Abstract
Multidrug‐resistant organisms (MDROs) are increasingly implicated in both acute and chronic wound infections. The limited therapeutic options are further compromised by the fact that wound bacteria often co‐exist within a biofilm community which enhances bacterial tolerance to antibiotics. As a consequence, topical antiseptics may be an important consideration for minimising the opportunity for wound infections involving MDROs. The objective of this research was to investigate the antimicrobial activity of a silver‐containing gelling fibre dressing against a variety of MDROs in free‐living and biofilm states, using stringent in vitro models designed to simulate a variety of wound conditions. MDROs included Acinetobacter baumannii, community‐associated methicillin‐resistant Staphylococcus aureus, and extended‐spectrum beta‐lactamase‐producing bacteria. Clostridium difficile was also included in the study because it carries many of the characteristics seen in MDROs and evidence of multidrug resistance is emerging. Sustained in vitro antimicrobial activity of the silver‐containing dressing was shown against 10 MDROs in a simulated wound fluid over 7 days, and inhibitory and bactericidal effects against both free‐living and biofilm phenotypes were also consistently shown in simulated colonised wound surface models. The in vitro data support consideration of the silver‐containing gelling fibre dressing as part of a protocol of care in the management of wounds colonised or infected with MDROs.
Keywords: Biofilm, Infection, Multidrug‐resistant organisms, Silver dressing, Wound
INTRODUCTION
The discovery and introduction of antibiotics was undoubtedly one of the major advancements in public health during the 20th Century. However, the consequent and continued emergence of multidrug‐resistant organisms (MDROs) has led to once‐considered harmless bacteria being feared as potentially lethal pathogens today. In the early years of the 21st Century, increasing awareness and concern with bacterial pathogens such as Acinetobacter baumannii, methicillin‐resistant Staphylococcus aureus (MRSA), extended‐spectrum beta‐lactamase (ESBL) producing Gram‐negative bacteria and Clostridium difficile is evident. Historically these organisms have been recognised as opportunistic nosocomial pathogens, with vulnerable hospitalised patients (e.g. critically ill, immuno‐compromised, debilitated) being at greatest risk (1). However, with increased antibiotic resistance, virulence and environmental tolerance, these pathogens are now becoming a serious threat in the wider, healthier community setting 2, 3, 4.
Despite the ubiquity of Acinetobacter spp in nature, multidrug‐resistant A. baumannii has no recognised natural habitat outside of the hospital environment (5). Due to its ability to survive on dry, inanimate surfaces, acquire resistance genes and tolerate biocides as well as antibiotics, A. baumannii is capable of spreading within health care facilities, and its impact in combat zones and natural disaster areas throughout the world is increasingly evident (5).
MRSA was first reported in 1961, and has been a major worldwide problem in hospitals since this time (6). Over the last decade, community‐associated MRSA (CA‐MRSA) has emerged globally in younger healthy individuals without obvious risk factors (e.g. recent hospitalisation or surgery) for MRSA‐associated infection (3). CA‐MRSA carries virulence determinants that are likely to play a key role in its pathogenesis (7), including Panton–Valentine leukocidin, a prevalent and potent toxin that has been associated with aggressive soft tissue infections and pneumonia (3).
Bacteria capable of producing a variety of ESBLs that confer resistance to penicillins and expanded‐spectrum cephalosporins have emerged in the last 25 years, and now present a significant risk to patients within hospital, long‐term care and community settings (8). ESBL‐producing bacteria differ from other MDROs in that associated genes are easily transferred via plasmids between various types of Gram‐negative bacteria including Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterobacter cloacae and A. baumannii.
While presently not classified as a MDRO, the emergence of a hypervirulent strain of C. difficile with increased resistance to fluoroquinolones has coincided with an increase in frequency and severity of C. difficile infections (9). In addition, multidrug resistance (clindamycin, moxifloxacin and rifampicin) has recently been observed in a hypervirulent strain of C. difficile (10). As a spore‐forming bacterium, C. difficile has the ability to spread extensively in health care settings and tolerate environmental challenges such as biocides (e.g. alcohol) and dry inanimate surfaces. Evidence for community‐associated C. difficile is emerging in patients with no predisposing risk factors such as advanced age, recent antibiotic therapy, recent hospitalisation and comorbidity (2).
Many of the pathogens associated with multidrug resistance are also common inhabitants of chronic and acute wounds (11). A review of the literature indicates that MDROs are increasingly involved in infections associated with a variety of cutaneous wound types, including burns, combat‐related, surgical and chronic wounds (Table 1) 5, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31. Wounds are often characterised by a complex and potentially pathogenic microflora that presents a serious risk for both wound infection and cross contamination. In non‐progressing recalcitrant wounds or those at risk of infection, topical antimicrobial agents may be the first therapeutic consideration for bioburden control. Where clear signs of spreading infection are evident, then systemic antibiotics are invariably used (Figure 1). However, the efficacy of systemic antibiotics will be influenced by several factors including the quality of blood flow to the wound tissue, the types of micro‐organisms in the wound, their antibiotic‐resistance profile and by the presence of bacteria‐derived biofilm which significantly enhances their tolerance to antibiotics (21). Particularly in situations where MDROs are components of a wound infection, the options and likely success of systemic antibiotic therapy is uncertain. Topical antiseptic agents are beneficial in that many have a broader spectrum of activity than antibiotics and they have a much lower propensity to induce bacterial resistance. Consequently, topical antiseptic agents may have an important role to play in wounds that are colonised or infected with MDROs.
Table 1.
Literature evidence for MDRO infections in a different wound types
| Combat and natural disaster traumatic wounds | Burns | Skin and soft tissue wounds | Surgical wounds | Chronic wounds | |
|---|---|---|---|---|---|
| ESBL‐producing bacteria | 12 | 14,15,16 | 17,18,19 | 20,21,22 | |
| MRSA/CA‐MRSA | 12 | 16 | 23,24,25 | 19 | 20,22,24 |
| A. baumannii | 5, 13,26,27,28,29 | 5,14,29 | 13,26 | 21 | |
| C. difficile | 28 | 30 | 31 | 31 |
ESBL, extended‐spectrum beta‐lactamase; CA‐MRSA, community‐associated methicillin‐resistant Staphylococcus aureus.
Figure 1.
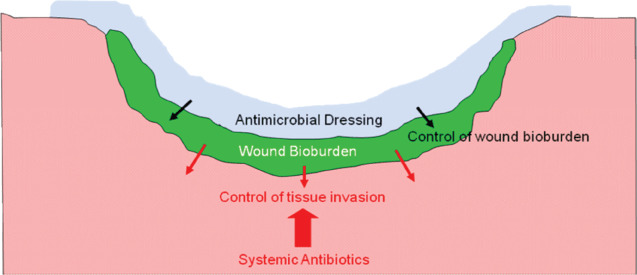
Use of topical and systemic antimicrobials in colonised/infected wounds.
Antimicrobial dressings are used to control wound bioburden, minimise the risk of infection and provide an antimicrobial barrier to minimise spread of wound pathogens. One such dressing is based on Hydrofiber® (registered trademark of ConvaTec Inc.) technology containing ionic silver (HFS). The Hydrofiber® technology comprises sodium carboxymethylcellulose fibres that, in dressing format, absorb fluid rapidly to form a cohesive gel that conforms closely to a wound's topography (32). Wound fluid is locked within the gelled dressing together with harmful components such as bacteria and caustic enzymes (32). The ionic silver component of the dressing provides antimicrobial protection both within the dressing and at the wound‐dressing interface. We have previously shown in vitro that, because of excellent conformability with a colonised simulated shallow wound surface, the antimicrobial action of the HFS dressing is maximised (33).
The objective of the current research was to investigate the antimicrobial activity of the HFS dressing against a variety of MDROs in free‐living and biofilm states, using four stringent in vitro fluid and solid culture models.
MATERIALS AND METHODS
Four in vitro models designed to simulate stringent clinical conditions were used to investigate the antimicrobial efficacy of a silver‐containing Hydrofiber® dressing silver [HFS (AQUACEL®, registered trademark of ConvaTec Inc., Ag, ConvaTec Inc, Skillman, NJ)] against MDROs. A Hydrofiber® dressing without silver [HF (AQUACEL®)] was used as a control where appropriate.
A variety of fluid and solid microbiological culture media were used for the cultivation of both aerobic and anaerobic bacteria. Materials and methods are described in greater detail elsewhere 33, 34, 35, 36.
Multidrug‐resistant organisms
A. baumannii: (NCTC 13424); A. baumannii: (NCTC 13422); A. baumannii: (NCTC 13421); community‐associated MRSA (CA‐MRSA USA300; HPA: H045260142); C. difficile (NCTC 11382); ESBL‐producing bacteria: E. coli producing TEM‐3 (NCTC 13351); E. coli producing CTX‐M‐15 (NCTC 13353); P. aeruginosa producing VIM‐10; VEB‐1 +ve: (NCTC 13437); E. cloacae producing AmpC BLACT IND (NCTC13405); K. pneumoniae producing blaCTX‐M group 25 gene (NCTC 13465).
Simulated wound fluid (SWF) model
This in vitro model was used to measure sustained antimicrobial activity over time. 5 cm × 5 cm pieces of HFS dressing were aseptically transferred to 10 ml volumes of SWF (peptone saline diluents and foetal calf serum) containing approximately 1 × 106 cfu/ml of one of the MDROs (34). All test models were incubated at 35°C under aerobic conditions (with the exception of the anaerobe, C. difficile), and total viable counts were performed at several time points over a 7‐day test period, using 0·1% sodium thioglycollate to neutralise residual silver activity. On day 2, each test model was re‐inoculated with approximately 1 × 105 cfu of the respective MDRO to simulate a worst‐case clinical situation. Each bacterium was tested against the HFS dressing on five occasions, and against the HF dressing (control) on one occasion.
Simulated colonised shallow wound (SCSW) model
This in vitro model was used to investigate the effect of dressing conformability on activity of the HFS dressing (33). Simulated in vitro shallow wounds were prepared using sterile gauze samples to create a standardised impression (5 cm × 5 cm × 2–3 mm depth) in the centre of a 140‐mm agar plate [tryptone soy agar (TSA) for aerobes and Wilkins Chalgren agar (WCA) for C. difficile]. The simulated shallow wound area was then inoculated with 4 ml of a suspension containing approximately 4000 viable cells of an MDRO. A 10 cm x 10 cm piece of the HFS dressing was then applied centrally over the simulated colonised shallow wound such that the dressing contacted the surrounding prominent agar (note: an adhesive cover dressing was applied over the HFS dressing as indicated in the manufacturer's instructions for use). The agar plates with dressings were incubated under appropriate atmospheric conditions at 35°C for 48 hours, after which time the dressings were removed and the extent of bacterial growth within the simulated shallow wound area was visualised and photographed. All agar plates (with dressings removed) were then re‐incubated for an additional 24 hours to enable any bacterial growth within the simulated shallow wound area to be visualised more clearly. Image analysis software (ImageTool for Windows, v3.0) was then used to quantify the extent of bacterial growth as a percentage of the total simulated shallow wound surface area. Each bacterium was tested against the HFS dressing on five occasions, and on one occasion in the absence of the dressing (control).
Simulated colonised wound surface (SCWS) model
This in vitro model was used to investigate the antimicrobial activity of the HFS dressing against a variety of MDROs seeded into agar directly beneath the dressing (35). A sterile layer (80 ml) of TSA (or WCA for C. difficile) in a 140‐mm Petri dish was flooded with a second layer of molten agar (45 ml) that had previously been seeded with ∼1 × 105 cfu/ml of one of the MDROs, to create a seeded agar layer ∼2 mm in depth. Following incubation under appropriate atmospheric conditions at 35°C for 4 hours to initiate growth, 10 cm × 10 cm pieces of HFS dressings were applied to the centre of each seeded agar plate and pressed down gently to ensure good contact with the agar surface before the application of a secondary adhesive cover dressing (as stated in manufacturer's instructions for use). All seeded agar plates were then incubated aerobically (with the exception of the anaerobe, C. difficile) at 35°C for 48 hours, following which the dressings were aseptically removed and photographs of the agar plates were taken. Agar plates (with dressings removed) were then re‐incubated for a further 24 hours to enable clear visualisation of any remaining viable cells. To assess bacteriostatic and/or bactericidal activity against the MDROs, a stab culture was taken from the centre of each seeded agar plate using a sterile loop and then sub‐cultured on to DE Neutralising agar (fastidious anaerobe agar containing 0·1% sodium thioglycollate for C. difficile) to neutralise any residual silver activity. Following incubation under appropriate atmospheric conditions, the sub‐cultured agar plates were observed for the presence or absence of bacterial growth. Each bacterium was tested against the HFS dressing on five occasions, and on one occasion in the absence of the dressing (control).
Biofilm model (poloxamer)
This in vitro model was used to assess the antimicrobial activity of the HFS dressing when each of the MDROs was expressing a biofilm phenotype (36). Twenty millilitre volumes of Mueller Hinton broth (or Wilkin's Chalgren broth for C. difficile) containing 30% poloxamer were separately dispensed into 90 mm Petri dishes and allowed to solidify. An additional 10 ml of liquid poloxamer‐broth medium containing at least 1·5 × 105 cfu/ml of one of the MDROs was dispensed over the original poloxamer layer to create a seeded overlay. All seeded poloxamer plates were allowed to solidify at 36°C for 4 minutes and then incubated under appropriate atmospheric conditions at 35°C for 4 hours to initiate growth. A 5 cm × 5 cm piece of the HFS dressing was saturated with 0.1% peptone water (∼4 ml) and aseptically transferred to a seeded poloxamer plate and plates were then incubated at 35°C for up to 48 hours. Following incubation, a corrected zone of inhibition (CZOI) was calculated for each challenge organism and photographs were taken. Each bacterium was tested against the HFS dressing on five occasions, and on one occasion in the absence of the dressing (control).
RESULTS
Simulated wound fluid model
All MDROs tested in this model were susceptible to the HFS dressing over a 7‐day test period (Figure 2). High populations of A. baumannii, C. difficile and ESBL‐producing bacteria (> 106 cfu/ml) were reduced by a factor of ∼100 000 within 48 hours in the presence of the HFS dressing, and sustained antimicrobial activity was evident thereafter, despite re‐inoculation of a heavy load of the respective MDRO on day 2. An approximate 100‐fold reduction in CA‐MRSA was observed in the presence of the HFS dressing in the first 48 hours, and no viable cells were detected (i.e. below the limit of detection) by day 7. The HF dressing (control) showed no antimicrobial activity.
Figure 2.
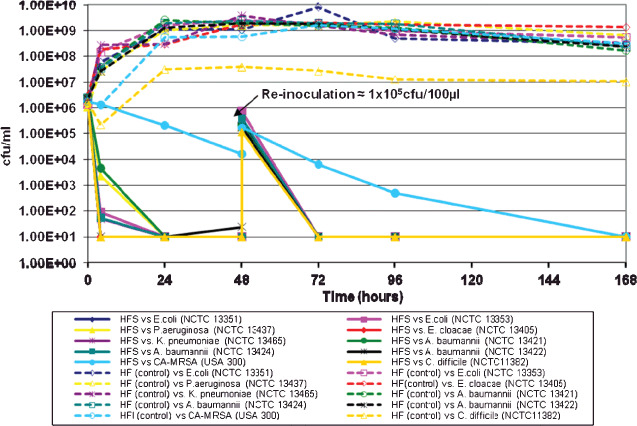
Simulated wound fluid model (SWF): antimicrobial action of the HFS dressing against MDROs over 7 days using the SWF model.
SCSW model
Growth of each MDRO, expressed as a percentage of the total surface area of the simulated shallow wound beneath the HFS dressing, is shown in Table 2 (column A), with photographic examples in figure 3A and B. In this in vitro model, the extent of bacterial growth (average of five tests) beneath the HFS dressing ranged from 0% (K. pneumoniae) to 8·6% (A. baumannii) of the total inoculated simulated shallow wound surface area; this compared with 100% growth of each organism in the absence of the HFS dressing.
Table 2.
SCSW model: percentage bacterial growth, bacteriostatic and bactericidal activity
| MDRO | A Mean % growth (in SCSW and SCWS model) (n = 5) | B Bacteriostatic activity (visual observation in SCWS model) | C Bactericidal activity (sub‐culture from the seeded agar in SCWS model) |
|---|---|---|---|
| A. baumannii (NCTC 13422) | 0·8 | No visible growth | No growth/Bactericidal |
| A. baumannii (NCTC 13424) | 8·6 | No visible growth | No growth/Bactericidal |
| A. baumannii (NCTC 13421) | 5·6 | No visible growth | No growth/Bactericidal |
| E. coli (ESBL) (NCTC 13351) | 3·1 | No visible growth | No growth/Bactericidal |
| E. coli (ESBL) (NCTC 13353) | 6·5 | Occasional creeping growth around the edge | No growth/Bactericidal* |
| P. aeruginosa (ESBL) (NCTC 13437) | 0·9 | No visible growth | No growth/Bactericidal |
| E. cloacae (ESBL) (NCTC 13405) | 0·6 | Sporadic colonies within agar | No growth/Bactericidal* |
| K. pneumoniae (ESBL) (NCTC 13465) | 0·0 | Sporadic colonies within agar | No growth/Bactericidal* |
| CA‐MRSA (USA 300) | 5·0 | No visible growth | No growth/Bactericidal |
| C. difficile (NCTC 11382) | 0·0 | No visible growth | No growth/Bactericidal |
MDRO, multidrug‐resistant organism; ESBL, extended‐spectrum beta‐lactamase; CA‐MRSA, community‐associated methicillin‐resistant Staphylococcus aureus; SCSW, simulated colonised shallow wound; SCWS, simulated colonised wound surface.
Controls without dressing showed 100% growth in the simulated shallow wound area (A) (n = 1).
*Initial observation of agar plates showed extensive areas of clearing with sporadic colonies which are likely to be cell variants within the population. Subsequent culture of a clear zone of agar beneath HFS dressing placement indicated bactericidal effect.
Figure 3.
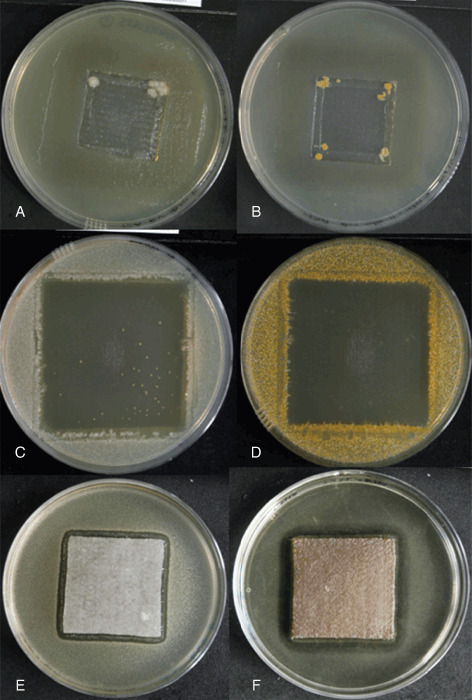
Examples of: growth of A. baumannii (NCTC 13421) (A) and community‐associated methicillin‐resistant Staphylococcus aureus (CA‐MRSA) (B) beneath the HFS dressing in the simulated colonised shallow wound (SCSW) model; bactericidal activity of the HFS dressing against K. pneumoniae (C; with the exception of persister cell variant colonies) and CA‐MRSA (D) in the simulated colonised wound surface (SCWS) model; zones of inhibition against A. baumannii (NCTC 13421) (E) and C. difficile (F) in the biofilm model.
SCWS model
The observed bacteriostatic and/or bactericidal effects of the HFS dressing against the MDROs are shown in Table 2 (columns B and C, respectively), with visual examples of the antimicrobial activity in Figure 3C and D. In this in vitro model, the HFS dressing killed each bacterium within the seeded agar layer (SCWS) directly beneath the dressing, as confirmed by subsequent sub‐culture from an area of agar where no visible growth was evident (bactericidal effect). However, with respect to two of the ESBL‐producing bacteria (E. cloacae and K. pneumoniae) sporadic colonies were visible within the seeded agar when dressings were removed after 48 hours (Figure 3C). Although confirmed as being the respective ESBL strains, colonial morphology was somewhat different to the predominant E. cloacae and K. pneumoniae populations, indicating the possibility of persister cell variants. In addition, occasional creeping growth of one E. coli strain (NCTC 13353) was observed on the outer edge of the zone of clearing (which is also likely to have been associated with a persister cell variant), but the HFS dressing was shown to be bactericidal against the parent population following sub‐culture. Confluent growth of each organism was observed on seeded agar plates in the absence of the HFS dressing (control), confirming the viability of each MDRO over the test period.
Biofilm model (poloxamer)
Anti‐biofilm activity of the HFS dressing was observed against all MDROs that were cultured in a biofilm‐like phenotype, with zones of inhibition ranging from 3·2 mm (E. cloacae) to 6·9 mm (P. aeruginosa) (Figure 4). Figure 3E and 3F shows example CZOIs for A. baumannii and C. difficile, respectively. Confluent growth of all organisms was observed in the absence of the HFS dressing.
Figure 4.
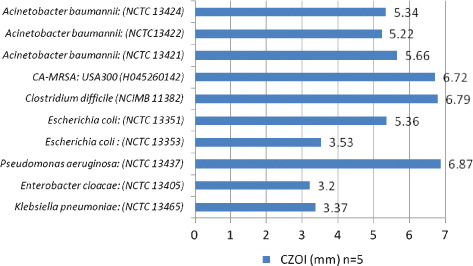
Poloxamer biofilm model: Inhibitory activity of the HFS dressing against MDROs (zone size in mm).
DISCUSSION
The MDROs investigated in this study, including C. difficile, are all known to colonise and potentially infect a variety of dermal wounds 5, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31. Since bacteria are capable of forming biofilm on living tissue (e.g. a wound bed) which enhances their tolerance to antimicrobial agents (21) and host immune cells, it was considered important to investigate the susceptibility of these MDROs to the selected silver‐containing wound dressing in their most natural and tolerant form, as well as their less natural free‐living form. Biofilm bacteria isolated from diabetic foot ulcers have been shown to be more tolerant to antibiotics than non‐biofilm bacteria (21). Local delivery of antimicrobial agents is considered to be of benefit in resolving infection, particularly when biofilm bacteria are present (12).
Combat and natural disaster‐related traumatic wounds are particularly susceptible to gross bacterial contamination, and the prevalence of MDROs has been reported 12, 14, 27, 29. Prompt and appropriate wound care is considered to be essential, involving aggressive surgical debridement to remove devitalised tissue and bacteria, cleansing and antimicrobial prophylaxis (both systemic and topical) 12, 14. Due to the nature of traumatic wounds such as combat‐related extremity injuries, significant anatomical and physiological derangement in local tissue is common (12). In view of the increasing consideration of topical antimicrobial agents in such wound types, Corrado (37) stated that a topical antimicrobial dressing must be selected according to the lesion characteristics, such as shape, size, level of exudate and presence of undermined tissue. Consequently, consideration of the physical, as well as the antimicrobial properties of a dressing is important to ensure that it interacts optimally with the wound environment. The technology of the HF and HFS dressings used in the current studies allows the dressings to rapidly hydrate in contact with wound fluid and form a cohesive gel that locks fluid and bacteria within the dressing, and forms intimate contact with a wound surface thus eliminating dead spaces where bacteria may proliferate. The excellent conformability of the gelled HFS dressing following hydration allows maximum exposure of silver ions to the colonised wound surface where the majority of bacteria reside, thus maximising the antimicrobial effect 33, 35.
Using a variety of stringent in vitro models designed to simulate clinical challenges as best possible, the kill rate, anti‐biofilm efficacy and effect of dressing conformability associated with the HFS dressing has been showed against a variety of MDROs. Irrespective of test method used, consistent antimicrobial activity of the HFS dressing was showed. However, in the SCWS in vitro model, despite bactericidal activity being observed against parent populations directly beneath the HFS dressing (i.e. extensive zones of clearing and no growth on sub‐culture), sporadic colonies of K. pneumoniae and E. cloacae, and occasional creeping growth of a strain of E. coli (NCTC 13353) were evident in the respective tests. In these cases, it is most likely that the colonies were silver‐tolerant persister cell variants of the parent and HFS dressing‐susceptible population. An association between persister cells and recalcitrance in chronic infections is known to exist, the process involving tolerant phenotypic variants of a normal cell population that become as susceptible as the original population when pressure from exposure to an antimicrobial agent falls (38). When each of the MDRO persister variants were sub‐cultured onto fresh agar in the absence of the HFS dressing, colonial morphology resembled that of the parent population, which is a recognised trait of persister cell phenotypes.
While it is acknowledged that only one of many currently available silver dressings was evaluated in this study, the antimicrobial activity of silver in different dressing types is known to be variable. This is largely influenced by the dressing technology and the by extent to which it is able to make the antimicrobial agent accessible to micro‐organisms, as has been showed in several in vitro studies 33, 35, 39. Although we consider that the stringent in vitro methodologies generated robust antimicrobial efficacy data against a wide spectrum of MDROs, studies to confirm the clinical impact of antimicrobial dressings would be beneficial. However, the HFS dressing has been shown to reduce wound bioburden, infection and pain in clinical studies 40, 41, 42.
Demonstrating the in vitro antimicrobial efficacy of the HFS dressing against a variety of MDROs may not only indicate potential clinical benefit in preventing or controlling endogenous wound infections where antibiotic options are limited and tolerated by biofilm phenotypes, but it may also act as an antimicrobial barrier to prevent the spread of such pathogens into the wider community. Hawkes et al. (43) stated that the goal for control of CA‐MRSA was to prevent its spread from colonised individuals to others in the community and advised that any draining lesions should be covered. The HFS dressing may provide infection control benefits in this respect, as well as providing antimicrobial prophylaxis in wounds that are at risk of infection by multidrug‐resistant bacteria.
REFERENCES
- 1. Alanis AJ. Resistance to antibiotics: are we in the post‐antibiotic era? Arch Med Res 2005;36:697–705. [DOI] [PubMed] [Google Scholar]
- 2. Schofield CB. Selective superbugs: MRSA, Acinetobacter baumannii, Clostridium difficile . Advance for Med. Lab Prof 2010;1–8. [Google Scholar]
- 3. Vandenesch F, Naimi T, Enright M, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. Community‐acquired methicillin‐resistant Staphylococcus aureus carrying Panton‐Valentine Leukocidin genes: worldwide emergence. Emerg Inf Dis 2003;9:978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuijper EJ, van Dissel JT. Spectrum of Clostridium difficile infections outside health care facilities. Canad Med Soc J 2008;179:747–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Towner KJ. Acinetobacter: an old friend, but a new enemy. J Hosp Inf 2009;73:355–63. [DOI] [PubMed] [Google Scholar]
- 6. Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin‐resistant Staphylococcus aureus (MRSA). Proc Nat Acad Sci 2002;99:7687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. Evolution of virulence in epidemic community‐associated methicillin‐resistant Staphylococcus aureus . Proc Nat Acad Sci 2009;106:5883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez F, Endimiani A, Hujer KM, Bonomo RA. The continuing challenge of ESBLs. Curr Opin Phamacol 2007;7:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonald LC. Trends in antimicrobial resistance in health care‐associated pathogens and effect on treatment. Clin Infect Dis 2006;42:65–71. [DOI] [PubMed] [Google Scholar]
- 10. Tenover FC, Tickler IA, Persing DH. Antimicrobial resistant strains of Clostridium difficile from North America. Antimicrob Agents Chemother 2012; (ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Micro Rev 2001;14:244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murray CK, Obremskey WT, Hsu JR, Andersen RC, Calhoun JH, Clasper JC, Whitman TJ, Curry TK, Fleming ME, Wenke JC, Ficke JR. Prevention of infections associated with combat‐related extremity injuries. J Trauma 2011;71 Suppl 2:S235–57. [DOI] [PubMed] [Google Scholar]
- 13. Sebeny PJ, Riddle MS, Petersen K. Acinetobacter baumannii skin and soft‐tissue infection associated with war trauma. Clin Infect Dis 2008;47:444–9. [DOI] [PubMed] [Google Scholar]
- 14. D’Avignon LC, Chung KK, Saffle JR, Renz EM, Cancio LC. Prevention of infections associated with combat‐related burn injuries. J Trauma 2011;71 Suppl 2:S282–9. [DOI] [PubMed] [Google Scholar]
- 15. Ullah F, Malik SA, Ahmed J. Antimicrobial susceptibility and ESBL prevalence in Pseudomonas aeruginosa isolated from burn patients in the North West of Pakistan. Burns 2009;35:1020–5. [DOI] [PubMed] [Google Scholar]
- 16. Swe Swe‐Han K, Coovadia Y. Prevalence of antibiotic‐resistant bacteria from adult ICUs and the burns unit at a larger tertiary hospital in Durban. Int J Infect Control 2010;v6:i2. [Google Scholar]
- 17. Idowu OJ, Onipede AO, Orimolade AE, Akinyoola LA, Babalola GO. Extended‐spectrum Beta‐lactamase Orthopedic Wound Infections in Nigeria. J Glob Infect Dis 2011;3:211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Empel J, Filczak K, Mrówka A, Hryniewicz W, Livermore DM, Gniadkowski M. Outbreak of Pseudomonas aeruginosa Infections with PER‐1 extended‐spectrum β‐Lactamase in Warsaw, Poland: further evidence for an International Clonal Complex. J Clin Micro 2007;45:2829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adegoke AA, Mvuyo T, Okoh AI, Jacob S. Studies on multiple antibiotic resistant bacteria isolated from surgical site infection. Sci Res Essays 2010;5:3876–81. [Google Scholar]
- 20. Gadepalli R, Dhawan B, Sreeniva V, Kapil A, Ammini AC, Chaudhry R. A clinico‐microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care 2006;29:1727–32. [DOI] [PubMed] [Google Scholar]
- 21. Zubair M, Malik A, Ahmad J, Rizvi M, Farooqui KJ, Rizvi MW. A study of biofilm production by gram‐negative organisms isolated from diabetic foot ulcer patients. Biol Med 2011;3:147–57. [Google Scholar]
- 22. Valencia I, Kirsner RS, Kerdel FA. Microbiologic evaluation of skin wounds: alarming trend toward antibiotic resistance in an inpatient dermatology service during a 10‐year period. J Am Acad Derm 2004;50:845–9. [DOI] [PubMed] [Google Scholar]
- 23. Roberts JC, Kreuger RL, Peak KK, Veguilla W, Cannons AC, Amuso PT, Cattani J. Community‐associated methicillin‐resistant Staphylococcus aureus epidemic clone USA300 in isolates from Florida and Washington. J Clin Micro 2006;44:225–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demling RH, Waterhouse B. The increasing problem of wound bacterial burden and infection in acute and chronic soft‐tissue wounds caused by methicillin‐resistant Staphylococcus aureus. J Burns Wounds 2007;7:86–98. [PMC free article] [PubMed] [Google Scholar]
- 25. Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 2009;7:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dallo SF, Weitao T. Insights into Acinetobacter war‐wound infections, biofilms and control. Adv Skin Wound Care 2010;23:169–74. [DOI] [PubMed] [Google Scholar]
- 27. Garzoni C, Emonet S, Legout L, Benedict R, Hoffmeyer P, Bernard L, Garbino J. Atypical infections in Tsunami survivors. Emerg Infect Dis 2005;11:1591–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huljev D. Characteristics of war wound infections during the Civil War in Croatia. EWMA J 2010;10:61–9. [Google Scholar]
- 29. Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008;21:538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kruszyńska DA, Mikucka A, Gospodarek E, Olszewski K, Kruczyński J, Matewski D. Toxin A‐producing Clostridium difficile as an aetiological factor of post‐traumatic wound infection. J Med Microbiol 2009;58:963–4. [DOI] [PubMed] [Google Scholar]
- 31. García‐Lechuz JM, Hernangómez S, Juan RS, Peláez T, Alcalá L, Bouza E. Extra‐intestinal infections caused by Clostridium difficile. Clin Microbiol Infect 2001;7:453–7. [DOI] [PubMed] [Google Scholar]
- 32. Walker M, Parsons D. Hydrofiber® Technology: its role in exudate management. Wounds UK 2010;6:31–8. [Google Scholar]
- 33. Bowler P, Jones S, Towers V, Booth R, Parsons D, Walker M. Dressing conformability and silver‐containing wound dressings. Wounds UK 2010;6:14–20. [Google Scholar]
- 34. Bowler PG, Jones SA, Walker M, Parsons D. Microbicidal properties of a silver‐containing hydrofiber dressing against a variety of burn wound pathogens. J Burn Care Rehabil 2004;25:192–6. [DOI] [PubMed] [Google Scholar]
- 35. Walker M, Jones S, Parsons D, Booth R, Cochrane C, Bowler P. Evaluation of low‐adherent antimicrobial dressings. Wounds UK 2011;7:32–45. [Google Scholar]
- 36. Percival SL, Bowler PG, Dolman J. Antimicrobial activity of silver‐containing dressings on wound microorganisms using an in vitro biofilm model. Int Wound J 2007;4:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corrado MD. Trauma wounds in modern warfare: local best practice in field setting. EWMA J 2010;10:55–8. [Google Scholar]
- 38. Fauvart M, De Groote N, Michiels J. Role of persister cells in chronic infections: clinical relevance and perspectives on anti‐persister therapies. J Med Micro 2011;60:699–709. [DOI] [PubMed] [Google Scholar]
- 39. Cavanagh MH, Burrell RE, Nadworny PL. Evaluating antimicrobial efficacy of new commercially available silver dressings. Int Wound J 2010;7:394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coutts P, Sibbald RG. The effect of a silver‐containing Hydrofiber® dressing on superficial wound bed and bacterial balance of chronic wounds. Int Wound J 2005;2:348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vanscheidt W, Lazareth I, Routkovsky‐Norval C. Safety evaluation of a new ionic silver dressing in the management of chronic ulcers. Wounds 2003;15:371–378. [Google Scholar]
- 42. Jurczak F, Dugre T, Johnstone A, Offori T, Vujovic Z, Hollander D. Randomised clinical trial of Hydrofiber® dressing with silver versus povidone‐iodine gauze in the management of open surgical and traumatic wounds. Int Wound J 2007;4:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hawkes M, Barton M, Conly J, Nicolle L, Barry C, Ford‐Jones EL. Community‐associated MRSA: superbug at our doorstep. Canad Med Soc J 2007;176:54–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


