Abstract
Lower leg ulcers are a serious and long‐term complication in patients with diabetes and pose a major health concern because of the increasing number of patients diagnosed with diabetes each year. This study sought to evaluate the clinical benefit of topical activated protein C (APC) on chronic lower leg ulcers in patients with diabetes. Twelve patients were randomly assigned to receive either APC (N = 6) or physiological saline (placebo; N = 6) in a randomised, placebo‐controlled, double‐blind pilot clinical trial. Treatment was administered topically, twice weekly for 6 weeks with final follow‐up at 20 weeks. Wound area was significantly reduced to 34·8 ± 16·4% of week 0 levels at 20 weeks in APC‐treated wounds (p = 0·01). At 20 weeks, three APC‐treated wounds had completely healed, compared to one saline‐treated wound. Full‐thickness wound edge skin biopsies showed reduced inflammatory cell infiltration and increased vascular proliferation following APC treatment. Patient stress scores were also significantly reduced following APC treatment (p < 0·05), demonstrating improved patient quality of life as assessed by the Cardiff Wound Impact Questionnaire. This pilot trial suggests that APC is a safe topical agent for healing chronic lower leg ulcers in patients with diabetes and provides supporting evidence for a larger clinical trial.
Keywords: Activated protein C, Diabetes, Ulcer, Wound healing
Introduction
Impaired skin wound healing in patients with diabetes is associated with a prolonged inflammatory reaction and delayed granulation tissue formation, angiogenesis and re‐epithelialisation. The current standard care for lower leg ulcers consists of debridement, daily moist dressing changes and off‐loading 1. A number of topical treatments and recombinant growth factors have been trialled and shows potential for healing ulcers in patients with diabetes, including recombinant human vascular endothelial growth factor (VEGF) 2, 3, 4, hepatocyte growth factor 5, basic fibroblast growth factor 5, 6, epidermal growth factor 7, 8 and platelet‐derived growth factor (PDGF)‐BB 9, 10. PDGF‐BB is the only growth factor to be FDA approved (Regranex® Gel, Healthpoint Biotherapeutics; Houston, TX), although it is not widely used and comes with a ‘black box’ warning because of an increased risk of mortality secondary to malignancy in patients treated with three or more tubes 11.
Activated protein C (APC) is a natural anti‐coagulant activated from its zymogen, protein C, by thrombin and thrombomodulin. Once activated, APC can engage the endothelial protein C receptor (EPCR) and elicit anti‐inflammatory, angiogenic, anti‐apoptotic and re‐epithelisation effects that may promote wound healing in lower leg ulcers in patients with diabetes 12, 13, 14, 15, 16, 17.
Our open‐label pilot study previously showed that APC reduces inflammation, enhances granulation tissue formation and promotes re‐epithelialisation in a small cohort of patients with chronic wounds of varying aetiology 12. In another open‐label pilot trial of non‐healing orthopaedic wounds, combined APC and topical negative pressure treatment increased granulation tissue, and reduced wound area and depth 17. Following treatment, wounds completely re‐epithelialised or provided sufficient closure to support surgical intervention.
This study was designed to assess the efficacy, safety and tolerability of topical APC on lower leg ulcers in patients with diabetes in a small randomised, placebo‐controlled, double‐blind pilot trial.
Patients and methods
Patients
The primary criteria for this study included established diagnosis of diabetes mellitus in accordance with the criteria of the American Diabetes Association 18 and the presence of a lower leg ulcer despite at least 6 months of standard wound care. Ulcers were defined as diabetic or venous, with ischaemic ulcers excluded by ankle brachial index. Individual patient and wound demographics are shown in Table 1. This study was approved by the Northern Sydney Health Human Research Ethics Committee (#0611‐218M) and performed in accordance with the Helsinki Declaration of 1975, as revised in 1983.
Table 1.
Individual demographic data for patients receiving topical saline or activated protein C (APC)
| Sex/age (years) | Wound duration (months) | Wound location/aetiology | Other wound care management |
|---|---|---|---|
| Saline | |||
| M/59 | 36 | Left lateral malleolus/neurotrophic | Three times/week polyurethane foam dressing and medical adhesive tape, monthly debridement |
| M/47 | 24 | Right lateral foot/neurotrophic | Three times/week nanocrystalline silver‐coated polyethylene net dressing, polyurethane foam dressing, weekly debridement |
| M/55 | 12 | Left plantar calcaneal foot/neurotrophic | Weekly debridement, absorbent dressing with strike through barrier, oral cephalexin for cellulitis |
| M/73 | 48 | Left posterior heel margin/neurotrophic | Angioplasty, weekly debridement, capillary dressing, absorbent dressing with strike through barrier |
| F/31 | 7 | Left plantar foot/venous | Weekly debridement, polyurethane foam dressing, CAM walker |
| F/90 | 6 | Right medial malleolus/venous | Cadexomer iodine and gauze |
| APC | |||
| M/66 | 48 | Right plantar heel/neurotrophic | Two times/week debridement, nanocrystalline silver‐coated polyethylene net dressing, polyethylene dressing, medical adhesive tape |
| M/82 | 12 | Left retrocalcaneal foot/neurotrophic | Three times/week polyurethane foam dressing, medical adhesive tape, weekly debridement |
| F/74 | 7 | Right lateral dorsal foot/venous | Angioplasty, weekly debridement, cadexomer iodine dressing, polyurethane foam dressing |
| M/65 | 12 | Right lateral plantar foot/venous | Two times/week debridement, cadexomer iodine dressing, polyurethane foam dressing |
| M/75 | 36 | Left medial malleolus/venous | Weekly debridement and polyurethane foam dressing with compression stockings |
| M/78 | 36 | Left lateral lower leg/neurotrophic | Weekly debridement, povidone iodine/polyethylene glycol viscose net and absorbent dressing with strike through barrier |
Treatment protocol and assessment
Recombinant human APC (Drotrecogin alfa activated or Xigris® (Eli Lilly; Indianapolis, IN; 400 µg/ml in sterile water) or physiological saline was applied topically beneath a sterile, occlusive polyurethane adhesive film (Tegaderm®, 3M; Maplewood, MN) as previously described 12. Treatment was applied twice weekly for 6 weeks, with post‐treatment follow‐ups at weeks 8, 12 and 20. An outline of the treatment and follow‐up protocol is depicted in Table 2. Wound area was assessed at each treatment and follow‐up visit with a digital measuring device (Visitrak, Smith and Nephew; North Ryde, Australia) as previously described 12. The primary outcome measure was percentage change in wound area at 20 weeks. The 20‐week assessment period was chosen as the end point for wound healing or non‐healing as it has previously been used to assess treatment of ulcers in patients with diabetes using topical PDGF gel 9, 10 or topical platelet‐derived wound healing formula (also called CT‐102 activated platelet supernatant) 19.
Table 2.
Outline of treatment and follow‐up protocol
| Visit no. | Week no. | Clinical procedures (in order) |
|---|---|---|
| 0 | 0 | Pre‐treatment: include or exclude according to criteria, obtain consent, physical examination, wound swab, skin biopsy, serum haematology and biochemistry |
| 1 | 1 | Treatment visits: wound history, |
| 2 | 2 | Bates‐Jensen tool, pain score, Cardiff |
| 3 | 3 | Wound Impact Questionnaire, |
| 4 | 4 | photograph wound, wound analysis with digital measuring device, debride, apply saline/activated protein C |
| 5 | 5 | |
| 6 | 6 | |
| 7 | 8 | Follow‐up visit: wound swab, skin biopsy, serum haematology and biochemistry |
| 8 | 12 | Follow‐up visits: Cardiff Wound Impact Questionnaire, photograph wound, wound analysis with digital measuring device |
| 9 | 20 |
Secondary outcomes included: (i) proportion of patients achieving >30% reduction in wound area at 2 weeks, (ii) clinical assessment of wound appearance including erythema and exudate utilising standard Bates‐Jensen wound scores at week 0, treatment and follow‐up visits, (iii) plasma functional protein C levels at weeks 1 and 8, (iv) serum glycated haemoglobin (HbA1C), C‐reactive protein (CRP) and plasma coagulation parameters, international normalised ratio (INR) and activated partial thromboplastin time (APTT) at week 0 and week 8, (v) histological analysis of skin biopsies at week 0 and week 8 and (vi) quality‐of‐life questionnaire using the Cardiff Wound Impact Questionnaire at week 0 and each treatment visit.
Histology
Four micrometer sections of 3‐mm skin punch biopsies from the wound edge were processed for histological analysis. Anatomical pathology of haematoxylin and eosin sections was reviewed by a dermatopathologist independent of the trial and blinded to each case.
Statistics
Data between treatment groups and time points was analysed by Fisher exact test, paired or unpaired parametric Student t‐test, non‐parametric Mann–Whitney U‐test, or repeated measures analysis of variance (ANOVA) with Newman‐Keuls post‐hoc analysis as appropriate. Statistical analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software; San Diego, CA) and p‐value <0·05 was considered as statistical significant.
Results
No significant differences were observed between saline‐ and APC‐treated patients with respect to sex, age, wound aetiology, wound duration or baseline wound area and volume. Wounds treated with saline and APC at week 0 and week 20 are shown in Figure 1A. Overall, three APC‐treated wounds completely healed by week 20, compared to one wound in the saline‐treated group. The mean wound area was significantly reduced in the APC‐treated group at week 20 compared to week 0 (immediately before treatment began, p = 0·01), while there was no significant difference in the saline‐treated group (p = 0·74) (Figure 1B). Lower leg ulcers in patients with diabetes treated with APC showed early reduction in wound size at week 2 of >15% in three patients and >30% in two patients (Figure 1C). In saline‐treated group, two patients demonstrated >15% reduction and one patient >30% at week 2.
Figure 1.
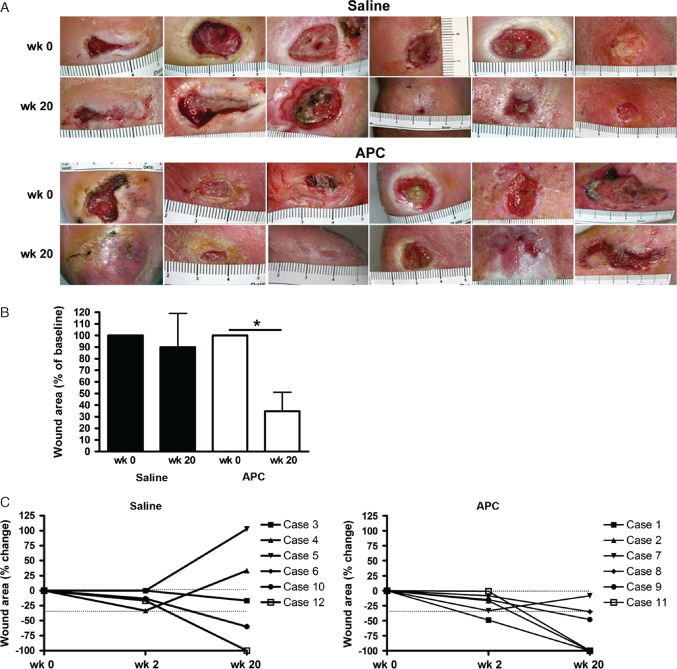
Photographs (A) and mean percentage change in wound area at week 20 (B) for saline‐ and activated protein C‐treated groups. Data expressed as mean ± standard error of the mean. *p < 0·05, paired Student t‐test. Ruler scale is in centimetre. (C) Percentage change in wound area indicating the improvement (negative) or deterioration (positive) at week 2 and week 20 compared to week 0 measurements. Dotted line shows 30% reduction (−30%) in wound size, with −100% denoting wound closure.
During the trial, the mean wound volume for the saline‐treated group significantly decreased to 82·6 ± 0·24% of week 0 levels at week 4 (p < 0·05) but returned to near week 0 levels by week 6 (Figure 2). In the APC‐treated group, wound volume was significantly reduced to 73·6 ± 2·2% at week 2 (p < 0·05) and continued to decrease, reaching 42·5 ± 7·8% of week 0 levels at week 6 (p < 0·001; Figure 2). At the final treatment (week 6), the mean wound volume between the saline‐ and APC‐treated groups was significantly different (p = 0·03).
Figure 2.
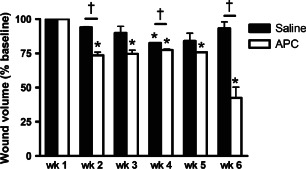
Wound volumes for saline‐ and activated protein C‐treated groups. Data expressed as mean ± standard error of the mean. *p < 0·05, repeated‐measures analysis of variance with Newman‐Keuls post‐hoc analysis; † p < 0·05, unpaired Student t‐test.
Bates‐Jensen wound scores provide a numerical indicator of wound health or degeneration assessing wound erythema, exudate, granulation tissue and re‐epithelialisation. Lower scores indicate healthier wounds, with a score of 13 indicating an intact healed wound. Mean scores for wounds at week 1 were 29·0 ± 1·8 and 26·5 ± 2·5 for saline‐ and APC‐treated groups, respectively, indicating mild‐moderate wound severity (Figure 3). Over the entire trial period (week 1 to week 20), there was no significant difference in mean scores for saline‐ or APC‐treated groups. However, when only the treatment period (week 1 to week 6) was considered, there was a significant decrease in the APC‐treated group from weeks 4–6 reaching 21·7 ± 2·0 at week 6 (p < 0·01; Figure 3). The mean score for the saline‐treated group at week 6 was 26·0 ± 1·4 and did not differ significantly from the APC‐treated group. The increase in mean score for the APC‐group at week 12 was due to one patient displaying wound deterioration (score of 45).
Figure 3.
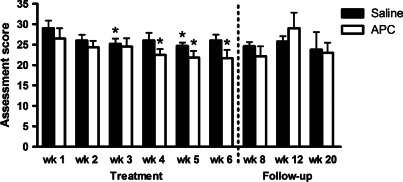
Bates‐Jensen assessment scores for saline‐ and activated protein C‐treated groups. Data expressed as mean ± standard error of the mean, where lower scores indicate wound improvement. * p < 0·05, repeated‐measures analysis of variance with Newman‐Keuls post‐hoc analysis.
Anatomical pathology of the biopsies at week 0 showed epidermal acanthosis and hyperkeratosis, and/or dermal neutrophilic debris and inflammatory cell infiltrate in 7 of 12 wounds (4/6 APC‐treatment group and 3/6 saline‐treatment group). At week 8, biopsies in the saline‐treated group displayed no change (2/6; 33%), reduced inflammatory cell infiltration (1/6; 17%) and increased vascular proliferation (1/6; 17%). Meanwhile, one patient displayed increased inflammatory cell infiltration with reduced vascular proliferation (1/6; 17%), and another patient displayed increased acanthosis and hyperkeratosis (1/6; 17%). APC‐treated group biopsies showed no change (1/6; 17%), reduced inflammatory cell infiltration (3/6; 50%) and increased vascular proliferation (2/6; 33%).
The Cardiff Wound Impact Questionnaire was used to assess the impact of chronic lower leg ulcers on patient quality of life and tolerability of the trial from week 0 to week 20. There were no significant differences in Cardiff Wound Impact scores between saline‐ and APC‐treated groups at week 0 or week 20 (Figure 4). At week 20, APC‐treated patients had significantly reduced stress associated with physical symptoms and daily living (p = 0·04) and social life when compared to week 0 (p = 0·02; Figure 4). Physical symptoms and daily living experience of APC‐treated patients also showed a trend towards improvement (p = 0·06). At the same time point, saline‐treated patients had no significant changes in quality of life compared to week 0 (Figure 4).
Figure 4.
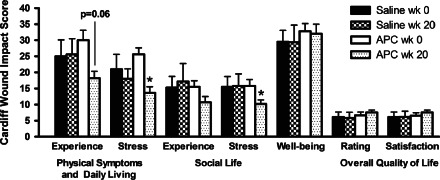
Cardiff Wound Impact Schedule scores from saline‐ and activated protein C‐treated groups at week 1 and week 20. Data expressed as mean ± standard error of the mean, where lower scores indicate improved quality of life. * p < 0·05, paired Student t‐test.
Clinical signs assessed for safety, included body temperature, wound erythema, exudate and infection, were normal in both saline‐ and APC‐treated groups. Laboratory parameters including serum functional protein C, HbA1c, CRP levels, and plasma coagulation parameters INR and APTT, did not differ significantly between treatment groups or time points.
Discussion
The chronic wound environment in patients with diabetes is complex and involves pathogenic changes in the epidermis and dermis, including inflammatory cell infiltration, neuropathy, impaired granulation tissue formation, angiogenesis and re‐epithelisation. Ideal treatments should have a wide‐ranging effect that targets multiple cell populations and healing processes. APC has a number of wound healing properties including reduction of inflammatory cytokines, tumour necrosis factor‐α, vascular cell adhesion molecule‐1 and endothelial selectin and inhibition of neutrophil infiltration causing suppression of inflammation 16, 20, 21; increase in the production of angiogenic factors VEGF, matrix metalloproteinase‐2 and Tie2, which promotes angiogenesis and creates stable blood vessels 13, 16, 22; stimulation of keratinocyte proliferation and stabilisation of epidermal barrier, required for re‐epithelialisation 13, 14, 23. These effects are largely mediated through EPCR, with PC and EPCR expression by keratinocytes evident in the skin of healthy individuals 13, 23 and in patients with diabetes 12.
Prior to its withdrawal from the market in late 2011, APC (marketed as Xigris® by Eli Lilly) had only been used clinically for the treatment of severe sepsis in adult patients with high mortality risk 24. Evidence is now emerging that APC is a potential treatment for chronic wounds of varying aetiologies 12, 17. In this study, APC reduced inflammatory cell infiltration, increased vascular proliferation and significantly reduced the wound size and volume of lower leg ulcers in patients with diabetes. Decreasing volumes of APC injected into the wound space as a measure of wound volume and a decrease in Bates‐Jensen scores over the treatment period were consistent with the observed improved wound healing by APC. Notably, our data suggests that APC improves wound health during the treatment period. However, the effect is not residual once APC treatment is terminated.
Further consideration for safety in our study was the effect of topical exogenous APC on plasma PC levels and coagulation parameters. Patients with diabetes have abnormalities of the coagulant system associated with a hyper‐coagulable state 25. Plasma PC, and routine plasma coagulation parameters, INR and APTT were normal. Thus, topical APC treatment in our study did not exacerbate the hyper‐coagulable state of patients with diabetes. Overall, the results show that APC treatment of lower leg ulcers in patients with diabetes improves wound healing, with a subsequent improvement in patient quality of life and no deleterious effect on patient safety.
The presence of unhealed lower leg ulcers in patients with diabetes can reduce their experience of daily and social activities compared to those with healed ulcers 26. The Cardiff Wound Impact Schedule has been validated in demonstrating the impact of chronic lower leg ulcers on patient health‐related quality of life 26, 27. Patients whose wounds were treated with APC reported less stress associated with physical symptoms and daily living, and social life compared to those treated with saline.
The method of application had the advantage of allowing the treatment to be in contact with the wound for 24 hours. However, this method was not ideal as it kept the wound in a wet environment which tended to cause maceration of local tissue. Future studies should investigate alternative application methods. Another limitation of this study was the small sample size. While a larger clinical trial is clearly required, this pilot study indicates that bi‐weekly application of topical APC is a potentially effective, safe and well‐tolerated treatment for chronic lower leg ulcers in patients with diabetes. By promoting wound healing, APC may reduce the number of lower extremity amputations in the population with diabetes thereby improving patient health‐related quality of life.
References
- 1. Mulder G, Tallis AJ, Marshall VT, Mozingo D, Phillips L, Pierce GF, Chandler LA, Sosnowski BK. Treatment of nonhealing diabetic foot ulcers with a platelet‐derived growth factor gene‐activated matrix (GAM501): results of a phase 1/2 trial. Wound Repair Regen 2009;17:772–9. [DOI] [PubMed] [Google Scholar]
- 2. Taub PJ, Silver L, Weinberg H. Plastic surgical perspectives on vascular endothelial growth factor as gene therapy for angiogenesis. Plast Reconstr Surg 2000;105:1034–42. [DOI] [PubMed] [Google Scholar]
- 3. Hanft JR, Pollak RA, Barbul A, van Gils C, Kwon PS, Gray SM, Lynch CJ, Semba CP, Breen TJ. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care 2008;17:30–2, 34–7. [DOI] [PubMed] [Google Scholar]
- 4. Bao P, Kodra A, Tomic‐Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshida S, Matsumoto K, Tomioka D, Bessho K, Itami S, Yoshikawa K, Nakamura T. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors 2004;22:111–9. [DOI] [PubMed] [Google Scholar]
- 6. Richard JL, Parer‐Richard C, Daures JP, Clouet S, Vannereau D, Bringer J, Rodier M, Jacob C, Comte‐Bardonnet M. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot, randomized, double‐blind, placebo‐controlled study. Diabetes Care 1995;18:64–9. [DOI] [PubMed] [Google Scholar]
- 7. Tsang MW, Wong WK, Hung CS, Lai KM, Tang W, Cheung EY, Kam G, Leung L, Chan CW, Chu CM, Lam EK. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 2003;26:1856–61. [DOI] [PubMed] [Google Scholar]
- 8. Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg 2006;56:394–8; discussion 9–400. [DOI] [PubMed] [Google Scholar]
- 9. Steed DL. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg 1995;21:71–8; discussion 9–81. [DOI] [PubMed] [Google Scholar]
- 10. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo‐controlled double‐blind study. Diabetes Care 1998;21:822–7. [DOI] [PubMed] [Google Scholar]
- 11. Ortho‐McNeil Pharmaceuticals Inc . Regranex Gel 0.01% (becaplermin) prescribing information including boxed warning and medication guide. URL http://www.regranex.com/PI_Full_Versionpdf. Oct. 2011 [Accessed on 19 September 2012].
- 12. Whitmont K, Reid I, Tritton S, March L, Xue M, Lee M, Fulcher G, Sambrook P, Slobedman E, Cooper A, Jackson C. Treatment of chronic leg ulcers with topical activated protein C. Arch Dermatol 2008;144:1479–83. [DOI] [PubMed] [Google Scholar]
- 13. Xue M, Campbell D, Sambrook PN, Fukudome K, Jackson CJ. Endothelial protein C receptor and protease‐activated receptor‐1 mediate induction of a wound‐healing phenotype in human keratinocytes by activated protein C. J Invest Dermatol 2005;125:1279–85. [DOI] [PubMed] [Google Scholar]
- 14. Xue M, Thompson P, Kelso I, Jackson C. Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP‐2 activity in cultured human keratinocytes. Exp Cell Res 2004;299:119–27. [DOI] [PubMed] [Google Scholar]
- 15. Julovi SM, Xue M, Dervish S, Sambrook PN, March L, Jackson CJ. Protease activated receptor‐2 mediates activated protein C–induced cutaneous wound healing via inhibition of p38. Am J Pathol 2011;179:2233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jackson CJ, Xue M, Thompson P, Davey RA, Whitmont K, Smith S, Buisson‐Legendre N, Sztynda T, Furphy LJ, Cooper A, Sambrook P, March L. Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Repair Regen 2005;13:284–94. [DOI] [PubMed] [Google Scholar]
- 17. Wijewardena A, Vandervord E, Lajevardi SS, Vandervord J, Jackson CJ. Combination of activated protein C and topical negative pressure rapidly regenerates granulation tissue over exposed bone to heal recalcitrant orthopedic wounds. Int J Low Extrem Wounds 2011;10:146–51. [DOI] [PubMed] [Google Scholar]
- 18. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–97. [DOI] [PubMed] [Google Scholar]
- 19. Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double‐blind trial in healing chronic diabetic foot ulcers. CT‐102 activated platelet supernatant, topical versus placebo. Diabetes Care 1992;15:1598–604. [DOI] [PubMed] [Google Scholar]
- 20. Schmidt‐Supprian M, Murphy C, While B, Lawler M, Kapurniotu A, Voelter W, Smith O, Bernhagen J. Activated protein C inhibits tumor necrosis factor and macrophage migration inhibitory factor production in monocytes. Eur Cytokine Netw 2000;11:407–13. [PubMed] [Google Scholar]
- 21. Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem 2001;276:11199–203. [DOI] [PubMed] [Google Scholar]
- 22. Minhas N, Xue M, Fukudome K, Jackson CJ. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J 2010;24:873–81. [DOI] [PubMed] [Google Scholar]
- 23. Xue M, Campbell D, Jackson CJ. Protein C is an autocrine growth factor for human skin keratinocytes. J Biol Chem 2007;282:13610–6. [DOI] [PubMed] [Google Scholar]
- 24. Bernard GR, Vincent J‐L, Laterre P‐F, LaRosa SP, Dhainaut J‐F, Lopez‐Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr, Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001;344:699–709. [DOI] [PubMed] [Google Scholar]
- 25. Gabazza EC, Takeya H, Deguchi H, Sumida Y, Taguchi O, Murata K, Nakatani K, Yano Y, Mohri M, Sata M, Shima T, Nishioka J, Suzuki K. Protein C activation in NIDDM patients. Diabetologia 1996;39:1455–61. [DOI] [PubMed] [Google Scholar]
- 26. Jaksa PJ, Mahoney JL. Quality of life in patients with diabetic foot ulcers: validation of the Cardiff Wound Impact Schedule in a Canadian population. Int Wound J 2010;7:502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition‐specific questionnaire to assess health‐related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


