Abstract
Non healing diabetic foot ulcers and the resulting potential amputations present significant costs to the health care system and reduce patient quality of life. The goal of diabetic foot ulcer treatment is to obtain wound closure as expeditiously as possible. The use of platelet‐rich plasma (PRP) to enhance wound healing has increased dramatically over the last decade. However, controversies exist in the literature regarding the added benefit of this procedure. The aim of this study is to investigate the efficiency of platelet releasate on the healing of chronic diabetic ulcers in comparison with platelet‐poor plasma (PPP). This study included 24 patients with chronic diabetic ulcers. They were systematically randomised into two groups: PRP group (n = 12) and PPP group (n = 12). The results showed that healing in PRP group was significantly faster (P < 0·005). PRP enhances healing of chronic diabetic foot ulcers.
Keywords: Chronic wounds, Diabetic foot ulcer, Platelet‐poor plasma, Platelet releasate, Platelet‐rich plasma
INTRODUCTION
A chronic wound is a wound that does not heal in an orderly set of stages and in a predictable amount of time the way most wounds do; wounds that do not heal within 3 months are often considered chronic (1). The vast majority of chronic wounds can be classified into three categories: venous, diabetic and pressure ulcers (2). A small number of wounds that do not fall into these categories may be because of causes such as radiation, poisoning or ischaemia (2).
Growth factors (GF) are imperative in timely wound healing; inadequate GF levels may be an important factor in the chronicity of the wound (3). In chronic wounds, the formation and release of GF may be prevented, the factors may be sequestered and unable to perform their metabolic roles, or degraded in excess by cellular or bacterial proteases (3).
Platelet‐derived GF have been used as an active treatment modality to stimulate the wound healing process of chronic non healing ulcers promoting growth of skin, soft tissue and blood vessels (4). Platelet‐derived GF may be recombinant (5) or autologous (6).
The use of platelet‐rich plasma (PRP) to enhance wound healing has increased dramatically over the last decade. However, controversies exist in the literature regarding the added benefit of this procedure (7).
Many authors 8, 9, 10, 11 found that platelet releasate has improved healing rates over standard care. On the other hand, others 12, 13, 14, 15 found no major difference in healing outcome of leg ulcers, between treatment groups with platelet releasate and control groups (placebo).
These controversies necessitated further controlled studies. Therefore, the aim of this study was to investigate the efficiency of platelet releasate on the healing of chronic diabetic ulcers.
PATIENTS AND METHODS
The patients enrolled in this study were eligible if they met the following inclusion criteria: age between 40 and 60 years old, suffering from either type of diabetes – type I (insulin dependent) or type II (non insulin dependent) – and they were in a controlled status. Patients included should have normal peripheral platelet count (>150 000/mm3).
Patients who were receiving or had received radiation or chemotherapy within 3 months of study and those with screening serum albumin level of <2·5 g/dl or haemoglobin <10·5 mg/dl or platelet count <100 × 109/l were excluded. Any patient with peripheral vascular disease was excluded too.
All patients had chronic (of at least 12 weeks' duration) non healing diabetic ulcer(s) confined to one anatomical site with no evidence of gangrene in the ulcer or on any part of the foot. All ulcers had debridement before the onset of this study to freshen the wounds and to remove necrotic tissues. Biopsy was taken from each ulcer and if the bacterial count exceeded 105 organisms per gram of tissue the patient was excluded from this study. Ulcers that had exposed tendons, ligaments or bone were excluded.
All patients who participated in this study provided informed consent forms.
PREPARATION OF PLATELET RELEASATE AND PLATELET‐POOR PLASMA
-
–
Venous blood was drawn into a tube containing an anticoagulant (citrate dextrose) to avoid platelet activation and degranulation (10 cc).
-
–
Then the blood was centrifuged, the first centrifugation is called ‘soft spin’ (1007 g), which allows blood separation into three layers, namely bottommost red blood cell (RBC) layer (55% of total volume), topmost acellular plasma layer called platelet‐poor plasma (PPP; 40% of total volume) and an intermediate PRP layer (5% of total volume) called the ‘buffy coat'.
-
–
Using a sterile syringe, the PPP, PRP and some RBCs (i.e. the upper two layers and very minimal ‘unavoidable’ amount of bottom layer) were transferred into another tube without an anticoagulant.
-
–
This tube underwent a second centrifugation (447·5 g) called ‘hard spin’. This allowed the platelets (PRP) to settle at the bottom of the tube with a very few RBCs. The acellular plasma, PPP (80% of the volume), was found on the top.
-
–
Most of the PPP was removed with a syringe and the remaining PRP was shaken well.
-
–
This PRP was then mixed with bovine thrombin (0·2 ml for every 1 cc PRP) and calcium chloride 10% (0·1 ml) at the time of application in a petri‐dish (Figure 1). This resulted in gelling of the platelet concentrate. Then the platelet concentrate was applied to the ulcer after being debrided, cleaned with normal saline solution.
Figure 1.
Platelet‐rich plasma (PRP) preparation in a petri‐dish.
TECHNIQUE OF APPLICATION
This study was performed on 24 patients. They were systematically randomised into two groups. The odd‐numbered patients had PRP (group I) and the even‐numbered patients had PPP (group II).
(i) The first group (12 patients): They had PRP as their dressing protocol, where PRP was applied to the diabetic foot after being prepared (within half an hour after preparation), followed by vaseline gauze and then a dressing.
The frequency of change of dressing was twice weekly with an interval of 3–4 days between every dressing. This protocol was prospected to be performed for patients up to 20 weeks, or stopped whenever healing occurred.
(ii) The second group (12 patients): This group had the PPP as their dressing protocol, where after applying the PPP, vaseline gauze was applied followed by a dressing, with a frequency of change of dressing twice weekly. The dressing protocol was performed for up to 20 weeks.
FOLLOW‐UP
The patients were advised to avoid pressure on ulcer area. Special shoe with moulded insole was used. Elevation of the feet was recommended during setting or lying down to decrease oedema. The patients were seen twice weekly throughout the treatment course and clinical evaluation was performed once weekly. Clinical laboratory tests were performed every 4 weeks for all treatment groups.
Evaluation of the rate of healing of the ulcer was carried out by measuring the ulcer's dimensions (length and width) using metric tapes at initial visit and then every week. Other wound variables including characteristics of wound exudates, necrotic tissue, infection and granulation tissue were documented.
Both clinical and laboratory data were documented and analysed statistically.
RESULTS
There were several risk factors recorded while taking the history of the patients of the two groups. Eight patients (33·3%) were smokers, 17 patients (70%) were suffering from hypertension, 11 patients (45·8%) were obese and 3 patients (12·5%) had a past history of diabetic sequela.
Using chi‐square test, no statistically significant difference was found between the two groups concerning the risk factors.
Several clinical signs were detected in the patients of the two study groups. Neuropathy was detected in 15 patients (62·5%), signs of inflammation in 9 patients (37·5%) and Charcot's joint away from the site of ulcer in 2 patients (8·3%). There was no statistically significant difference between the two groups regarding clinical signs.
Using the chi‐square test and cross tabs, there was no statistical significance concerning the difference in sites of diabetic ulcers in the two study groups.
The ulcer's initial length ranged from 2 to 7 cm, the initial width ranged from 2 to 5 cm and the average surface area ranged from 4 to 21 cm2 (Table 1).
Table 1.
Detailed criteria of the diabetic ulcers according to the dimensions
| Ulcer criteria | Minimum | Maximum | Mean | |
|---|---|---|---|---|
| Wound length (initial; cm) | PRP | 2·5 | 7 | 3·66 |
| PPP | 2 | 4 | 3·2 | |
| Wound width (initial; cm) | PRP | 2 | 5 | 2·70 |
| PPP | 2 | 5 | 2·61 | |
| Wound area (initial; cm2) | PRP | 5 | 21 | 10·25 |
| PPP | 4 | 20 | 8·50 | |
There was no statistically significant difference between the two groups regarding culture and sensitivity tests for swabs from ulcers.
There was no statistically significant difference between the two groups regarding laboratory data at the start or the end of this study.
The mean healing time for PRP was 11·5 weeks (8–18 weeks) and the mean healing time for PPP was 17 weeks (14–20 weeks). Statistical analysis (using the one‐way ANOVA test and post hoc tests) showed statistically significant difference (P < 0·005). Table 2 shows healing duration of both PRP and PPP. Figures 2 and 3 show a case healed using PRP in 10 weeks. Figures 4 and 5 show healing ulcer using PPP.
Table 2.
Healing duration in weeks for the healed cases (maximum, minimum and mean)
| Healing duration (weeks) | n | Mean | Minimum | Maximum | |
|---|---|---|---|---|---|
| PRP | 12 | 11·5 | 8 | 18 | |
| PPP | 9 | 17· 1 | 14 | 20 |
Figure 2.
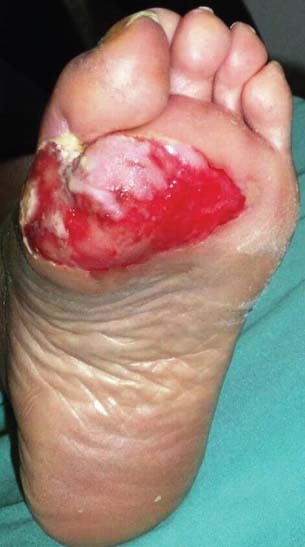
Diabetic foot ulcer before application of platelet‐rich plasma (PRP).
Figure 3.
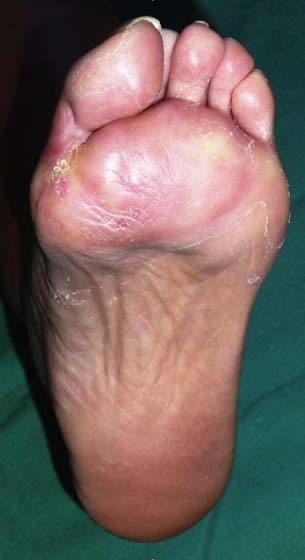
Diabetic foot ulcer shown in Figure 2 completely healed 10 weeks after application of platelet‐rich plasma (PRP).
Figure 4.
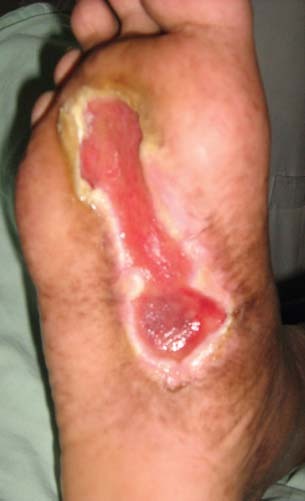
Diabetic foot ulcer on sole before application of platelet‐poor plasma (PPP).
Figure 5.
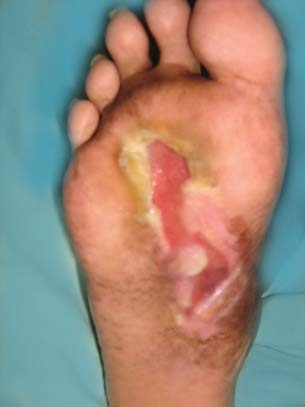
Diabetic foot ulcer shown in Figure 4 did not heal after 20 weeks of application of platelet‐poor plasma (PPP).
Furthermore, using the Kaplan–Meier test and the log‐rank test for studying the healing duration it showed that it is statistically significant.
DISCUSSION
One of the most common complications of diabetes in the lower extremity is the diabetic foot ulcer. It is estimated that 15% of patients with diabetes will develop a lower extremity ulcer during the course of their disease (16).
In this study, the effect of platelet releasate (PRP) and the released GF upon activation on the healing of diabetic foot ulcers was studied in comparison with the PPP (control group).
All associated factors that might have given advantage in healing of ulcers other than tested materials (PRP and PPP) showed no significant difference between the two groups. These factors included risk factors (smoking, hypertension or obesity), clinical signs (neuropathy, Charcot's joints and inflammation), location and dimensions of ulcers, culture and sensitivity tests from swabs taken from ulcers and laboratory data both at the start and the end of study.
Although McAleer et al. (17) supported the use of autologous blood to prepare PRP and PPP, Jeong et al. (18) used homologous blood. McAleer et al. (17) stated that the use of autologous blood is free from concern about transmittable diseases such as HIV and hepatitis. On the other hand, the rationale of Jeong et al. (18) was that many diabetic ulcer patients are anaemic or haemodynamically unstable. In this study, we used autologous blood as a source of PRP and PPP preparation.
The volume of blood used to prepare the platelet concentrate was a matter of debate. Driver et al. (19) used small volumes (20–60 cc) for preparation of PRP. Jeong et al. (18) used large volumes of blood (250–500 cc) to prepare homologous platelet concentrate. In our study, small volumes (10–20 cc) (of the patient own blood) were taken to prepare PRP or PPP.
The local application of platelet gel differs through different techniques of preparation. Man et al. (20) used one of the dual syringe method with PRP (platelet gel) and the other with bovine thrombin and calcium chloride. Driver et al. (19) poured platelet gel directly into the wound and then covered the wound by a dressing. In this study, the platelet gel was prepared in the gel form and then applied directly to the wound after being debrided and to be followed by dressing.
Concerning the frequency of dressing, it is variable in many studies using PRP. Crovetti et al. (3) used the dressing once per week. Jeong et al. (18) and Driver et al. (19) used it twice weekly. We used the same protocol of twice‐weekly application.
The result of this study showed that the healing of chronic diabetic foot ulcer by PRP is significantly faster than by PPP. The role of PRP in accelerating healing of chronic ulcers was supported by previous studies 8, 9, 10, 11.
CONCLUSION
PRP promotes and accelerates healing of chronic diabetic foot ulcers. This helps rapid wound healing in patients who have comorbidities that preclude anaesthesia and surgeries or who refuse undergoing skin grafting. Wound healing will prevent spread of infection and prevent lower extremities amputations.
ACKNOWLEDGEMENT
This study was approved by Ain Shams University ethical committee. None of the authors declare any conflict of interest.
REFERENCES
- 1. Mustoe TA. Dermal ulcer healing: advances in understanding. Presented at meeting: tissue repair and ulcer/wound healing: molecular mechanisms, therapeutic targets and future directions; 2005. Mar 17–18; Paris, France. URL http://www.pasteur.fr/applications/euroconf/tissuerepair/mustoe_abstract.pdf
- 2. Mustoe TA. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg 2004;187: S65–70. [DOI] [PubMed] [Google Scholar]
- 3. Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, Maroni M, Carabelli A. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci 2004;30:145–51. [DOI] [PubMed] [Google Scholar]
- 4. Cross KJ, Mustoe TA. Growth factors in wound healing. Surg Clin North Am 2003;83:531–45. [DOI] [PubMed] [Google Scholar]
- 5. Steed DL. Clinical evaluation of recombinant human platelet‐derived growth factors for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer study group. J Vasc Surg 1995;21:71–8. [DOI] [PubMed] [Google Scholar]
- 6. Adler SC, Kent K. Enhancing wound healing with growth factors. Facial Plast Surg Clin N Am 2002; 10:129–46. [DOI] [PubMed] [Google Scholar]
- 7. Freymiller EG, Aghaloo TL. Platelet rich plasma: ready or not? J Oral Maxillofac Surg 2004;62: 484–8. [DOI] [PubMed] [Google Scholar]
- 8. Steed DL, Goslen JB, Holloway GA. CT‐102 activated platelet supernatant, topical versus placebo: a randomized prospective double blind trial in healing of chronic diabetic foot ulcers. Diabetes Care 1992;15:1598–604. [DOI] [PubMed] [Google Scholar]
- 9. Glover JL, Weingarten MS, Buchbinder DS, Poucher RL, Deitrick GA 3rd, Fylling CP. Four year multicenter retrospective study of topical multiple growth factor therapy for chronic wounds. Adv Wound Care 1997;10:27–32. [PubMed] [Google Scholar]
- 10. O’Meara SM, Cullum NA, Majid M, Sheldon TA. Systematic review of antimicrobial agents used for chronic wounds. Br J Surg 2001;88:4–21. [DOI] [PubMed] [Google Scholar]
- 11. Kantor J, Margolis DJ. Treatment options for diabetic neuropathic foot ulcers: a cost‐effectiveness analysis. Dermatol Surg 2001;27:347–51. [DOI] [PubMed] [Google Scholar]
- 12. Reutter H, Bort S, Jung MF, Klyscz T, Schippert W, Zuder D, Junger M. Questionable effectiveness of autologous platelet growth factors (PDHWF) in the treatment of venous leg ulcers. Hautarzt 1999;50:859–65. [DOI] [PubMed] [Google Scholar]
- 13. Stacey MC, Mata SD, Trengove NJ, Mather CA. Randomised double‐blind placebo controlled trial of topical autologous platelet lysate in venous ulcer healing. Eur J Vasc Endovasc Surg 2000;20:296–301. [DOI] [PubMed] [Google Scholar]
- 14. Senet P, Bon FX, Benbunan M, Bussel A, Traineau R, Calvo F, Dubertret L, Dosquet C. Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. J Vasc Surg 2003;38:1342–8. [DOI] [PubMed] [Google Scholar]
- 15. Mazzucco L, Medici D, Serra M, Panizza R, Rivara G, Orecchia S, Libener R, Cattana E, Levis A, Betta PG, Borzini P. The use of autologous platelet gel to treat difficult‐to‐heal wounds: a pilot study. Transfusion 2004;44: 1013–8. [DOI] [PubMed] [Google Scholar]
- 16. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes and costs of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–7. [DOI] [PubMed] [Google Scholar]
- 17. McAleer JP, Kaplan E, Persich G. Efficacy of concentrated autologous platelet‐derived growth factors in chronic lower‐extremity wounds. J Am Podiatr Med Assoc 2006;96:482–8. [DOI] [PubMed] [Google Scholar]
- 18. Jeong SH, Han SK, Kim WK. Treatment of diabetic foot ulcers using a blood bank platelet concentrate. Plast Reconstr Surg 2010;125: 944–52. [DOI] [PubMed] [Google Scholar]
- 19. Driver VR, Hanft J, Fylling CP, Beriou JM. Autologel diabetic foot ulcer study group. A prospective, randomized, controlled trial of autologous platelet‐rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage 2006;52:68–74. [PubMed] [Google Scholar]
- 20. Man D, Plosker H, Winland‐Brown JE. The use of autologous platelet‐rich plasma (platelet gel) and autologous platelet‐poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg 2001;107: 229–37. [DOI] [PubMed] [Google Scholar]


