Abstract
A complex compound (immune (’IM’) fraction) from colostrum‐derived whey was investigated for its potential wound healing properties. One of its most intriguing in vitro abilities was to significantly inhibit the contraction of collagen gel while fibroblast density remained as in control gels. This antagonist effect was dose dependent and fibroblasts in these gels did not exhibit any stress fibres. Subsequently, in vivo studies have been conducted in two wound models in guinea pigs. Daily application on full‐thickness wounds of a liquid formulation of the IM fraction (first model) significantly delayed wound closure by contraction compared to what normally occurred in control wounds. In another wound model, a gel formulation of the IM fraction was applied on scar tissues, which resulted in a minimised residual scar on 5/8 wounds compared to corresponding wound areas seen prior to treatment. Conversely, most control wounds exhibited scar tissue from which 3/8 resembled hypertrophic scar tissue. Wound tissue treated with IM fraction covered a significantly larger area than in the control wounds, whereas the collagen deposition was unchanged as in the presence of α‐smooth muscle actin. Thus, IM fraction may act by modulating the contraction rate and wound remodelling.
Keywords: Collagen gel contraction, Colostrum‐derived whey, Remodelling, Scar tissue
INTRODUCTION
Cutaneous wound healing is a complex phenomenon that involves different steps leading towards permanent repair scar tissue, whereas, in comparison, tissue regeneration may occur in foetal wounds as a result of many factors such as a very limited inflammatory reaction (1). In many cases, scar tissue is considered normal and relatively accepted in a hidden area. However, under some circumstances, excessive scarring representing fibrotic skin conditions such as hypertrophic scar tissue and keloids (the latter extends beyond the initial wound boundary) appears unsightly. In addition, after a burn injury, scar tissue is often accompanied by wound contractures that raise many problems such as restricted joint mobility and psychosocial issues.
The causes of excessive scarring have been attributed to genetic predisposition (e.g. darker skin with a prevalence of 4–6% in the Black population and in Asians and Hispanics), delayed epithelialisation (e.g. burn wounds, laser resurfacing, dermabrasion), persistent inflammation, age (less excessive scar tissue in ageing population) and persistence of tension on wound closure 2, 3.
The management of tissue repair has focused on the use of silicone gels or sheets (i.e. compression therapy) that are well accepted to limit wound contracture after complete covering of burn wounds. Conventional treatments consisting of topical applications of corticosteroids and compression therapy have also been used. Despite this, these patients have to turn to plastic and reconstructive surgery to improve scar tissue aesthetics. In addition, hypertrophic scars and keloids can be difficult to treat. Surgical excision is one of the most efficient therapies despite some recurrence (4). Alternately, different products have been investigated to minimise or reduce scar tissue production as well as the size of hypertrophic scars and keloids, and particularly their recurrence after surgery. Different products have been administered locally such as imiquimod, resiquimod, verapamil, tacrolimus, 5‐fluorouracil, bleomycin, retinoids, vitamin E, interleukin‐10 (IL‐10), mannose‐6‐phosphate and interferon from which some have shown reducing recurrence rates associated with the removal of the scar. More recently, transforming growth factor‐β3 (TGF‐β3; Avotermin®) has been reported to be successful in a clinical trial (5). Other therapies such as cryosurgery, radiotherapy and lasers have been proposed. However, research is still in progress towards reducing scar tissue formation with targeting products (i.e. antagonists) based on biological and physiological knowledge of connective tissue repair formation (5).
Colostrum is the first protein‐rich fluid secreted by the mammary glands in the first few days just after giving birth. Colostrum is rich in antimicrobial peptides that provide passive immunity to protect the newborn from infections. It also promotes the development of the immune system and facilitates the growth and immune maturation of many tissues due to an important source of growth factors, in particular the insulin‐like growth factor (IGF) family, which is known to contain potent mitogens and cell survival factors. IGFs also play an important role for successful wound healing during the early phase 6, 7. Colostrum can be processed to extract and concentrate bioactive compounds such as growth factors and antimicrobial peptides, but it is also rich in casein that masks the bioactive components. A procedure has recently been developed, implicating sequential steps to obtain a liquid form of a casein‐depleted colostrum whey fraction (called the ‘IM’ fraction or IMf) that contains effective amounts of bioactive growth factors, specifically IGF‐1 (43 ng/ml), but not of any TGF‐β isoforms, and 70% peptides of less than 500 Da which have not been fully characterised (data not published).
The aim of the present study was to evaluate the wound healing properties of the IMf, and particularly the late phase of scar tissue and remodelling, based on in vitro results of fibroblast‐mediated collagen gel contraction assay.
MATERIALS AND METHODS
In vitro assessments
Preparation of the IMf.
Fat was separated from raw frozen bovine colostrum using a milk separator. Caseins were then precipitated with the addition of double strength microbial rennet (Danisco, Madison, WI). The curds were separated by centrifugation to form colostrum whey at 65°C. After removing casein fines from the colostrum whey with a milk separator, the colostrum whey was then heated to 50°C and submitted to an ultrafiltration step using a spiral wound membrane with a molecular weight cut‐off of 10 kDa (Parker Process Advanced Filtration, Oxnard, CA). The ultrafiltration permeate was collected and acidified to pH 3·2. The acidified permeate was then concentrated by nanofiltration resulting in an IMf solution containing a 2% w/v average of proteins (stock solution). In addition, a crude colostrum extract (‘crude’) free of casein was collected prior to the whey heating stage. The crude extract was then used as a control component in the in vitro investigations.
For each experiment, the IMf stock solution was sterilised by gamma radiation (2 kGray) because the membrane filtration sterilisation resulted in clumsy precipitates and loss of bioactivity. The pH of the IMf stock solution (kept at 4°C in the dark until used) was adjusted with sterile 1 N NaOH and then diluted in culture medium for the in vitro studies and in phosphate buffered solution (PBS) for the in vivo studies. The crude extract that came in powder form was dissolved in culture medium at a stock solution of 10 mg/ml (corresponding at a 1:1 dilution of the IMf).
Fibroblasts.
Human fibroblasts were derived from foreskins, after written informed consent which was approved by the Centre Hospitalier Universitaire de Québec (CHUQ)'s Ethics Committee. Cells were used at passages 10–25. They were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma Chemical Co., St Louis, MO) supplemented with 5% foetal bovine serum (FBS) and containing antibiotics and antifungals. For the cultures in the presence of the compounds to be tested, the concentration of FBS was set at 0·5%. Control culture conditions consisted of media with 5% and 0·5% FBS. Cultures were performed in 5% CO2 and humid atmosphere.
Collagen gel contraction assay.
Fibroblasts were mixed in a collagen solution prior to gel formation at a density of 5 × 104 cells per 500 µl gel in each well of 24‐multiwell plates. Collagen gels were made by mixing a rat tail tendon collagen solution (initial concentration: 3·5 mg/ml) with the cell‐containing medium solution at a ratio of 1:2·5 (final collagen concentration: 1·4 mg/ml). One millilitre of the cell‐containing medium solution was composed of 200 µl DMEM 5× without NaHCO3, 100 µl FBS, 100 µl NaHCO3 (0·26 M), 4 µl NaOH (1 N), 20 µl ddH2O and 176 µl of cell suspension as previously described (8). Gels were formed after 15‐minute incubation. After 2–4 hours of incubation in medium with serum (5%)‐supplemented medium, medium was changed, cell cultures rinsed and the different compounds to be tested were diluted in culture medium supplemented with 0·5% FBS. Compounds were introduced daily for four consecutive days. Triplicate samples were analysed and the experiment was repeated. By 24 hours the gels were released from wells and left floating. Medium was changed at day 2. After 4 days, images of the gels were taken and gel surfaces were quantified using an image analysis system (Image J, Scion Corporation, Frederick, MD).
Measurement of mitochondrial activity (XTT assay).
To investigate the activity of fibroblasts in the collagen gel, an XTT (2,3‐bis 2 methoxy‐4‐nitro‐5‐sulfopheny‐2H‐tetrazolium‐5‐carboxy‐ anilide inner salt) assay was performed according to the manufacturer's procedure (TOX2, Sigma‐Aldrich Canada Ltd, Oakville, Ontario, Canada). After a 4‐day culture period, gels were rinsed in PBS (×2; 15 minutes). A PBS solution of XTT (1 mg/ml) was mixed with a PBS solution of phenazinemethosulphate and incubated at 37°C for 1 hour. Optical densities were then measured with a fluorometer (Spectra Max 340Pe Molecular Devices, Sunnyvale, CA) set at 450‐nm absorbance, and the values were subtracted from the blank values. For each condition, cultures were performed in triplicate. Adsorbances were correlated to cell numbers using correlation equations obtained from standard curves of known dilutions of live fibroblasts (as references).
Visualisation of actin filaments (F‐actin) and α‐SM actin.
At 4 days of culture, cell‐embedded gels were washed in PBS and then fixed either in 3·7% formaldehyde solution in PBS (F‐actin filaments) for 3–4 hours or in 100% acetone [α‐smooth muscle (SM) actin] for 2 hours, followed by several rinses in PBS. F‐Actin was shown using an Alexa‐fluor phallacidin (Molecular Probes, Invitrogen, Burlington, Ontario, Canada) in fibroblasts embedded in gels that were previously permeabilised with a 0·1% Triton‐X‐100 in PBS. α‐SM actin was shown by staining directly the fibroblast‐embedded gels with the Cy3‐conjugated monoclonal anti‐α‐SM actin (Sigma‐Aldrich, St Louis, MO) followed by rinsing in PBS. Then, the samples laid on slides and embedded in a mounting fluid (glycerol–Tris) were directly observed under fluorescence at an excitation of 346 nm and an emission of 442 nm using a filter and a long‐distance working condensator.
In vivo assessments
The animal studies were performed after approval by our local institutional Animal Care Committee and in agreement with the guidelines of the Canadian Council for Animal Care. All animals received human care.
Two distinct full‐thickness wound models were developed to investigate the effects of the IMf in female guinea pigs (Hartley, Charles River, Montréal, Quebec, Canada). In the first model, wounds were left open and treated immediately after wounding to assess the early phases of wound healing. The second model considered the effect of a topical treatment on scar tissue modelling only after complete epithelialisation.
First animal model (‘treated immediately’).
One full‐thickness wound (2·5 × 2 cm) was performed on each flank of each animal (n = 18), close to the spinal cord. Under sterile surgical conditions and anaesthesia, skin including the subcutaneous tissue was excised down to the subcutaneous muscle that was left in place. Any dermal connective and fat tissues left after excision were carefully removed, and haemostasis was insured by a temporary compression. The two wounds created on each animal were treated with the same treatment to avoid any interference. Different dilutions of IMf were applied onto those wounds on a daily basis for 23 days. Animals from the control groups received PBS, the buffered solution used to dilute IMf. Three animals (six wounds) were used for each condition. Wounds were then covered with a transparent self‐adhesive polyurethane film (Tegaderm®, 3M), followed by a sterile gauze. The trunk of each animal was wrapped with a self‐adhesive elastic bandage (Elastoplast®) to maintain the dressing in place and to avoid any trauma. The bandage was left in place and periodically changed every 3–5 days. A dorsal zip‐like opening was made in the dressing in order to introduce the compounds onto the wounds on a daily basis. For each group, one animal was euthanised at day 15 and the other two at day 23.
Second animal model (‘treated once closed’).
Two full‐thickness wounds (1 × 1 cm) on each flank of each animal were created following the same surgical and dressing procedures as described for the first model, except that the polyurethane film was directly applied onto wounds without any treatment. The dressing was left secured in place for 5 days, replaced for an additional five days and the wounds were then left to heal spontaneously without any dressing. By day 20 postwounding, epithelialisation was almost completed (≥95%) in most cases, and the treatments started. A concentrated form of the IMf stock formulation (cIM) was produced by processing IMf to a speed‐vacuum concentration (<100 mTorr at room temperature) resulting in a gel formulation that can easily be applied like an unguent. cIM was then sterilised by gamma radiations. A control gel formulation was derived from a 2% w/v bovine serum albumin (BSA) in PBS solution, which was concentrated (cBSA) in similar conditions by speed vacuum and then sterilised by gamma radiation.
On each animal, two wounds on one flank were treated with the cIM and two others with cBSA as control wounds on the other flank. The compound to be applied was alternately selected on the left or right flank distributed amongst a total of eight guinea pigs (i.e. eight wounds per condition). The formulations were applied by ‘massage’ over the closed wounds that were then left free of dressing. A home‐made Elizabeth's collar was secured in place on each animal immediately after application of the compound. The collar was left in place for at least 1 hour. The treatment was applied every day for 14 days.
Quantification.
In both models, quantification of the macroscopic views of wound surface areas was performed directly on photographs using the Image J program. Photographs of the wounds were taken periodically at the same focus distances during the treatment phase; a measuring tape was used to scale the wounds. At the end of the experiments, animals were euthanised. Wounds and the surrounding skin were excised and fixed in formaldehyde for histological investigations. Specimens were embedded in paraffin and sectioned through the middle of the wound and its margins in order to reach the wound's largest size (perpendicular to the crania‐caudal axis). Tissue sections were stained with haematoxylin‐eosin saffron (HES). Other sections were stained with picro‐sirius red to observe the organisation of collagen fibres in wound tissues under polarised light. Unstained sections were also processed to identify α‐SM actin in scar tissues using the Cy3‐conjugated monoclonal anti‐α‐SM actin. As the specimens were fixed in formaldehyde, an antigen retrieval buffer was used (5:1 solution of 0·1 M sodium citrate/0·1 M citric acid at pH 6·0) followed by heating slides at 55°C for 5 minutes in a microwave. Slides were then rinsed in PBS and mounted with a fluorescent mounting fluid. Observation was performed as described earlier in the in vitro investigation.
Digitalised images of wound sections were captured with a video camera (Q‐Imaging, Burnaby, British Columbia, Canada). Using the Image J program, the surfaces occupied by wound tissues (wound area) were measured by tracing their contours. The wound tissue thickness was also measured following three lengths perpendicular to the wound surface and limited at the bottom of the wound by the subcutaneous muscle or the fatty tissue depending on the structures identified below the wound tissue. A ratio of epidermal covering was calculated after measuring the length of non epithelialised wound surface reported to the entire length of the wound surface (independently of the epidermis thickness). In addition, the surface occupied by birefringent collagen fibres on picro‐sirius‐stained sections was measured in the wound tissue and then related to the total surface wound tissue area in each tissue section.
Statistical analyses
Significant in vitro differences were determined by the one‐way analysis of variance (ANOVA) test using the Student–Newman–Keuls method as a multiple pairwise comparison procedure. The P value was set at ≤0·05. In the in vivo studies, one‐way ANOVA was also used with the Fisher's least significance distance (LSD) method as a multiple pairwise comparison procedure. The P value was set at ≤0·05. In certain conditions, the Student's t‐test was used to compare two sets of data with a P value at 0·001.
RESULTS
In vitro studies
Collagen gel released from the wells rapidly contracted in the presence of medium supplemented with 5% FBS and at a lesser degree with 0·5% in a significant manner (Figure 1). In the presence of decreasing dilutions of the IMf, the gel was less contracted than the two control media (0·5% and 5% FBS) particularly at low dilutions (Figure 1A). The areas of the gels measured at day 6 were significantly increased (P < 0·05) in the presence of low dilutions ranging from 1:0·5 to 1:10 (v/v of IMf/culture medium) as compared to the control culture in the presence of 5% FBS (Figure 1A). The surface area values at 1:1 and 1:0·5 dilutions were also significantly different to all the other conditions including the two control media. In the presence of increasing concentrations of the crude extract, the contraction occurred in a similar manner as found in the positive control (0·5% FBS) but significantly lower than the negative controls (5% FBS), except for the highest concentration (10 mg/ml) (Figure 1B). The contraction induced by the crude extract was significantly higher than that in the presence of the IMf.
Figure 1.
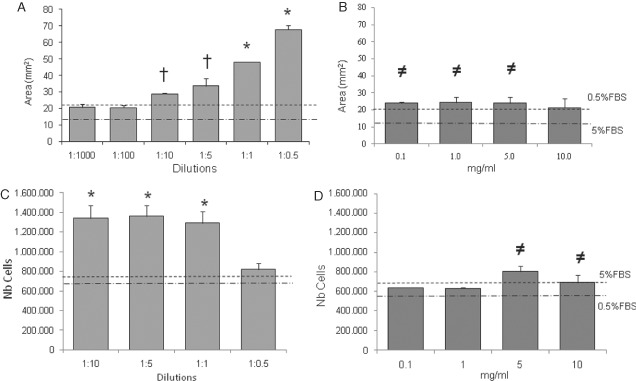
Fibroblast‐populated collagen gel contraction assay. The surface areas occupied by the collagen gel were measured after 6 days in the presence of decreasing dilutions of IMf (A) and increasing concentrations of crude extract (B). All conditions were compared to those in the presence of 0·5% and 5% foetal bovine serum (FBS) (dashed lines). *Significantly different compared to the other conditions; and † or ≠ compared to 5% FBS. The number of cells present in the contracted collagen gel was also determined by the 2,3‐bis 2 methoxy‐4‐nitro‐5‐sulfopheny‐2H‐tetrazolium‐5‐carboxyanilide inner salt (XTT) assay in the presence of IMf (C) and crude extract (D). *Significantly different compared to the other conditions; and ≠ compared to 0·5% FBS. Means ± SEM are presented; n = 6.
Fibroblast activity in collagen gel as measured indirectly by an XTT assay was close to the activity measured in the two control medium conditions in the presence of the lowest dilution (1:0·5) of IMf (Figure 1C). In higher IMf dilutions (1:10–1:1), the activities of fibroblasts were significantly increased in comparison with the other conditions. In the presence of 0·1 and 1 mg/ml crude extract, the number of cells present in gels was close to those in the control media, while at 5 and 10 mg/ml a significant increase was observed in comparison with those in the presence of 0·5% FBS (Figure 1D).
Fibroblasts exposed to low dilutions of IMf exhibited largely aggregated and elongated F‐actin compared to those grown in the presence of low serum as a control (Figure 2A). The formation of stress fibres was observed particularly in the fibroblasts grown in 5% FBS and with high dilutions of IMf. Cells stained for α‐SM actin presented diffuse fluorescence in the presence of IMf or 0·5% FBS compared with the cells in the presence of 5% FBS (Figure 2B). α‐SM actin in the presence of the crude extract appeared similar to that in 0·5% FBS (data not shown).
Figure 2.
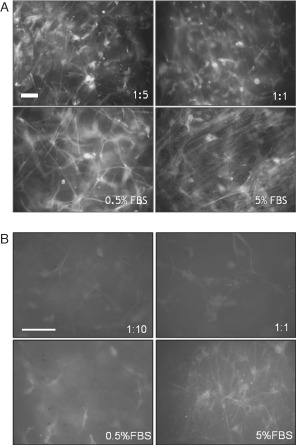
Actin distribution in fibroblasts embedded in collagen gel after 4 days. F‐actin stained with Alexa‐fluor phallacidin (A) and α‐smooth muscle (SM) actins stained with a Cy3‐labelled antibody (B) were observed in the presence of IMf at various dilutions and in the two controls. Bar = 100 µm.
In vivo studies
Treatment of open wounds over a 23‐day period (first model) resulted in less wound contraction when IMf (1:0·5) was applied compared to wounds treated with PBS (Figure 3A). Measurements of the wound contraction did not show any significant difference compared to control wounds after treatment with different dilutions of IMf (from 1:1000 to 1:10). A significant decrease in wound contraction was observed only by day 14 at 1:1 dilution of IMf and by day 23 at the lowest dilution (1:0·5), compared to their respective control wounds (Figure 3B). On histological sections (day 23), the areas occupied by the wound tissues were significantly higher after treatment with 1:1 IMf compared to control wounds, but not with the other conditions (data not shown). However, there was no significant difference in the wound tissue thickness, epidermal covering and deposition of birefringent collagen.
Figure 3.
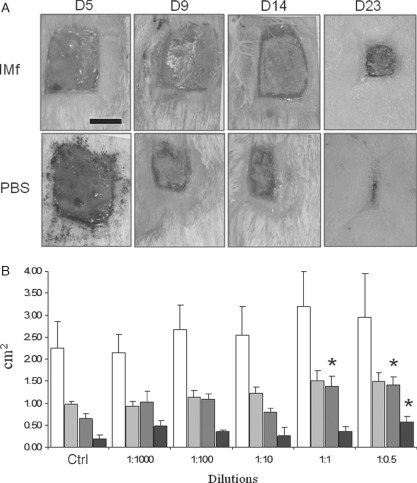
Full‐thickness wounds (2 × 2·5 cm) treated immediately after wounding (first wound model). (A) Representative images of the wounds at different periods of treatment with IMf (1:1 dilution) and compared to control wounds treated with the vehicle phosphate buffered solution (PBS) (bar = 1 cm). (B) Wound surface areas (cm2) as measured on days 5 (white bars), 9 (light grey bars), 14 (dark grey bars) and 23 (dark bars) (means ± SEM are presented; n = 18 up to day 15, and n = 12 up to day 23; *significantly different compared to control conditions at respective time periods).
Wounds only treated after closure (second model) appeared contracted following a four‐point star‐like shape at day 0 of treatment, with very few unclosed tiny areas in 4/16 wounds (Figure 4A). Treatment of these wounds for 14 days with the cIM resulted in wounds that had spread out in the form of a slight residue, and had lost their four‐point star shape in 5/8 wounds (Figure 4A). Four out of eight wounds presented a visible scar in the cIM‐treated wound tissue. Wounds treated with cBSA had less spreading except for 1/8 and in all cases obvious scar tissue was present. The surfaces occupied by the residual wound areas and the scar tissues themselves were traced for quantification (Figure 4B). The area occupied by the obvious scar tissue was then subtracted from the total area for each wound. These values show a significant difference in the residual wound areas between the cIM‐treated wounds and those treated by cBSA (Figure 4C).
Figure 4.
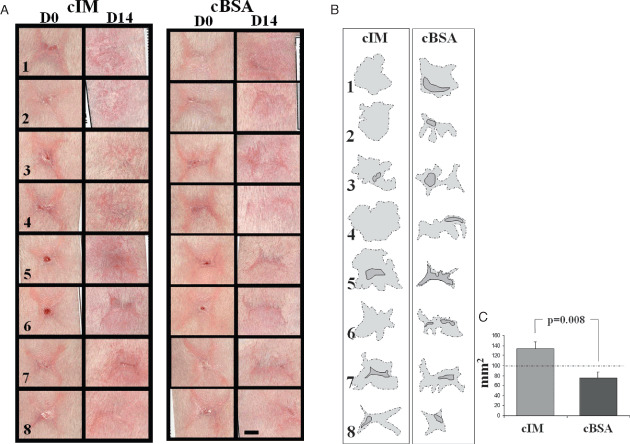
Full‐thickness wounds (1 × 1 cm) treated after complete epithelialisation (second wound model). (A) Macroscopic images of the eight wounds (numbered) as they appeared after spontaneous healing during 20 days (D0) and then treated with either cIM or concentrated bovine serum albumin (cBSA) for another 14 days (D14) (bar = 5 mm). (B) The margins of residual wounds (dashed lines filled with light grey) and obvious scar tissue (straight lines filled with dark grey) were traced from the images in (A) (columns D14). (C) Surfaces occupied by light grey areas from (B) and subtracted from those in dark grey in wounds treated with cIM and cBSA (means ± SEM are presented; n = 8).
In addition, haematoxylin‐phloxine‐saffron (HPS)‐stained histological sections show larger scar tissue in the wounds treated by cIM compared with those treated by cBSA. The surface areas were significantly different between the cIM‐ and cBSA‐treated wounds (Figure 5A). Epidermal covering was present in both conditions. Staining with α‐SM actin showed close distributions within the scar tissue between the cIM‐ and cBSA‐treated wounds and both present important angiogenesis (Figure 5B). After picro‐sirius red staining and observation under a polarised light, wound collagen fibre contents exhibited similar patterns in cIM‐ and cBSA‐treated wounds (Figure 5C). The area fractions of birefringent collagen in wound scar tissues were also calculated and no significant difference was found (Figure 5C).
Figure 5.
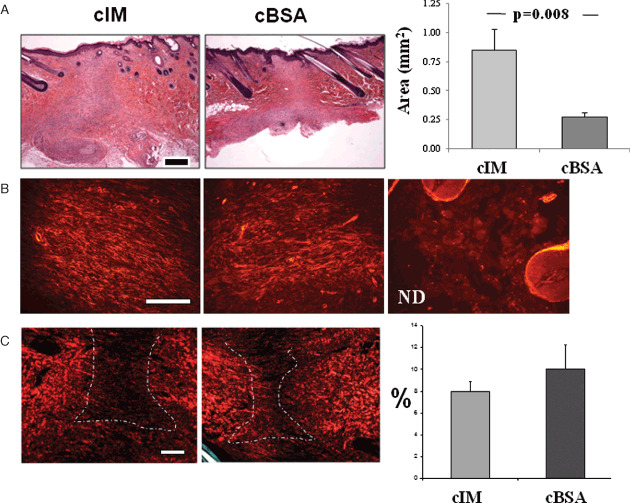
Histological observations and quantification of wounds treated after wound closure (second model). (A) Haematoxylin‐phloxine‐saffron (HPS)‐stained sections of wounds after 14 days (total 34 days) of treatment with cIM and concentrated bovine serum albumin (cBSA) (bar = 500 µm) and the surface areas occupied by the scar tissue are presented after cIM and cBSA treatments. (B) The distribution of α‐smooth muscle (SM) actin was observed after immunohistochemistry in scar tissues treated with cIM and cBSA, and in normal dermis (ND). (C) Birefringent collagen deposited in wounds was observed on picro‐sirius‐stained tissue sections under a polarised light (bar = 250 µm) and the fraction area of collagen deposition in wounds was quantified (means ± SEM are presented; n = 8).
DISCUSSION
Amongst different fractions derived from colostrums, the IMf has the property to limit the contractibility of human dermal fibroblasts in a dose‐dependent manner, which was not because of a decrease in the number of living cells present in the collagen gel. Moreover, those fibroblasts did not acquire stress fibres. By comparison with the crude extract, IMf may be involved in the modulation of tissue remodelling which can be of interest in scar tissue prevention. The question is then whether it should be applied onto wounds either at the early phase of wound healing or after wound closure. Upon wounding, the application of relatively high concentrations of IMf delayed wound contraction, and subsequently wound closure without significant consequences on the morphological patterns within wound tissues. Furthermore, repeated applications of the cIM on closed wounds resulted, in many cases, in the dissipation of the forming scar. The healing behaviour was also reflected by a larger wound tissue as observed on histological sections with non excessive collagen deposition and no change in the distribution of α‐SM actin. Thus, in vitro and in vivo observations are relatively concomitant. On one hand, the wound closure is delayed by the compound, on the other hand, relatively long‐term studies show reduced scarring.
Collagen gel contraction is the result of collagen remodelling by fibroblasts in which the cytoskeleton plays an important role and it may also predict tissue contraction 9, 10 . Disruptors of the cytoskeleton such as colchicine and cytochalasin decrease collagen gel contraction (9) as well as antifibrogenic agents such as pentoxifylline (11). TGF‐β1 and ‐β2, which play a critical role in scarring and fibrosis, induce fibroblasts to contract collagen gel 12, 13, 14. In addition, TGF‐β increased transdifferentiation of fibroblasts into contractile myofibroblasts containing stress fibres 15, 16. Although TGF‐β has been found in bovine milk whey extract and skimmed colostrums 17, 18, 19, the filtration process to obtain IMf may eliminate TGF‐β. Screening IMf for a variety of growth factors and cytokines ended up with the presence of IGF‐1, but not TGF‐β. The presence of IGF‐I, in relatively large amounts, in the IMf may explain less contraction of a collagen gel and collagen production. However, IGFs are also known to enhance wound healing in its early phase (7), which has not been shown in our first wound model. The limitation of gel contraction and the absence of stress fibres in fibroblasts as observed with the IMf suggest that specific compounds within IMf play a role in the modulation of scar tissue and wound contraction as observed in the two guinea pig models. A variety of small peptides (70% below 500 Da) has also been found in the IMf but has not yet been completely identified. Amongst them, it may be possible to find relaxin which has also been detected in milk‐derived products. Relaxin is known to modulate both IGF‐1 and TGF‐β1 and to decrease the expression of α‐SM actin (20). Moreover, colostrum‐derived products may also contain hormones such as estrogens that reduce the inflammatory response and increase collagen deposition, thereby the remodelling phase of the healing wound (21). In addition, it has been recently shown that IMf stimulates the production of antimicrobial peptide, the β‐defensin (personal communication), which is known to promote wound closure in diabetic or infected wounds, by limiting the inflammatory response and by the inhibition of TGF‐β 1, 22, 23, 24, 25.
In the present study, the healing response of full‐thickness open wounds was selected to investigate the effect of a topical compound during the early phase of wound healing in a group of animals and during the later scarring phase in another group. A previous study using this model had focused only on wound contraction rate and microscopic assessment without reporting the clinical appearance (26). In humans, macroscopic evaluation of scar tissues after burns comprises a scale that includes pigmentation, pliability, height and vascularity (27). However, such criteria are difficult to apply in wounds created in animals in which wound contraction, almost inexistent in humans, is also involved in the wound closure process. In addition, there is no ideal scarring wound model in small rodents 28, 29, 30. The morphological aspects of scar tissues and the quantification of scar surface areas can be a valuable assessment of the scarring process in these rodents. Thus, a dissipated scar‐like tissue as observed after cIM treatment can be considered as wounds that are healed with limited remodelling and wound contraction.
Although scar tissue management should be focused on their prophylaxis, the prevalence of hypertrophic scars, keloids and wound contracture after burns is inevitable due to the extent of trauma and individual and genetic considerations. Moreover, their recurrence after reconstructive surgery is still an issue. Topical applications of naturally derived products, such as genistein and camptothecin, have been proposed to improve scarring 31, 32. Colostrum‐derived compounds may have interesting features among the alternative natural products, in which IGF‐1 and relaxin may play a role. The IMf/cIM may be considered as an antagonist of scar tissue formation. However, further studies on larger animals are needed prior to clinical trials.
REFERENCES
- 1. Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem 2009;48: 137–61. [DOI] [PubMed] [Google Scholar]
- 2. Mustoe T. Scars and keloids. BMJ 2004;328:1329–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Juckett G, Hartman‐Adams H. Management of keloids and hypertrophic scars. Am Fam Physician 2009;80:253–60. [PubMed] [Google Scholar]
- 4. Bran G, Goessler U, Hormann K, Riedel F, Sadick H. Keloids: current concepts of pathogenesis (review). Int J Mol Med 2009;24:283–93. [DOI] [PubMed] [Google Scholar]
- 5. Ferguson M, Duncan J, Bond J, Bush J, Durani P, So K, Taylor L, Chantrey J, Mason T, James G, Laverty H, Occleston NL, Sattar A, Ludlow A, O’Kane S. Prophylactic administration of avotermin for improvement of skin scarring: three double‐blind, placebo‐controlled, phase I/II studies. Lancet 2009;373:1264–74. [DOI] [PubMed] [Google Scholar]
- 6. Van Lonkhuyzen D, Hollier B, Shooter G, Leavesley D, Upton Z. Chimeric vitronectin:insulin‐like growth factor proteins enhance cell growth and migration through co‐activation of receptors. Growth Factors 2007;25:295–308. [DOI] [PubMed] [Google Scholar]
- 7. Todorović V, Pesko P, Micev M, Bjelović M, Budec M, Mićić M, Brasanac D, Ilić‐Stojanović O. Insulin‐like growth factor‐I in wound healing of rat skin. Regul Pept 2008;150: 7–13. [DOI] [PubMed] [Google Scholar]
- 8. Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol 1972;54:626–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Redden R, Doolin E. Complementary roles of microtubules and microfilaments in the lung fibroblast‐mediated contraction of collagen gels: dynamics and the influence of cell density. In Vitro Cell Dev Biol Anim 2006;42:70–4. [DOI] [PubMed] [Google Scholar]
- 10. Ngo P, Ramalingam P, Phillips J, Furuta G. Collagen gel contraction assay. Methods Mol Biol 2006;341:103–9. [DOI] [PubMed] [Google Scholar]
- 11. Isaac C, Mathor M, Bariani G, Paggiaro A, Herson M, Goldenstein‐Schainberg C, Carrasco S, Teodoro WR, Yoshinari NH, Ferreira MC. Pentoxifylline modifies three‐dimensional collagen lattice model contraction and expression of collagen types I and III by human fibroblasts derived from post‐burn hypertrophic scars and from normal skin. Burns 2009;35: 701–6. [DOI] [PubMed] [Google Scholar]
- 12. Cutroneo K. TGF‐beta‐induced fibrosis and SMAD signaling: oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen 2007;15(Suppl 1): S54–60. [DOI] [PubMed] [Google Scholar]
- 13. Shah M, Foreman D, Ferguson M. Neutralisation of TGF‐beta 1 and TGF‐beta 2 or exogenous addition of TGF‐beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995;108(pt 3):985–1002. [DOI] [PubMed] [Google Scholar]
- 14. Barrientos S, Stojadinovic O, Golinko M, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 15. Cowin AJ. Differential expression of F‐actin in in utero fetal wounds. Eur J Dermatol 2005;15: 133–9. [PubMed] [Google Scholar]
- 16. Cowin AJ, Hatzirodos N, Teusner JT, Belford DA. Differential effect of wounding on actin and its associated proteins, paxillin and gelsolin, in fetal skin explants. J Invest Dermatol 2003;120: 1118–29. [DOI] [PubMed] [Google Scholar]
- 17. Rayner T, Cowin A, Robertson J, Cooter R, Harries R, Regester G, Smithers GW, Goddard C, Belford DA. Mitogenic whey extract stimulates wound repair activity in vitro and promotes healing of rat incisional wounds. Am J Physiol Regul Integr Comp Physiol 2000;278:R1651–60. [DOI] [PubMed] [Google Scholar]
- 18. Takayama Y, Kitsunai K, Mizumachi K. Factors in bovine colostrum that enhance the migration of human fibroblasts in type I collagen gels. Biosci Biotechnol Biochem 2001;65:2776–9. [DOI] [PubMed] [Google Scholar]
- 19. Torre C, Jeusette I, Serra M, Brazis P, Puigdemont A. Bovine colostrum increases proliferation of canine skin fibroblasts. J Nutr 2006; 136 (7 Suppl) :2058S–60S. [DOI] [PubMed] [Google Scholar]
- 20. Samuel CS, Unemori EN, Mookerjee I, Bathgate RA, Layfield SL, Mak J, Tregear GW, Du XJ. Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology 2004;145:4125–33. [DOI] [PubMed] [Google Scholar]
- 21. Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest 2003;111:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malmsten M, Davoudi M, Walse B, Rydengård V, Pasupuleti M, Mörgelin M, Schmidtchen A. Antimicrobial peptides derived from growth factors. Growth Factors 2007;25:60–70. [DOI] [PubMed] [Google Scholar]
- 23. Steinstraesser L, Koehler T, Jacobsen F, Daigeler A, Goertz O, Langer S, Kesting M, Steinau H, Eriksson E, Hirsch T. Host defense peptides in wound healing. Mol Med 2008;14:528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park H, Cho D, Kim H, Lee J, Cho B, Bang S, Song SY, Yamasaki K, Di Nardo A, Gallo RL. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL‐37. J Invest Dermatol 2009;129: 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stelwagen K, Carpenter E, Haigh B, Hodgkinson A, Wheeler TT. Immune components of bovine colostrum and milk. J Anim Sci 2009;87 (13 Suppl):3–9. [DOI] [PubMed] [Google Scholar]
- 26. Vooijs D, Walboomers X, Parker J, Von den Hoff J, Jansen J. Transforming growth factor‐beta3‐loaded microtextured membranes for skin regeneration in dermal wounds. J Biomed Mater Res A 2004;70:402–11. [DOI] [PubMed] [Google Scholar]
- 27. Akita S, Akino K, Imaizumi T, Hirano A. Basic fibroblast growth factor accelerates and improves second‐degree burn wound healing. Wound Repair Regen 2008;16:635–41. [DOI] [PubMed] [Google Scholar]
- 28. Ramos M, Gragnani A, Ferreira L. Is there an ideal animal model to study hypertrophic scarring? J Burn Care Res 2008;29:363–8. [DOI] [PubMed] [Google Scholar]
- 29. Saulis A, Mogford J, Mustoe T. Effect of Mederma on hypertrophic scarring in the rabbit ear model. Plast Reconstr Surg 2002;110:177–83. [DOI] [PubMed] [Google Scholar]
- 30. Aksoy M, Vargel I, Canter I, Erk Y, Sargon M, Pinar A, Tezel GG. A new experimental hypertrophic scar model in guinea pigs. Aesthetic Plast Surg 2002;26:388–96. [DOI] [PubMed] [Google Scholar]
- 31. Cao C, Li S, Dai X, Chen Y, Feng Z, Zhao Y, Wu J. Genistein inhibits proliferation and functions of hypertrophic scar fibroblasts. Burns 2009;35:89–97. [DOI] [PubMed] [Google Scholar]
- 32. Zhang G, Gao W, Li X, Yi C, Zheng Y, Li Y, Xiao B, Ma XJ, Yan L, Lu KH, Han Y, Guo SZ. Effect of camptothecin on collagen synthesis in fibroblasts from patients with keloid. Ann Plast Surg 2009;63:94–9. [DOI] [PubMed] [Google Scholar]


