Burrell R. Infection and the chronic wound: a focus on silver. Adv Skin Wound Care 2005;18 Suppl 1:2–12. [DOI] [PubMed] [Google Scholar]
- 2. Gabriel A, Shores J, Heinrich C, Baqai W, Kalina S, Sogioka N, Gupta S. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 2008;5:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolvos T. Wound instillation with negative pressure wound therapy. Ostomy Wound Manage 2005;51 Suppl 2A:21S–6S. [PubMed] [Google Scholar]
- 4. Bernstein BH, Tam H. Combination of subatmospheric pressure dressing and gravity feed antibiotic instillation in the treatment of post‐surgical diabetic foot wounds: a case series. Wounds 2005;17:37–48. [Google Scholar]
- 5. Wolvos T. The evaluation of a combined negative pressure wound therapy and negative pressure wound therapy with instillation system for the treatment of difficult to heal wounds: an initial clinical experience. Presented at the Clinical Symposium on Advances in Skin and Wound Care, September 9–12, 2011, National Harbor, MD. 9‐9‐2011. [Abstract].
- 6. Gabriel A, Karmy‐Jones R, Schroeder S. Experience with a new integrated negative pressure wound therapy system with volumetric automated fluid instillation in at‐risk wounds. Presented at the Clinical Symposium on Advances in Skin and Wound Care, September 9–12, 2011, National Harbor, MD. 9‐9‐2011. [Abstract].
- 7. Gabriel A, Shores J, Bernstein B, de Leon J, Kamepalli R, Wolvos T, Baharestani MM, Gupta S. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) Therapy: experience and case series. Int Wound J 2009;6 Suppl 2:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Timmers MS, Graafland N, Bernards AT, Nelissen RG, van Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen 2009. ;17:278–86. [DOI] [PubMed] [Google Scholar]
- 9. Schintler MV, Prandl EC, Kreuzwirt G, Grohmann MR, Spendel S, Scharnagl E. The impact of V.A.C. Instill in severe soft tissue infections and necrotizing fasciitis. Infection 2009;37 Suppl 1:31–2. [Google Scholar]
- 10. Lehner B, Fleischmann W, Becker R, Jukema GN. First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study. Int Orthop 2011;35:1415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raad W, Lantis JC II, Tyrie L, Gendics C, Todd G. Vacuum‐assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int Wound J 2010;7:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D’Hondt M, D’Haeninck A, Dedrye L, Penninckx F, Aerts R. Can vacuum‐assisted closure and instillation therapy (VAC‐Instill therapy) play a role in the treatment of the infected open abdomen? Tech Coloproctol 2011;15:75–7. [DOI] [PubMed] [Google Scholar]
- 13. Schintler MV, Grohmann M, Donia C, Aberer E, Scharnagl E. Management of an unfortunate triad after breast reconstruction: pyoderma gangrenosum, full‐thickness chest wall defect and Acinetobacter Baumannii Infection. J Plast Reconstr Aesthet Surg 2010;63:e564–e567. [DOI] [PubMed] [Google Scholar]
- 14. Wolvos T. Wound instillation – The next step in negative pressure wound therapy. Lessons learned from initial experiences. Ostomy Wound Manage 2004;50:56–66. [PubMed] [Google Scholar]
- 15. Health care acquired infections. Hospitals in Pursuit of Excellence 2010 January 1 URL http://www.hpoe.org/topic‐areas/health‐care‐acquired‐infections.shtml [accessed on 9 September 2010].


